Abstract
Background
Anemia is a characteristic of myelodysplastic syndromes, such as the rare 5q- syndrome, but its mechanism remains unclear. In particular, data are lacking on the terminal phase of differentiation of erythroid cells (enucleation) in myelodysplastic syndromes.
Design and Methods
We used a previously published culture model to generate mature red blood cells in vitro from human hematopoietic progenitor cells in order to study the pathophysiology of the 5q- syndrome. Our model enables analysis of cell proliferation and differentiation at a single cell level and determination of the enucleation capacity of erythroid precursors.
Results
The erythroid commitment of 5q(del) clones was not altered and their terminal differentiation capacity was preserved since they achieved final erythroid maturation (enucleation stage). The drop in red blood cell production was secondary to the decrease in the erythroid progenitor cell pool and to impaired proliferative capacity. RPS14 gene haploinsufficiency was related to defective erythroid proliferation but not to differentiation capacity.
Conclusions
The 5q- syndrome should be considered a quantitative rather than qualitative bone marrow defect. This observation might open the way to new therapeutic concepts.
Keywords: myelodysplastic syndrome, 5q- syndrome, erythroid differentiation, enucleation
Introduction
The mechanism of acquired alterations of erythropoiesis in myelodysplastic syndromes (MDS) has been the subject of countless publications.1,2 However, no study so far has addressed the terminal phase of erythroid cell differentiation, namely, enucleation.3 Only the proliferation and commitment phases of erythroid cell pathophysiology have undergone scrutiny, because there is no culture model for enucleation.
Anemia is the main feature of MDS. MDS are thought to be due to acquired clonal anomalies of hematopoietic stem cells,4 in particular to a defect in the differentiation of these cells.5,6 This is the rationale that underpins current treatment.7 However, the lack of a complete in vitro model for erythropoiesis that includes enucleation means that the pathophysiology of anemia remains unclear. In addition, the relatively mild anemia observed in some patients with a high percentage of abnormal clones in their bone marrow remains a paradox.8
5q- syndrome is a rare syndrome characterized by an isolated del(5q) cytogenetic abnormality, macrocytic anemia, fewer than 5% of marrow and blood blast cells, and a favorable clinical outcome. One treatment option is lenalidomide, a drug that has received approval from the Food and Drug Administration for the treatment of MDS in patients with an interstitial deletion of the long arm of chromosome 5. It effectively reduces red blood cell (RBC) requirements. The 5q deletion was the first cytogenetic abnormality to be associated with a distinct clinical phenotype in cases of malignancy.9
Recently, a defect in the function of a ribosomal protein subunit (RPS14) has been implicated in 5q- syndrome.10,11 The RPS14 gene is located in the deleted region. Its partial inactivation in normal hematopoietic progenitor cells gives rise to a phenotype that matches the 5q- syndrome. RPS14 deficiency affects erythroid differentiation.
Earlier reports by our team have described an in vitro model of erythropoiesis in which mature RBC are generated from human progenitor cells.12,13 This model can be used to analyze cell proliferation and differentiation in a homogeneous erythroid population in culture, and to measure the enucleation capacity of erythroid precursors. In the present study, we used this model to investigate whether RBC production is altered in patients with 5q- syndrome and whether terminal maturation (enucleation capacity) is impaired. By furthering our understanding of anemia in 5q-deleted MDS, we may be able to design novel treatment strategies.
Design and Methods
Patients
Patients were classified according to the French-American-British (FAB) and World Health Organization (WHO) classification system. Five patients with 5q- syndrome (fewer than 5% blasts in the marrow and a single chromosome abnormality, namely, the 5q deletion) entered the study; their median age was 82 years (range, 72–86 years) (Table 1). The median hemoglobin concentration was 9.2 g/dL (range, 7.5–9.4 g/dL). At the time of bone marrow sampling, four out of the five patients required transfusions. Patients were classified as having either a low (0) or intermediate 1 (0.5) prognosis according to the International Prognostic Scoring System (IPSS). All were heterozygous for the 5q deletion and had the same breakpoint region. The percentage of 5q deleted clones was 79% (range, 54–81%) by standard karyotyping of whole bone marrow and 96% (range, 91–98%) in CD34+ cells by fluorescence in situ hybridization (FISH) analysis. Normal control bone marrow samples were obtained from six healthy individuals with a median age of 83 years (range, 71–86 years). Both patients and control subjects gave their informed consent to participation in this study, which followed the guidelines of the ethical committee for research at Saint Antoine Hospital.
Table 1.
Patients’ characteristics.
Cell culture
CD34+ cells were isolated by supermagnetic microbead selection using Mini-MACS columns (Miltenyi Biotech, Bergisch Glodbach, Germany). The purity of the isolated cells was 92 ± 6%. The cells were plated in a liquid culture medium based on Iscove’s modified Dulbecco’s medium–glutamax (Biochrom, Berlin, Germany) and heparinized human plasma.
The expansion procedure was a modification14 of our previously published three-step technique.12,13 In the first step (days 0–8), CD34+ cells (104/mL) were cultured in the presence of 10−6 M hydrocortisone (Sigma), 100 ng/mL stem cell factor (SCF; kindly provided by Amgen, Thousand Oaks, CA, USA), 5 ng/mL interleukin-3 (IL-3; R&D Systems, Abingdon, UK) and 3 IU/mL erythropoietin (Eprex, kindly provided by Janssen-Cilag, Issyles-Moulineaux, France). On day 4, one volume of cell culture was diluted in four volumes of fresh medium containing hydrocortisone, SCF, IL-3 and erythropoietin. In the second step (days 8–11), the cells were resuspended at 3×105/mL in fresh medium supplemented with SCF and erythropoietin. In the third step (up to day 18), the cells were cultured in fresh medium in the presence of erythropoietin alone. Cell counts were adjusted to 1×106 and 5×106 cells/mL on days 11 and 15, respectively. The cultures were maintained at 37°C in 5% CO2 in air. Results are expressed as expansion rates after plating. Cells were stained with May-Grünwald-Giemsa (MGG, Sigma) for morphological analyses. They were then spotted on slides. Cytological observations were evaluated by microscopic analysis on at least 300 cells/slide.
