Abstract
Background
Lenalidomide improves erythropoiesis in patients with low/intermediate-1 risk myelodysplastic syndrome and interstitial deletion of the long arm of chromosome 5 [del(5q)]. The aim of this study was to explore the effect of lenalidomide treatment on the reserves and functional characteristics of bone marrow hematopoietic progenitor/precursor cells, bone marrow stromal cells and peripheral blood lymphocytes in patients with low/intermediate-1 risk myelodysplastic syndrome with del(5q).
Design and Methods
We evaluated the number and clonogenic potential of bone marrow erythroid/myeloid/megakaryocytic progenitor cells using clonogenic assays, the apoptotic characteristics and adhesion molecule expression of CD34+ cells by flow cytometry, the hematopoiesis-supporting capacity of bone marrow stromal cells using long-term bone marrow cultures and the number and activation status of peripheral blood lymphocytes in ten patients with low/intermediate-1 risk myelodysplastic syndrome with del(5q) receiving lenalidomide.
Results
Compared to baseline, lenalidomide treatment significantly decreased the proportion of bone marrow CD34+ cells, increased the proportion of CD36+/GlycoA+ and CD36−/GlycoA+ erythroid cells and the percentage of apoptotic cells within these cell compartments. Treatment significantly improved the clonogenic potential of bone marrow erythroid, myeloid, megakaryocytic colony-forming cells and increased the proportion of CD34+ cells expressing the adhesion molecules CD11a, CD49d, CD54, CXCR4 and the SLAM antigen CD48. The hematopoiesis-supporting capacity of bone marrow stroma improved significantly following treatment, as demonstrated by the number of colony-forming cells and the level of stromal-derived factor-1α and intercellular adhesion molecule-1 in long-term bone marrow culture supernatants. Lenalidomide treatment also increased the proportion of activated peripheral blood T lymphocytes.
Conclusions
The beneficial effect of lenalidomide in patients with lower risk myelodysplastic syndrome with del(5q) is associated with significant increases in the proportion of bone marrow erythroid precursor cells and in the frequency of clonogenic progenitor cells, a substantial improvement in the hematopoiesis-supporting potential of bone marrow stroma and significant alterations in the adhesion profile of bone marrow CD34+ cells.
Keywords: lenalidomide, hematopoiesis, MDS, chromosome 5q deletion
Introduction
Myelodysplastic syndromes (MDS) comprise a heterogeneous group of hematopoietic stem cell malignancies characterized by ineffective bone marrow (BM) hematopoiesis, peripheral blood cytopenias and a substantial risk of progression to acute myeloid leukemia.1 Clonal chromosomal abnormalities are frequently identified in MDS patients and may critically affect the malignant transformation.2,3 One of the most common cytogenetic abnormalities is the interstitial deletion of the long arm of chromosome 5 [del(5q)] identified in 16% to 28% of MDS patients.4,5 Recently, it was shown that the immunomodulating drug lenalidomide, a 4-amino-glutarimide analog of thalidomide, may substantially improve or even restore erythropoiesis in MDS patients with del(5q) by suppressing the abnormal clone.6,7 Based on marked hematologic and cytogenetic responses in lower risk patients, lenalidomide was approved for the treatment of transfusion-dependent patients with low/intermediate-1 risk MDS, according to the International Prognostic Scoring System (IPSS),8 displaying del(5q) alone or with additional chromosomal abnormalities.9
The precise mechanism of action of lenalidomide in MDS and its biological effects on BM hematopoietic and microenvironmental cells remain largely unknown. In vitro studies have shown that lenalidomide has a direct, selective, inhibitory effect on the hematopoietic progenitor cells of the del(5q) clone, but does not affect the growth of the cytogenetically normal cells in MDS patients.10 Interestingly, a pro-proliferative and erythropoiesis-promoting effect of lenalidomide on normal BM hematopoietic progenitor cells has been reported.11,12 In association with its direct effects, lenalidomide may indirectly affect the survival and growth of hematopoietic progenitor cells in MDS through its immune-modulating, anti-angiogenic and adhesion-modulating properties.13,14 In vitro studies have shown that lenalidomide down-regulates the production of the pro-inflammatory cytokines tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), and transforming growth factor beta-1 (TGF-β1) by activated monocytes while it up-regulates IL-2 and interferon-gamma (IFN-γ) production promoting the activation of T and natural killer (NK) cells.15,16 A co-stimulatory effect of lenalidomide on T-cell responses following T-cell receptor activation as well as an inhibitory effect on T-regulatory cells have been also reported.14,17 Lenalidomide, like other immunomodulating drugs, may inhibit the secretion of angiogenic cytokines by both BM hematopoietic and microenvironmental cells and may also alter a broad range of responses induced by angiogenic and cell adhesion molecules.18,19
A number of elegant clinical studies have substantiated the exciting effect of lenalidomide on erythropoiesis of MDS patients with del(5q) and have resolved clinically relevant practical considerations of the treatment.20–22 In contrast, the effects of lenalidomide therapy on the reserves, functional and survival characteristics of BM hematopoietic cells and the function of BM stromal cells have not been extensively studied. In the current study we globally examined BM hematopoiesis in association with clinical responses in a number of lower risk MDS patients with del(5q) following lenalidomide therapy. We specifically evaluated, before and after treatment, the number and clonogenic potential of the BM erythroid, myeloid and megakaryocytic progenitor cells, the apoptotic characteristics and adhesion molecule expression of CD34+ cells as well as hematopoiesis-supporting capacity and the pro-inflammatory cytokine, angiogenic and adhesion molecule production by BM stromal cells. Changes in the number and activation status of peripheral blood lymphocyte subsets were also evaluated.
Design and Methods
Patients
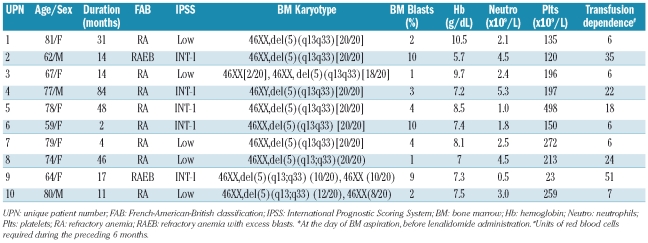
Ten white patients (eight females and two males) with de novo MDS according to French-American-British (FAB) criteria, aged 60 to 80 years (median, 71 years), were enrolled in the study. All patients had del(5q) as an isolated cytogenetic abnormality, had low or intermediate-1 risk disease according to the IPSS and were transfusion-dependent requiring at least two units of red cells in the last 8 weeks preceding enrollment.8 The patients were assigned to receive lenalidomide (Caps Revlimid; Genesis Pharma, Greece) at the standard dose of 10 mg/day for 21 days of every 28-day cycle without any additional treatment, except for red cell and platelet transfusions when required. Evaluations at baseline (week 0) and following treatment (week 24) included peripheral blood cell counts and flow-cytometric analysis, BM aspirate and biopsy for morphological, flow-cytometric and cytogenetic analyses and in vitro studies of hematopoiesis. Peripheral blood counts, differential and basic serum chemistry were monitored weekly. Clinical responses were assessed based on previously reported criteria.8 BM samples were also obtained from 30 hematologically normal subjects, age- and sex-matched to the patients, whereas ten other healthy controls were used for the CD34+ cell sorting for the recharging experiments. Informed consent according to the Helsinki Protocol was obtained from all subjects and the study was approved by the Institutional Ethics Committee and the Hellenic Drug Organization. The patients’ characteristics are detailed in Table 1.
Table 1.
Clinical and laboratory data of the patients studied.*
Bone marrow samples
BM samples from posterior iliac crest aspirates were diluted 1:1 in Iscove’s modified Dulbecco’s medium (IMDM; GibcoBRL, Life Technologies, Palsley, Scotland), supplemented with 100 IU/mL penicillin-streptomycin (Gibco) and 10 IU/mL preservative-free heparin (Sigma, St Louis, MO, USA). Diluted BM samples were centrifuged on Lymphoprep (Nycomed Pharma AS, Oslo, Norway) to obtain the bone marrow mononuclear cells (BMMC).
Purification of CD34+ cells
CD34+ cells were isolated from BMMC by indirect magnetic labeling (magnetic activated cell sorting; MACS isolation kit, Miltenyi Biotec GmbH, Germany) according to the manufacturer’s protocol. In each experiment, the purity of CD34+ cells was greater than 96% as estimated by flow-cytometry.