Semisolid culture assays
Burst-forming unit-erythroid (BFU-E) progenitors were assayed in methylcellulose cultures as previously described.15 The cultures were incubated at 37°C in 5% CO2 in air, and colonies were scored on day 14.
Limiting dilution assay
To study erythroid differentiation at a clonal level, we performed a limiting dilution assay (LDA) starting from CD34+ MDS cells.15 The cells were seeded on day 0 in 96-well microtiter plates in a final volume of 100 μL per well. Dilution steps were 1/200 cells/well with 10 to 240 replicates per dilution. Culture media and cytokines were as described above in the Cell culture section. The medium was changed from day 7 to day 18 in line with cell amplification. On day 11, frequencies were calculated by Poisson statistics. On day 14, before the enucleation stage, karyotyping was performed using half of the cells from each well; the remaining cells were maintained in culture until day 18 to measure RBC content. The abnormal karyotypes identified at diagnosis in patients with 5q(del) were detected by FISH analysis of interphase nuclei. Thus, at the end of culture, it was possible to correlate the level of differentiation with the karyotype and to calculate the number of RBC produced by one 5q(del) CD34+ cell as compared to one normal CD34+ cell.
Conventional and molecular cytogenetic analyses at diagnosis
Conventional chromosome analyses were performed on unstimulated bone marrow aspirates after culture for 24 h in RPMI 1640 supplemented with fetal calf serum. Chromosomes were GTG- and RHG-banded and at least 20 metaphases were analyzed using standard procedures. The karyotypes are described according to the International System for Human Cytogenetic Nomenclature.16 Molecular cytogenetic (FISH) analysis of metaphases was performed using the LSI EGR-1/D5S721, D5S23 Dual Color Probe (Vysis, Downers Grove, IL, USA). Hybridization procedures were carried out according to the manufacturer’s guidelines.
Molecular cytogenetic analysis of cultured limiting dilution assay cells
Slide preparations of pooled, cultured LDA cells were obtained using standard procedures and adjusted for the limited number of cells. FISH analysis of interphase nuclei was performed using the LSI EGR-1/D5S721, D5S23 Dual Color Probe (Vysis, Downers Grove, IL, USA). Hybridization procedures were carried out according to the manufacturer’s guidelines. The signals were analyzed with the Genikon System (Nikon Europe B.V., The Netherlands). Whereas normal cells display four distinct signals (two orange, two green), deleted cells show only one orange and two green signals. The number of nuclei examined depended on the number of available cells, but whenever possible at least 200 nuclei were analyzed per pooled LDA cell.
Measurement of RPS14 expression
RBC were collected on days 0, 4, 8, 11 and 15 from bulk erythroid cultures of CD34+ cells from 5q(del) patients and normal donors. Depending on the number of cells, total RNA was extracted using the Trizol method (Invitrogen, Paisley, Scotland) or an extraction kit (Ambion RNAqueous-4 PCR kit) according to manufacturers’ guidelines. DNase-treated RNA (1 μg) was transcribed into cDNA using 200 units of SuperScript II reverse transcriptase (Invitrogen) and 150 ng of random primers (Invitrogen), and the resulting cDNA was aliquoted to avoid repeated freeze/thaw cycles. Real-time polymerase chain reaction amplification was performed in an ABI PRISM 7500 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The RPS14 gene was amplified to establish a relative gene expression pattern. The gene for β2-microglobulin was used as a reference. All primer sequences and probes were from PE Applied Biosystems. The polymerase chain reaction runs were performed in duplicate using Taqman Master Mix (Applied Biosystems) with 10 ng of cDNA and 300 nM of primers in a final reaction volume of 20 μL. After incubation for 2 min at 50°C, AmpliTaq Gold was activated by incubation for 10 min at 95°C. A total of 40 amplification cycles were run with an annealing temperature of 60°C. Calibration curves were established to check that the polymerase chain reaction efficiencies were similar for RPS14 and the β2-microglobulin gene. The relative expression of RPS14 in samples from patients and normal subjects was calculated according to the formula:17 fold expression =2−ΔΔCt where ΔΔCt = (CtRPS14 − Ctβ2m) on the day of culture – (CtRPS14 −Ctβ2m) on day 0 of culture. This calculation defines the expression of a target gene relative to a reference gene and is suited to exploring physiological changes in gene expression levels. If 2−ΔΔCt is greater than one, RPS14 is up-regulated at a given time of culture and if 2−ΔΔCt is less than one, it is down-regulated. In the LDA analysis, the higher the ΔCt (CtRPS14 −Ctβ2m), the lower the expression of RPS14.
Statistical analyses
Values are expressed as medians with their ranges. Differences between groups were analyzed with the Mann-Whitney test. P values of less than or equal to 0.05 are considered statistically significant.
Results
Overall capacity of 5q(del) bone marrow cells to generate mature red blood cells in vitro
To evaluate whether bone marrow progenitor cell populations from 5q(del) patients behave like those from normal controls with regard to in vitro capacity to generate mature enucleated RBC, we applied the three-step protocol described in the Design and Methods section. CD34+ cell proliferation and erythroid commitment were first induced with SCF, IL-3, erythropoietin and hydrocortisone for 8 days. Erythroid proliferation was then amplified with SCF and erythropoietin for 3 days. Finally, the cells were maintained until terminal maturation in the presence of erythropoietin alone up to day 18.
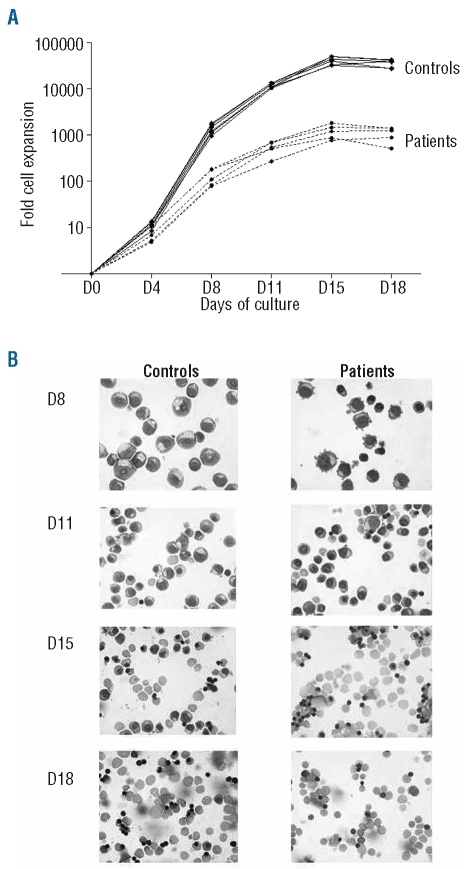
The rate of increase of CD34+ cells from 5q(del) patients was 33-fold lower than that of cells from control subjects throughout culture. On day 18, the median fold expansion was 1,265 (range, 515–1,398) versus 38,962 (range, 27,738–43,305) (P<0.005) (Figure 1A). Erythroid commitment, on the other hand, was as high in patients as in controls. By day 8, 91% (range, 87–98%) of cells were proerythroblasts/basophilic erythroblasts in patients; the corresponding figure in controls was 98% (range, 96–99%) (P=0.1). Erythroid cells in both patients and controls continued to proliferate/differentiate and achieved complete maturation into enucleated RBC: 67% (range, 45–76%) versus 45% (range, 31–51%) on day 15, and 86% (range, 86–90%) versus 86% (range, 80–88%) on day 18 (P=0.46 on day 18) (Figure 1B).
Figure 1.
In vitro proliferation of erythroid progenitors. (A) CD34+ cells from five patients with 5q- syndrome (•–•) and six healthy donors (+-+) were cultured for 18 days in a liquid medium according to a three-step protocol. Total cell numbers were counted at the indicated times. Results are expressed as fold-expansion on day 18 relative to day 0. (B) Photographs of the cells on days 8, 11, 15 and 18 of liquid culture after May-Grünwald-Giemsa staining. Each stage of erythroid maturation is represented: proerythroblast, basophilic erythroblast, polychromatophilic erythroblast, orthochromatic erythroblast and RBC (magnification × 630). Results are representative of cytospin samples prepared from a 5q(del) patient and a healthy donor.
In MDS patients, CD34+ cells are a mixed population of malignant and non-malignant cells. Among this population, from the expansion rates and enucleation levels, we calculated that, on day 18, CD34+ cells from MDS patients generated 32 times fewer RBC than normal CD34+ bone marrow cells: 1,088 (range, 413–1,248) versus 33,043 (range 24, 410–34,644) RBC/CD34+ cell, respectively (P<0.005). This decrease in RBC generation in 5q(del) patients could be partly related to the impaired clonogenic capacity of the bone marrow samples at the time of collection as the median number of BFU-E was 17 (range, 9–100) colonies/104 cells in MDS patients versus 735 (range, 420–1110) colonies/104 cells in controls (P<0.005).
FISH analysis during culture showed that the proportion of 5q(del) clones decreased steadily over time. On day 15, 56 to 92% of the cells did not express 5q(del). As 5q-clones are outgrown by their non-del (5q) counterparts in bulk culture,18 the vast majority of RBC present on day 18 presumably derived from the residual non-deleted clones that were seeded. Interestingly, the observed deficit in proliferation was correlated with the residual percentage of deleted clones (r2= 0.96). In the absence of 5q(del) clones, the proliferation rate reached a normal level.
To determine the contribution of non-deleted and 5q-deleted clones to defective erythropoiesis in 5q(del) patients, we performed the following experiments.