Reserves of bone marrow progenitor and precursor cells
Flow-cytometry was used to quantify the BM CD34+ progenitor cells and their subpopulations. Specifically, 1×106 BMMC were triple-stained with phycoerythrin (PE)-conjugated mouse anti-human CD34 monoclonal antibody (581; Beckman-Coulter, Marseille France), phycoerythrin-cyanin 5 (PC5)-conjugated CD45 (J.33) and fluorescein isothiocyanate (FITC)-conjugated CD33 (D3HL60.251) or CD71 (YDJ1.2.2) or CD61 (SZ21) monoclonal antibodies (Beckman-Coulter) for the estimation of the myeloid, erythroid, and megakaryocytic progenitor cells, respectively. CD45/CD34 stained BMMC were also stained with fluorescence-conjugated monoclonal antibodies (all purchased from Beckman-Coulter unless otherwise indicated) against the adhesion molecules CD11α (25.3), CD44 (MEM.85; Caltag Laboratories, Burlingame, CA, USA), CD48 (J4.57), CD54 (84H10), CD49d (HP2/1), CD49e (SAM1), CD62L (DREG56) and the chemokine receptor of stromal derived factor-1 (SDF-1) CXCR4 (CD184; 12G5). PE-, FITC- and PC5-conjugated mouse IgG of appropriate isotype served as negative controls. Data from 500,000 events were acquired and processed using an Epics Elite model flow-cytometer (Coulter, Miami, FL, USA). Analysis was performed in the gate of cells with low forward scatter (FSC) and low side scatter (SSC) properties gated on the CD45+ cells according to the CD45-PC5/SSC scattergram. For the quantification of the erythroid precursor cells, 1×106 BMMC were stained with anti-glycophorin A (GlycoA)-PE (11E4B7.6) and anti-CD36-FITC (FA6-152) monoclonal antibodies (Beckman-Coulter) or with PE- and FITC-conjugated mouse IgG to identify the CD36+/GlycoA+ and CD36−/GlycoA+ early and mature erythroid precursor cells, respectively.23,24
Study of apoptosis
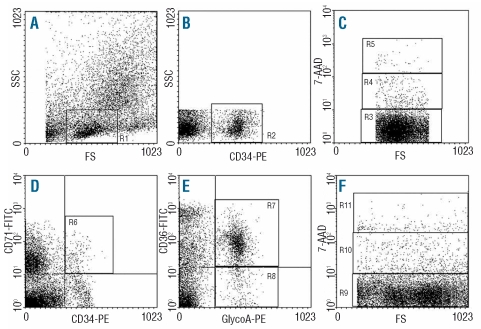
Flow-cytometry and 7-amino-actinomycin D (7AAD) staining was used to study apoptosis in the BM cell subpopulations. For the study of apoptosis in the CD34+ cell fraction, aliquots of 1×106 BMMC stained with anti-CD34-PE monoclonal antibody as above, were further stained with 100 μL 7AAD (200 μg/mL) (Calbiochem-Novabiochem, La Jolla, CA, USA) as previously described.25,26 For the analysis, a scattergram was created by combining SSC with CD34 fluorescence in the gate of cells with low FSC and SSC properties and a second scattergram was created by combining FSC with 7AAD fluorescence to quantify 7AAD-negative (live), -dim (early apoptotic) and –bright (late apoptotic/dead) cells in the gate of the CD34+ cells (Figure 1). For the study of apoptosis in the erythroid cell subpopulations, aliquots of cells stained with anti-CD34-PE/anti-CD71-FITC or anti-GlycoA-PE/anti-CD36-FITC were further stained with 7AAD. The live, early and late apoptotic cells were quantified in the gates of CD34+/CD71+, CD36+/GlycoA+, and CD36−/GlycoA+ erythroid cell populations as previously detailed24,27 (Figure 1).
Figure 1.