Capacity of 5q(del) clones to achieve full maturation into red blood cells
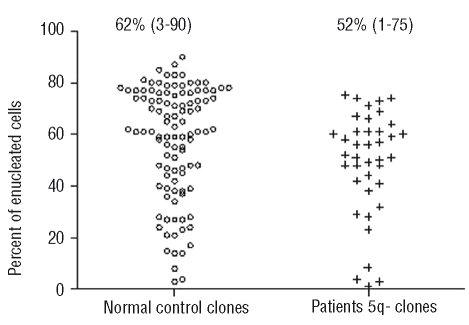
We first investigated whether RBC generated in vitro from patients originated from non-deleted clones and/or from pathological, i.e. 5q(del) clones. The data were compared to the RBC-generating capacity of CD34+ clones from normal controls. To study erythroid differentiation at a clonal level and discover whether in vitro maturation is related to the presence of 5q(del), we performed a LDA starting with CD34+ cells. On day 18, 5q(del) clones and healthy clones generated enucleated RBC to similar extents: median level 52% (range, 1–75%) versus 62% (range, 3–90), respectively (P=0.7) (Figure 2). Taking in account proliferation levels and enucleation rates, this implies that one CD34+ cell from the 5q(del) clone generated 88 times fewer RBC than one CD34+ cell from a healthy clone [one 5q(del) clone cell generated 2.02±1.01×104 RBC and one healthy clone cell 1.77±0.196×106 RBC]. We then investigated whether diminished erythroid production by the 5q(del) clone was related to a maturation defect, i.e. impaired enucleation, or was due to impaired clonal proliferation. We focused on differentiation as from day 15. We calculated the number of RBC generated by one erythroblast from the number of erythroblast cells on day 15 and of enucleated cells on day 18, after correction for cell amplification. Using an LDA, we selected a 100% pure 5q(del) population and compared it to a healthy clone. No impairment in the 5q(del) clone’s ability to achieve final maturation was observed. A single erythroblast from the 5q(del) clone generated 0.54 RBC and that from the healthy clone 0.53 RBC. We concluded that the decreased RBC production was not due to a diminished intrinsic incapacity to expel the nucleus.
Figure 2.
Generation of enucleated RBC from one CD34+ cell in a LDA. Wells were analyzed for the percentage of enucleated RBC on day 18 (each dot is the result for one well). Data are from three control subjects and three patients. 5q(del) clones were able to generate enucleated RBC to a similar extent as controls: median level of enucleation: 62% (range, 3–90) vs. 52% (range, 1–75) respectively (P=0.7).
Non-deleted erythropoiesis in 5q(del) patients
To determine whether the capacity to generate RBC was impaired in non-deleted clones in the bone marrow of 5q(del) patients, we selected a 100% cytogenetically non-deleted population by LDA and assessed its erythroid competence. On day 18, one CD34+ cell from the non-deleted clone generated 3.7×105 RBC, compared to 1.77×106 for one CD34+ cell from the healthy control and 2.02×104 for one CD34+ 5q(del) clone cell. Overall, residual non-deleted clones in MDS patients generated 18 times more RBC than 5q(del) clone, but five times fewer RBC than the healthy clone.
RPS14 gene expression in erythroid cultures and its correlation with cell proliferation rate and terminal differentiation
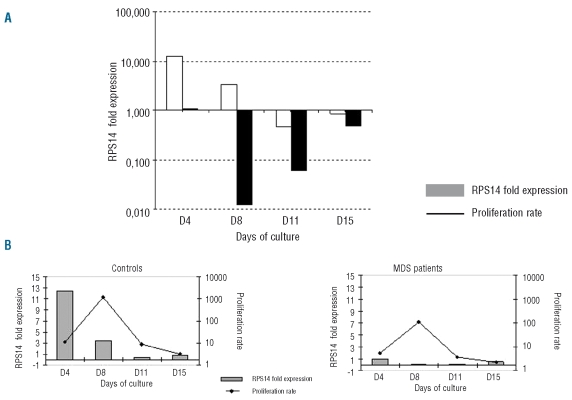
We compared the kinetics of RPS14 expression during culture of CD34+ cells derived from 5q(del) patients (n=3) and controls (n=3) using real-time quantitative reverse transcriptase polymerase chain reaction (see protocol in the Cell culture section) (Figure 3A). RPS14 expression diminished gradually in control cells whereas marked under-expression was noted throughout culture in cells from 5q(del) patients. Mean gene expression was several fold higher in controls than in patients (12.65 versus 1.09 on day 4, 3.45 versus 0.012 on day 8, 0.47 versus 0.06 on day 11, and 0.86 versus 0.845 on day 15). We then correlated the kinetics of RPS14 with the cell proliferation rate. In normal controls, RPS14 was highly expressed between day 0 and day 8, during the proliferation phase, and was then under-expressed from day 8 to day 15, during the differentiation phase. In cells from 5q(del) patients, RPS14 under-expression throughout culture was correlated with a low cell proliferation rate (Figure 3B).
Figure 3.
(A) RPS14 gene expression during erythroid culture. Data are from three patients (▪) and three control subjects (□). Results are expressed as the mean value of 2−ΔCt (see Design and Methods). (B) Correlation with cell proliferation rate. RPS14 gene expression is the mean value of 2−ΔCt.
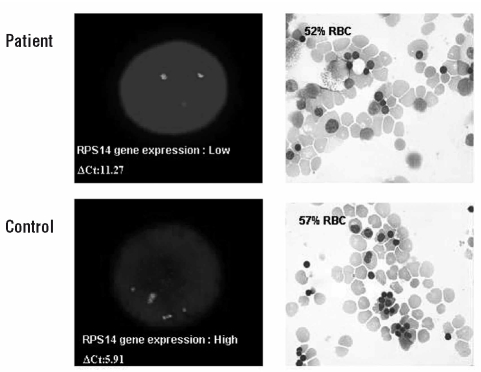
We also studied RPS14 gene expression at the single cell level in a LDA. We compared expression in 5q(del) clones [over 99% 5q (del) clones by FISH analysis] and normal clones on day 8 and related this expression to stage of enucleation on day 18. Whereas gene expression in pathological clones was at least half that in normal clones on day 8, enucleation rates were similar on day 18 (Figure 4) (ΔCt = 11.27 versus 5.91, respectively).
Figure 4.
RPS14 gene expression and generation of enucleated RBC in a LDA. On day 8, 5q (del) clones were pooled [>99% 5q (del) clones as determined by FISH analysis]. RPS14 gene expression was measured by real time RT-PCR and normalized to β2 microglobulin: ΔCt=CtRPS14 − Ctβ2m; the higher the ΔCt, the lower the gene expression. We then determined the percentage of enucleated RBC on day 18. A similar analysis was performed with normal clones.
The action of lenalidomide action in patients with 5q(del) syndrome
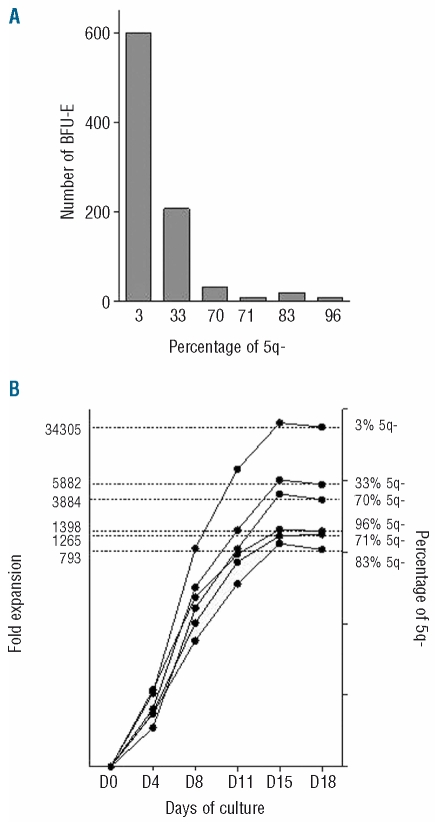
We studied the action of lenalidomide in two patients with 5q(del) syndrome at treatment initiation and after 4 and 10 months of treatment. As the patients were becoming transfusion independent at 4 months, the percentage of 5q(del) clones decreased, and the BFU-E and amplification capacity increased. On pooling the data (Figure 5A–B), we found an ex vivo correlation between the percentage of 5q(del) clones, BFU-E numbers, and amplification capacity.
Figure 5.
(A) BFU-E numbers from two 5q- syndrome patients treated with lenalidomide. BFU-E numbers and percent 5q(del) clones for Patient 1 were 10 (71%) on day 0 of treatment, 600 (3%) at 4 months and 20 (83%) at 8 months. The corresponding figures for patient 2 were 9 (96%), 207 (33%), and 33 (70%), (B) Ex vivo proliferation of erythroid progenitors. CD34+ cells obtained at the same time-points were cultured for 18 days in a liquid medium according to a three-step protocol. Total cell numbers were counted. Results are expressed as fold-expansion on day 18 relative to day 0 and correlated with percent 5q(del) clones.
Discussion
In the absence of a way to study the terminal phase of enucleation, information on defective erythropoiesis in MDS has remained incomplete. The in vitro model of erythropoiesis that we have developed enables a differential analysis of cell proliferation, commitment and enucleation, and the monitoring of healthy and pathological clones at the single cell level by the use of molecular markers such as the 5q deletion detected by FISH analysis. Our model can, therefore, be used to analyze the erythroid lineage from the early stage of CD34+ progenitors to the late stage of differentiation into enucleated RBC. We established that: (i) erythroid commitment of the pathological clones in patients with 5q- syndrome was not impaired; (ii) terminal differentiation capacity was preserved since final erythroid maturation, including the stage of enucleation, was achieved; (iii) the drop in RBC production was secondary to a decrease in the erythroid progenitor cell pool and to impaired proliferative capacity.
Our results differ somewhat from those of Span et al., who described abrogation of enhanced proliferation of MDS progenitors by increased apoptosis.19 However, the two studies are not entirely comparable. Our study focused on the 5q- syndrome (IPSS score <1) whereas the study by Span et al. concerned high-risk MDS patients (IPSS≥1.5); only one patient (with sideroblastic anemia) had low-risk disease. The starting cell populations were also different (CD34+ cells versus bone marrow mononuclear cells) as were culture conditions. We engaged CD34+ cells into the erythroid lineage and analyzed BFU-E colonies whereas Span et al. used a cocktail of cytokines without erythropoietin and correlated their results with the clonogenic capacity associated with an apoptosis score.
Our focus was on erythroid cell line proliferation and differentiation, and on the faithful expression of CD34 on MDS cells. We cannot, however, rule out that CD34 is expressed in more terminally differentiated erythroid cells in 5q(del) patients, leading to an apparent decrease in proliferative capacity per cell. However, in a study of two patients responding to lenalidomide, we found a direct correlation between the percentage of 5q(del) clones and the proliferative capacity of CD34+ cells. With fewer than 5% 5q(del) clones, proliferation returned to normal. The same was true for BFU-E content; BFU-E numbers normalized with the elimination of the 5q(del) clone.
A large number of pathophysiological mechanisms have been proposed for MDS, ranging from abnormalities of the bone marrow stroma20,21 to autoimmune mechanisms.22,23 The major defects are nevertheless attributed to disorders of cellular proliferation/differentiation. Our results on terminal differentiation are novel but those on cell proliferation confirm the findings of many other studies. The primary target of 5q deletions is known to be a lymphomyeloid hematopoietic stem cell.24 5q(del) stem cells are CD34+CD38− but inefficient in reconstituting hematopoiesis.25 The ineffective erythropoiesis has been correlated with an increase in intramedullary Fas-dependent apoptosis of differentiated cells.26–28 Interestingly, in our conditions, CD34+ progenitor cells were able to proliferate in the presence of human plasma whereas impaired proliferation has been observed in a serum-free medium despite optimal concentrations of SCF, Flt-3 ligand, thrombopoietin, IL-3, granulocyte colony-stimulating factor, granulocyte-monocyte colony-stimulating factor and erythropoietin.29 Our results do not confirm the impaired cell differentiation due to excess apoptosis that was observed by Choi et al. in an analysis of membrane phenotype in a recent murine model of MDS.30 That study did not, however, address enucleation. The findings of our study and the one by Choi et al. were, however, concordant regarding progenitor deficiency (27-fold less in our study versus 10-fold less) and proliferative capacity which was much reduced (33-fold versus 30-fold decrease, respectively).