Flow-cytometric analysis of BM cells. (A) Scattergram of forward scatter (FSC) versus side scatter (SSC) to allow gating on cells with low FSC and low SSC properties (R1). (B) Scattergram of anti-CD34 fluorescence versus SSC gated on R1, to allow gating on CD34+ cells (R2). (C) Scattergram of FSC versus 7AAD fluorescence gated on the CD34+ cells (R2) showing the live (R3), early apoptotic (R4) and late apoptotic/dead (R5) cells. Apoptosis was similarly studied within the CD34+/CD71+ erythroid progenitor cells (R6) (plot D) and the CD36+/GlycoA+ (R7) and CD36−/GlycoA+ (R8) erythroid precursor cells (plot E). An example of apoptosis in the ungated cells is depicted in scattergram (F) to show the live (R9), early (R10) and late apoptotic/dead (R11) cells.
Clonogenic assays
Erythroid and myeloid colony-forming units
For the estimation of the erythroid and myeloid colony-forming units, we cultured 105 BMMC in 35-mm Petri dishes in 1 mL methylcellulose culture medium (Stem Cell Technologies, Vancouver, BC, Canada) supplemented with 5 ng/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; R&D Systems, Minneapolis, MN, USA), 50 ng/mL IL-3 (R&D Systems) and 2 IU/mL erythropoietin (Janssen-Cilag, Athens, Greece) as previously detailed.28 After 14 days of culture at 37°C in a 5% CO2 fully humidified atmosphere, colonies were scored and classified as erythroid-burst forming units (BFU-E) or granulocyte- plus macrophage- plus granulocyte-macrophage colony forming units (total CFU-GM). The total sum of colonies was characterized as colony-forming cells (CFC).
Megakaryocye colony-forming units
For the quantification of the megakaryocyte colony-forming units (CFU-Meg), we cultured 5×105 BMMC per chamber of a double-chamber slide using a commercially available culture medium (MegaCult-C, StemCell Technologies), according to the manufacturer’s instructions. After 10 to 12 days of incubation at 37°C in a 5% CO2 humidified atmosphere, colonies were fixed and stained on culture slides with anti-CD41 monoclonal antibody (5B12; Dako, Glostrup, Denmark) using the alkaline phosphatase anti-alkaline phosphatase technique (APAAP), as previously described, and then scored.29 Results are expressed as total CFU-Meg colonies (pure CFU-Meg + mixed CFU-Meg).
Assessment of bone marrow stromal cell function
Standard long-term bone marrow cultures
BMMC (1×107) were grown according to the standard technique28 in 10 mL IMDM supplemented with 10% fetal bovine serum (FBS; Gibco), 10% horse serum (Gibco), 100 IU/mL penicillin-streptomycin, 2 mmol L-glutamine and 10−6 mol hydrocortisone sodium succinate (Sigma) and incubated at 33°C in a 5% CO2 humidified atmosphere. At weekly intervals, cultures were fed by demi-depopulation and non-adherent cells were counted and assayed for CFC as above.
Cytokine measurements in supernatants of long-term bone marrow cultures
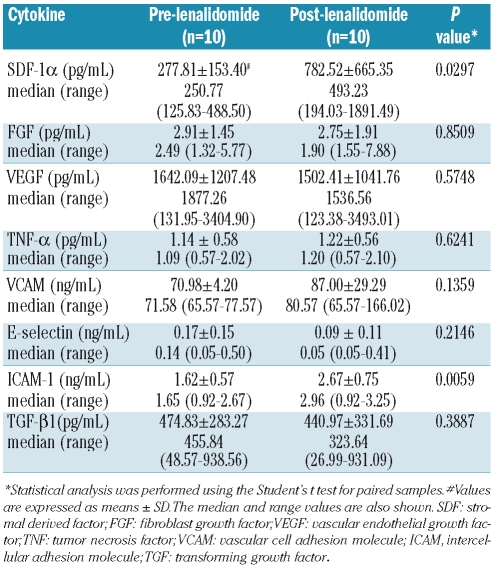
Cell-free supernatants of confluent long-term BM cultures (LTBMC) (week 3–4) before and after lenalidomide treatment were stored at −70°C for subsequent quantification of TNF-α, TGF-β1, SDF-1α, fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and E-selectin using enzyme-linked immunosorbent assays (ELISA). All ELISA kits were purchased from R&D Systems except for the TNF-α kit (Biosource International Inc., California, USA).