Haploinsufficiency of the gene encoding for RPS14 is thought to cause the characteristic hematologic phenotype defining the 5q- syndrome, namely blockade of erythroid differentiation.10,11 Our observations challenge this view. We show that complete differentiation to the enucleated state is possible. Basal RPS14 expression was relatively low even in healthy individuals, but was much reduced in 5q(del) patients. We compared the kinetics of RPS14 expression in erythroid cells derived from progenitor cells obtained from patients with 5q- syndrome and healthy controls. We found that in controls, RPS14 was strongly expressed during the proliferation phase and very much under-expressed during the terminal differentiation phase, which suggests a role for RPS14 during proliferation and not during differentiation. Indeed, in patients, RPS14 was under-expressed throughout culture: the rate of proliferation was very much decreased, but the cells did ultimately differentiate into RBC. The kinetics of RPS14 expression support our hypothesis that anemia in 5q- syndrome is not linked to an incapacity of progenitor cells to differentiate into RBC but arises solely from the proliferative defect of the progenitor cells.
That the enucleation capacity of 5q(del) clones does not depend on RPS14 gene expression was confirmed by single cell analysis in a LDA. Our findings indicate that the RPS14 gene is involved in the proliferation of erythroid precursors rather than in a true process of terminal differentiation, i.e. enucleation, and are supported by those of Ebert et al.10 who attributed 5q(del) syndrome to haploinsufficiency of the RPS14 gene. However, unlike these authors, who associated the lack of differentiation into RBC with RPS14 under-expression, on the basis of decreased expression of antigenic markers (CD71 or glycophorin A), we did not observe impaired differentiation since the erythroid cells derived from progenitor cells obtained from 5q(del) patients reached the final stage of differentiation (i.e. enucleation) under our culture conditions.
Recent work on erythropoiesis in Blackfan-Diamond anemia has revealed similar effects to those found in our study. A quarter of patients with Blackfan-Diamond anemia carry a mutation of the RPS19 gene responsible for an anomaly of ribosomal synthesis. Miyake et al. reported cell cycle blockade with excess apoptosis and much impaired cell proliferation, but no effect on terminal erythroid differentiation.31 The findings of this study might support novel treatment strategies for 5q- syndrome. Lenalidomide is able to suppress 5q(del) clones, as indicated by the frequently observed cytogenetic remission in treated patients.32–35 However, lenalidomide might increase the risk of progression to acute myeloid leukemia through a mechanism of selective pressure.11 For this reason, in 2008 the Committee for Medicinal Products for Human Use of the European Medicines Agency recommended the refusal of marketing authorization for lenalidomide, intended for treatment of transfusion-dependent patients with MDS associated with del(5q) and with a low to intermediate risk of progressing to leukemia or death. An alternative therapeutic strategy might be aimed at reducing apoptosis and restoring proliferation of 5q(del) clones rather than at suppressing them. For example, inhibition of FADD-dependent caspase-8 activation might be used to restore peripheral RBC counts.28
As we have shown, once they expand, 5q(del) clones will differentiate into RBC. Corticosteroids represent a treatment option in Blackfan-Diamond anemia and it is of interest to note that in our in vitro culture conditions corticosteroids increased the proliferation of erythroid progenitor cells. In fact, the combination of corticosteroids and lenalidomide has been proposed. In our study, we assumed that non-deleted clones in MDS patients were residual normal clones. However, their capacity to generate RBC was lower than that of controls because of impaired cell proliferation. The reason for this impairment is unclear. Direct contact inhibition can be ruled out since the analysis was performed at the single cell level.
MDS is a clonal disease based on markers that are not acquired. However, cytogenetic abnormalities are acquired and do not, therefore, necessarily define the size of the initial neoplastic clone. Thus, cytogenetically normal cultures from MDS CD34+ cells may not have arisen from residual normal stem cells. This might explain the partial defect we noted in erythroid expansion. One could also argue that a 5-fold difference in expansion in an 18-day culture is relatively minor. Moreover, a CD34+ cell from a MDS patient is different from a CD34+ cell from a healthy control. MDS CD34+ progenitor cells are heterogeneous with different expansion capacities, which could explain this small difference. A careful phenotypic analysis of the early progenitor compartments, such as the CD34+/CD38− subpopulation, would have been helpful.
On the basis of the above findings, it is unlikely that the decreased erythroid maturation and subsequent anemia seen in 5q- syndrome is caused by a specific blockade of late differentiation. The anemia is more likely to be the consequence of the proliferative defect of both pathological and residual non-deleted clones in the patient’s bone marrow, their enucleation capacity remaining unchanged.
In conclusion, our erythroid model is valuable for analyzing erythropoiesis in hematologic malignancies. We used it to investigate the 5q- syndrome, but it could also be applied to other MDS with a karyotypic marker. Our study revealed that the 5q- syndrome is a quantitative rather than merely qualitative defect of bone marrow. This observation might open the way to new therapeutic concepts.