Recharged long-term bone marrow cultures
To test the hematopoiesis-supporting capacity of patients’ LTBMC stromal cells independently of the autologous cells, we used a two-stage culture procedure as previously described.30,31 In brief, confluent LTBMC stromal layers from patients and normal controls were irradiated (10 Gy) and recharged with 5×104 allogeneic normal CD34+ BM cells. In each experiment, flasks were recharged in triplicate and CD34+ cells from the same normal control were used to test cultures from patients and normal subjects. Cultures were monitored weekly by determining the number of CFC in the non-adherent cell fraction.
Peripheral blood lymphocyte subsets
Two-color flow-cytometry was used for the analysis of PB lymphocyte subsets. In brief, 100 μL aliquots of EDTA-anticoagulated peripheral blood were stained as described above with a combination of PE- or FITC-conjugated monoclonal antibodies (purchased from Beckman-Coulter unless otherwise indicated). In particular, anti-CD3 (UCHT1) was combined with each of the following monoclonal antibodies representing T-cell activation markers: anti-CD25 (IL-2 receptor; B1.49.9), anti-CD95 (anti-Fas) (LOB3/17; Serotec, Oxford, UK), anti-CD38 (T16), anti-CD69 (TP1.55.3) and anti-CD71. The proportions of B and NK cells were also estimated using the anti-CD19 (J4.119) and anti-CD16 (3G8), anti-CD56 (N901), anti-CD57 (NC1) monoclonal antibodies, respectively. Red blood cells were lysed and the cells fixed using the Q-prep reagent system (Coulter, Luton, UK). Data were acquired as described above and analysis was performed on 10,000 events in the gate of cells with low FSC and low SSC properties which is the gate including lymphocytes. Results are expressed as proportions of cells expressing each monoclonal antibody. Furthermore, by dividing the proportions of double-positive cells using the above described monoclonal antibody combinations by the percentages of total CD3+ cells, we estimated the proportions of activated cells within the T-lymphocyte population.32
Statistical analysis
Data were analyzed using the GraphPAd Prism statistical PC program (GraphPad Software, San Diego, CA, USA). Student’s t-test for paired samples was used to examine differences before and after treatment with lenalidomide. Standard two-way analysis of variance (two-way ANOVA) was applied to examine differences in the number of CFC in LTBMC of samples taken either from patients before and after treatment or from patients and healthy controls. The Mann-Whitney test was used to compare flow cytometry and colony data from patients after therapy and healthy controls. Grouped data are expressed as mean ± one standard deviation (SD).
Results
Reserves, survival and immunophenotypic characteristics of bone marrow progenitor/precursor cells
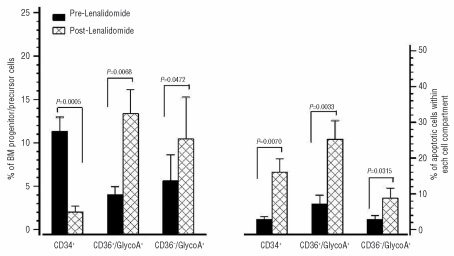
Overall, the percentage of total CD34+ cells was significantly reduced following treatment (2.02±2.13%) compared to baseline (11.33±5.09%, P=0.0005) suggesting a decrease of blast cells in patients’ BM (Figure 2). Significant decreases were also noted in the percentages of myeloid CD34+/CD33+ (0.13±0.27%), megakaryocytic CD34+/CD61+ (0.45±0.48%) and erythroid CD34+/CD71+ (0.57±0.65%) progenitor cells following treatment compared to baseline (1.02±1.15%, 5.20±2.76%, 3.73±4.54%, respectively) (P=0.0241, P=0.0003, P=0.0492, respectively). These decreases probably reflect the reduction of total CD34+ cells after therapy and not a frank reduction of the myeloid, megakaryocytic and erythroid progenitor cells. Interestingly, the proportion of CD34+/CD61+ and CD34+/CD71+ cells in patients after therapy were similar to those of the healthy subjects (0.50±0.31% and 0.59±0.24%, respectively) (P=0.3907 and P=0.0796, respectively). Regarding the erythroid precursor cells, significant increases were seen in the proportions of the CD36+/GlycoA+ and CD36−/GlycoA+ subpopulations after therapy (13.35±8.90% and 10.45±15.18%, respectively) compared to baseline (4.04±2.87% and 5.61±9.59%, respectively) (P=0.0068 and P=0.0472, respectively) (Figure 2). These findings indicate a lenalidomide-mediated inhibition of the del(5q) progenitor pool which shows an inherent defect in erythroid differentiation and a parallel expansion of the non-del(5q) progenitors which have better erythroid differentiation potential. Interestingly, however, the proportion of the more mature CD36−/GlycoA+ cells, remained lower than that in the healthy controls (33.21±5.56%) (P=0.0017).
Figure 2.
Flow-cytometric evaluation of the reserves and survival characteristics of BM progenitor and erythroid precursor cells before and after lenalidomide therapy. The left bars represent the mean proportion (± SEM) of BM CD34+ progenitor and CD36+/GlycoA+ and CD36−/GlycoA+ erythroid precursor cells before and after treatment. The right bars represent the mean proportion (± SEM) of apoptotic (7AADdim) cells within the CD34+, CD36+/GlycoA+ and CD36−/GlycoA+ cell compartments before and after therapy. Results were compared using the Student’s t test for paired samples and P values are indicated. SEM: standard error of the mean.
The survival characteristics of the patients’ BM progenitor and precursor cells before and after therapy are also presented in Figure 2. The proportion of apoptotic (7AADdim) cells within the CD34+ cell fraction increased significantly following lenalidomide therapy (16.03±11.99%) compared to pre-treatment values (2.90±2.56%; P=0.0070). Similarly, there were significant post-therapy increases in the proportions of apoptotic cells within the CD36+/GlycoA+ (25.27±16.33%) and CD36−/GlycoA+ (8.76±9.01%) early and mature erythroid precursor cell subsets compared to baseline (7.19±7.58% and 2.87±3.29%, respectively) (P=0.0033 and P=0.0315, respectively). The proportions of apoptotic cells within the CD34+, CD36+/GlycoA+ and CD36−/GlycoA+ cell compartments did, however, remain higher in patients than in healthy controls (4.91±4.63%, 5.04±3.04% and 2.46±2.43%, respectively) (P=0.0092, P=0.041, and P=0.0378, respectively).
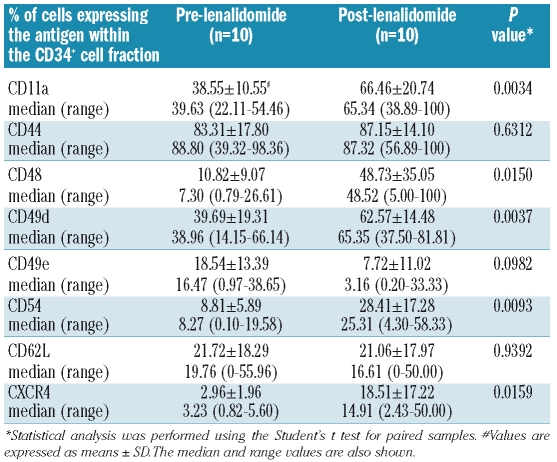
Changes in the expression of adhesion molecules in the CD34+ cell fraction before and after therapy are presented in Table 2. There were statistically significant increases in the proportions of CD34+ cells expressing CD11a, CD49d and CD54 antigens following therapy compared to baseline (P=0.0034, P=0.0037 and P=0.0093, respectively) suggesting that lenalidomide improves the adhesion capacity of BM hematopoietic progenitor cells in the BM microenvironment structures. The increased proportion of CD34+ cells expressing the CXCR4 chemokine receptor following therapy compared to baseline (P=0.0159) supports this hypothesis. The proportions of CD34+ cells expressing the CD44, CD49a, and CD62L adhesion molecules following therapy were not statistically different. Interestingly, however, a statistically significant increase was noted in the proportion of CD48+ cells within the CD34+ cell fraction following therapy compared to baseline (P=0.0037) possibly indicating an increase in the number of committed progenitor cells.33,34
Table 2.
Flow-cytometric analysis of adhesion molecule expression on bone marrow CD34+ cells.