Acknowledgments
The authors thank Isabelle Pinson and Nathalie Guillot (Service de Cytogénétique, Hôpital Saint-Antoine, Paris) for expert technical assistance with FISH analyses. We also thank Pr Pierre Fenaux and Dr. Lionel Ades for providing some patients’ samples (Service d’Hématologie, Hôpital Avicenne, Bobigny).
Footnotes
Authorship and Disclosures
LG, LK, CM, CdW: performed the experiments; LG: collected samples; LG, LK, NCG, HL, LD: analyzed results, produced the figures, wrote the paper; LG, LK, NCG, MCG, HL, LD: analyzed results; LG, LK, MCG, HL, LD: designed the research.
The authors reported no potential conflicts of interest.
Funding: this work was supported by grants from the Association Combattre La Leucémie (CLL) and the Etablissement Français du Sang (EFS).
References
- 1.Cazzola M, Malcovati L. Myelodysplastic syndromes: coping with ineffective hematopoiesis. N Engl J Med. 2005;352(6):536–8. doi: 10.1056/NEJMp048266. [DOI] [PubMed] [Google Scholar]
- 2.Nimer SD. Myelodysplastic syndromes. Blood. 2008;111(10):4841–51. doi: 10.1182/blood-2007-08-078139. [DOI] [PubMed] [Google Scholar]
- 3.Bessis M. Erythroblastic island, functional unity of bone marrow. Rev Hematol. 1958;13(1):8–11. [PubMed] [Google Scholar]
- 4.Corey SJ, Minden MD, Barber DL, Kantarjian H, Wang JC, Schimmer AD. Myelodysplastic syndromes: the complexity of stem-cell diseases. Nat Rev Cancer. 2007;7(2):118–29. doi: 10.1038/nrc2047. [DOI] [PubMed] [Google Scholar]
- 5.Lee YT, Miller LD, Gubin AN, Makhlouf F, Wojda U, Barrett AJ, et al. Transcription patterning of uncoupled proliferation and differentiation in myelodysplastic bone marrow with erythroid-focused arrays. Blood. 2001;98(6):1914–21. doi: 10.1182/blood.v98.6.1914. [DOI] [PubMed] [Google Scholar]
- 6.Tehranchi R, Invernizzi R, Grandien A, Zhivotovsky B, Fadeel B, Forsblom AM, et al. Aberrant mitochondrial iron distribution and maturation arrest characterize early erythroid precursors in low-risk myelodysplastic syndromes. Blood. 2005;106(1):247–53. doi: 10.1182/blood-2004-12-4649. [DOI] [PubMed] [Google Scholar]
- 7.Hellstrom-Lindberg E, Malcovati L. Supportive care, growth factors, and new therapies in myelodysplastic syndromes. Blood Rev. 2008;22(2):75–91. doi: 10.1016/j.blre.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 8.List A, Kurtin S, Roe DJ, Buresh A, Mahadevan D, Fuchs D, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352(6):549–57. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- 9.Van den Berghe H, Cassiman JJ, David G, Fryns JP, Michaux JL, Sokal G. Distinct haematological disorder with deletion of long arm of no. 5 chromosome. Nature. 1974;251(5474):437–8. doi: 10.1038/251437a0. [DOI] [PubMed] [Google Scholar]
- 10.Ebert BL, Pretz J, Bosco J, Chang CY, Tamayo P, Galili N, et al. Identification of RPS14 as a 5q- syndrome gene by RNA interference screen. Nature. 2008;451(7176):335–9. doi: 10.1038/nature06494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cazzola M. Myelodysplastic syndrome with isolated 5q deletion (5q- syndrome). A clonal stem cell disorder characterized by defective ribosome biogenesis. Haematologica. 2008;93(7):967–72. doi: 10.3324/haematol.13377. [DOI] [PubMed] [Google Scholar]
- 12.Neildez-Nguyen TM, Wajcman H, Marden MC, Bensidhoum M, Moncollin V, Giarratana MC, et al. Human erythroid cells produced ex vivo at large scale differentiate into red blood cells in vivo. Nat Biotechnol. 2002;20(5):467–72. doi: 10.1038/nbt0502-467. [DOI] [PubMed] [Google Scholar]
- 13.Giarratana MC, Kobari L, Lapillonne H, Chalmers D, Kiger L, Cynober T, et al. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol. 2005;23(1):69–74. doi: 10.1038/nbt1047. [DOI] [PubMed] [Google Scholar]
- 14.Miharada K, Hiroyama T, Sudo K, Nagasawa T, Nakamura Y. Efficient enucleation of erythroblasts differentiated in vitro from hematopoietic stem and progenitor cells. Nat Biotechnol. 2006;24(10):1255–6. doi: 10.1038/nbt1245. [DOI] [PubMed] [Google Scholar]
- 15.Kobari L, Pflumio F, Giarratana M, Li X, Titeux M, Izac B, et al. In vitro and in vivo evidence for the long-term multilineage (myeloid, B, NK, and T) reconstitution capacity of ex vivo expanded human CD34(+) cord blood cells. Exp Hematol. 2000;28(12):1470–80. doi: 10.1016/s0301-472x(00)00557-9. [DOI] [PubMed] [Google Scholar]
- 16.Shaffer LG, Tommerup N. ISCN: An International System for Human Cytogenetic Nomenclature. S. Karger; Basel: 2005. [Google Scholar]
- 17.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30(9):E36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tehranchi R, Fadeel B, Schmidt-Mende J, Forsblom AM, Emanuelsson E, Jadersten M, et al. Antiapoptotic role of growth factors in the myelodysplastic syndromes: concordance between in vitro and in vivo observations. Clin Cancer Res. 2005;11(17):6291–9. doi: 10.1158/1078-0432.CCR-04-1850. [DOI] [PubMed] [Google Scholar]