Clonogenic progenitor cells
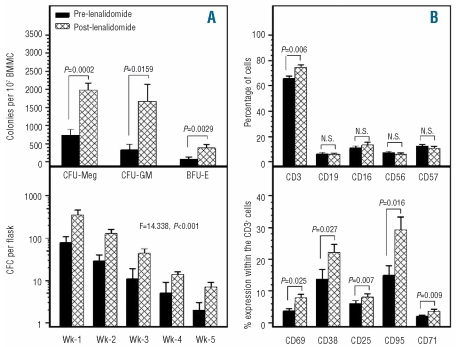
The numbers of clonogenic progenitor cell before and after lenalidomide therapy are depicted in Figure 3A. The frequency of CFC obtained from BMMC was significantly higher after therapy (2060±1652 per 107 BMMC) than at baseline (420±655 per 107 BMMC; P=0.0074). This increase was due to the improvement of both CFU-GM and BFU-E post-therapy (1670±1497 and 390±292 per 107 BMMC, respectively) compared to pre-treatment (340±481 and 80±187 per 107 BMMC, respectively) (P=0.0159 and P=0.0029, respectively). There was also a significant increase in the number of CFU-Meg post-therapy (1988±589 per 107 BMMC) compared to baseline (740±500 per 107 BMMC; P=0.0002). These data suggest an improvement of the clonogenic potential of patients’ BMMC following therapy. However, the numbers of clonogenic progenitor cells, namely CFU-GM, BFU-E and CFU-Meg remained lower in patients post-therapy than in healthy controls (5017±2634, 3400±1580 and 2940±1097 per 107 BMMC, respectively) (P=0.0019, P<0.0001, and P=0.0193, respectively) suggesting the possible persistence of clonal cells in the hematopoietic progenitor cell pool.
Figure 3.
BM clonogenic progenitor cells and peripheral blood lymphocyte subsets before and after lenalidomide therapy. (A) The bars in the upper graph represent the mean number (±SEM) of CFU-Meg, CFU-GM and BFU-E colonies obtained from 107 BMMC. The bars in the lower graph represent the mean CFC numbers (±SEM) in LTBMC supernatants over 5 weeks of culture, before and after therapy. Results were compared using Student’s t test for paired samples (upper graph) and 2-way ANOVA (lower graph) and the P and F values are indicated. (B) The bars in graph B represent the mean proportion (±SEM) of PB lymphocyte subpopulations (upper panel) and the mean percentage of cells expressing markers of activation within the CD3+ cell fraction (lower panel), before and after therapy. Results were compared using Student’s t test for paired samples and P values are shown. SEM; standard error of the mean.
Bone marrow stromal cell function
Typical confluent stromal layers, consisting of cells of hematopoietic and mesenchymal origin that mimic the BM microenvironment,35 were formed over the first 3–4 weeks in patients’ LTBMC before and after therapy. The number of CFC in the non-adherent cell fraction increased significantly following lenalidomide therapy compared to baseline (F = 14.338, P<0.001), over a period of 5 weeks of culture (Figure 3A). This increase probably reflects the post-treatment improvement of the clonogenic potential of BM progenitor cells. Alternatively, it may indicate an improvement in the hematopoiesis- supporting capacity of LTBMC adherent cells after treatment. To investigate this hypothesis, we evaluated the capacity of irradiated LTBMC stromal layers from patients to support the growth of normal CD34+ cells before and after therapy. Before lenalidomide therapy, the CFC recovery from non-adherent cells was significantly lower for patients than for healthy controls (F = 11.014, P<0.01) suggesting that the hematopoiesis-supporting capacity of patients’ stromal cells is defective. Following therapy, however, the number of CFC in the non-adherent cell fraction did not differ significantly between patients and healthy controls (F = 0.016, P>0.05), suggesting a substantial improvement in the capacity of patients’ stromal cell layers to support hematopoiesis. To probe the mechanism underlying this improvement further, we evaluated the levels of hematopoiesis-related cytokines in LTBMC supernatants, which essentially reflects cytokine production in patients’ BM microenvironment. The levels of SDF-1α and ICAM-1 were significantly higher after therapy than at baseline (P=0.0297 and P=0.0059, respectively) whereas no statistically significant differences were observed in the levels of TNF-α, TGF-β1, FGF, VEGF, VCAM-1 or E-selectin (Table 3).
Table 3.
Cytokine levels in LTBMC supernatants.