- 19.Span LF, Rutten E, Gemmink A, Boezeman JB, Raymakers RA, de Witte T. Bone marrow mononuclear cells of MDS patients are characterized by in vitro proliferation and increased apoptosis independently of stromal interactions. Leuk Res. 2007;31(12):1659–67. doi: 10.1016/j.leukres.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Mayani H. Abnormal stromal cells in myelodysplastic syndromes: genomics presents further evidence. Leuk Res. 2007;31(5):577–8. doi: 10.1016/j.leukres.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Villar O, Garcia JL, Sanchez-Guijo FM, Robledo C, Villaron EM, Hernández-Campo P, Lopez-Holgado N, et al. Both expanded and uncultured mesenchymal stem cells from MDS patients are genomically abnormal, showing a specific genetic profile for the 5q- syndrome. Leukemia. 2009;23(4):664–72. doi: 10.1038/leu.2008.361. [DOI] [PubMed] [Google Scholar]
- 22.Molldrem JJ, Jiang YZ, Stetler-Stevenson M, Mavroudis D, Hensel N, Barrett AJ. Haematological response of patients with myelodysplastic syndrome to antithymocyte globulin is associated with a loss of lymphocyte-mediated inhibition of CFU-GM and alterations in T-cell receptor Vβ profiles. Br J Haematol. 1998;102(5):1314–22. doi: 10.1046/j.1365-2141.1998.00920.x. [DOI] [PubMed] [Google Scholar]
- 23.Chamuleau ME, Westers TM, van Dreunen L, Groenland J, Zevenbergen A, Eeltink CM, et al. Immune mediated autologous cytotoxicity against hematopoietic precursor cells in patients with myelodysplastic syndrome. Haematologica. 2009;94(4):496–506. doi: 10.3324/haematol.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nilsson L, Edén P, Olsson E, Månsson R, Astrand-Grundström I, Strömbeck B, et al. The molecular signature of MDS stem cells supports a stem-cell origin of 5q myelodysplastic syndromes. Blood. 2007;110(8):3005–14. doi: 10.1182/blood-2007-03-079368. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson L, Astrand-Grundström I, Arvidsson I, Jacobsson B, Hellström-Lindberg E, Hast R, et al. Isolation and characterization of hematopoietic progenitor/stem cells in 5q-deleted myelodysplastic syndromes: evidence for involvement at the hematopoietic stem cell level. Blood. 2000;96(6):2012–21. [PubMed] [Google Scholar]
- 26.Raza A, Gezer S, Mundle S, Gao XZ, Alvi S, Borok R, et al. Apoptosis in bone marrow biopsy samples involving stromal and hematopoietic cells in 50 patients with myelodysplastic syndromes. Blood. 1995;86(1):268–76. [PubMed] [Google Scholar]
- 27.Claessens YE, Bouscary D, Dupont JM, Picard F, Melle J, Gisselbrecht S, et al. In vitro proliferation and differentiation of erythroid progenitors from patients with myelodysplastic syndromes: evidence for Fas-dependent apoptosis. Blood. 2002;99(5):1594–601. doi: 10.1182/blood.v99.5.1594. [DOI] [PubMed] [Google Scholar]
- 28.Claessens YE, Park S, Dubart-Kupperschmitt A, Mariot V, Garrido C, Chrétien S, et al. Rescue of early-stage myelodysplastic syndrome-deriving erythroid precursors by the ectopic expression of a dominant-negative form of FADD. Blood. 2005;105(10):4035–42. doi: 10.1182/blood-2004-08-3166. [DOI] [PubMed] [Google Scholar]
- 29.Merchav S, Wagemaker G, Souza LM, Tatarsky I. Impaired response of myelodysplastic marrow progenitors to stimulation with recombinant hematopoietic growth factors. Leukemia. 1991;5(4):340–6. [PubMed] [Google Scholar]
- 30.Choi CW, Chung YJ, Slape C, Aplan PK. Impaired differentiation and apoptosis of hematopoietic precursors in a mouse model of myelodysplastic syndrome. Haematologica. 2008;93(3):1394–7. doi: 10.3324/haematol.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyake K, Utsugisawa T, Flygare J, Kiefer T, Hamaguchi I, Richter J, et al. Ribosomal protein S19 deficiency leads to reduced proliferation and increased apoptosis but does not affect terminal erythroid differentiation in a cell line model of Diamond-Blackfan anemia. Stem Cells. 2008;26(2):323–9. doi: 10.1634/stemcells.2007-0569. [DOI] [PubMed] [Google Scholar]
- 32.List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355(14):1456–65. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 33.Pellagatti A, Jädersten M, Forsblom AM, Cattan H, Christensson B, Emanuelsson EK, et al. Lenalidomide inhibits the malignant clone and up-regulates the SPARC gene mapping to the commonly deleted region in 5q- syndrome patients. Proc Natl Acad Sci USA. 2007;104(27):11406–11. doi: 10.1073/pnas.0610477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ebert BL, Galili N, Tamayo P, Bosco J, Mak R, Pretz J, et al. An erythroid differentiation signature predicts response to lenalidomide in myelodysplastic syndrome. PLoS Med. 2008;5(2):e35. doi: 10.1371/journal.pmed.0050035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohr B, Oelschlaegel U, Thiede C, Stewart MM, Ehninger G, Platzbecker U, et al. The response to lenalidomide of myelodysplastic syndrome patients with deletion del(5q) can be sequentially monitored in CD34+ progenitor cells. Haematologica. 2009;94(3):430–1. doi: 10.3324/haematol.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]