Peripheral blood lymphocyte subsets
Activated T-lymphocytes have been implicated in the pathophysiology of MDS.36 We, therefore, studied patients peripheral blood lymphocyte subsets before and after lenalidomide therapy, focusing mainly on the expression of markers of T-cell activation. We found that the proportion of peripheral blood CD3+ cells increased significantly following therapy (74.54±6.06%) compared to baseline (65.83±6.15%; P=0.006) whereas the proportions of CD19+, CD16+, CD56+ and CD57+ cells remained unchanged (Figure 3B). A significant increase was observed in the proportion of activated T cells after treatment, as indicated by the increased proportions of CD69+, CD38+, CD25+, CD95+ and CD71+ cells within the CD3+ cell fraction (7.86±3.36%, 22.16±8.01%, 8.04±3.53%, 29.36±12.62%, 3.70±1.82%, respectively) compared to baseline (3.71±2.17%, 13.76±8.97%, 6.01±3.11%, 14.96±9.15%, 2.03±0.99%, respectively) (P=0.0253, P=0.0274, P=0.0070, P=0.0162, P=0.0093, respectively) (Figure 3B).
Response evaluation
Hematologic and cytogenetic responses were assessed according to the modified criteria of the International Working Group.37 Eight patients had a major erythroid response, as demonstrated by transfusion independence, whereas two patients had minor responses with 59% and 55% reductions in transfusion requirements. In all cases improvement was sustained for at least 8 consecutive weeks. Hematologic improvement was associated with the pattern of cytogenetic responses. Specifically, patients with major erythroid improvement also had major cytogenetic responses, as demonstrated by the absence of the del(5q) abnormality on standard metaphase analysis. The two patients with minor erythroid improvement had minor cytogenetic responses, as shown by the 52% and 95% reductions in the number of abnormal cells in metaphases.
Discussion
The immunomodulating drug lenalidomide has pronounced therapeutic effects in MDS patients with del(5q).9 Lenalidomide has been described to have several cellular activities, including anti-angiogenic and anti-inflammatory effects through regulation of cytokine production and modulation of T-, NKT-, T-regulatory and NK-cell functions.13–19,38 Although lenalidomide seems to target mainly BM microenvironment structures, the drug has been described to have a direct effect on clonal hematopoietic cells through inhibition of proteins critical for cell survival or stimulation of tumor suppressor genes in the 5q region.10,39 Many mechanisms of action of lenalidomide on patients’ hematopoiesis do, however, remain undefined.
Studies investigating the mechanism of action of lenalidomide in MDS patients with del(5q) have been based mainly on the in vitro and/or ex vivo incubation of hematopoietic cells with the drug and examination of changes at cellular and molecular levels. Our study was an ex vivo investigation of the effect of lenalidomide treatment on the reserves and functional characteristics of BM progenitor/precursor and microenvironmental cells in low/intermediate-I risk MDS patients with del(5q), in association with the clinical effect. In accordance with previously reported data,7 80% of our patients became transfusion-independent whereas 20% of the patients had a minor erythroid response with significant reduction of transfusion requirements. Patients with major erythroid improvement also had a major cytogenetic response with disappearance of the del(5q) abnormality whereas patients with minor erythroid improvement had a corresponding minor cytogenetic response, suggesting that cytogenetic and hematologic patterns of response are significantly correlated.
The study of the reserves and functional characteristics of BM progenitor cells showed that the proportion of CD34+ cells decreased significantly following therapy and this decrease was associated with a significant increase in the proportion of apoptotic cells within this cell compartment. Although clonal and normal CD34+ cells were not discriminated, it seems reasonable to accept that the CD34+ cells before therapy belonged mainly to the malignant clone and accordingly, were resistant to apoptosis and had a survival advantage over the apparently normal CD34+ cells post-therapy.40 In addition, lenalidomide-induced SPARC up-regulation, which has been shown in both clonal and normal cells,10 might play a role. It appears that SPARC, in addition to its anti-proliferative, anti-adhesive and anti-angiogenic functions, might also have an apoptosis-inducing effect.41,42 In keeping with the increased proportion of apoptotic CD34+ cells following treatment, there was accelerated apoptosis of erythroid progenitor/precursor cells, probably indicating the recovery of the normal, non-clonal cells. This hypothesis was supported by the improvement in the number and the clonogenic potential of BM erythroid cells after therapy, as demonstrated by the proportion of GlycoA+ cells and the frequency of BFU-E in the BMMC fraction but also by the hematologic improvement.
In association with the increase in the numbers of BFU-E colonies, there were significant increases in CFU-GM and CFU-Meg colony recovery by BMMC following therapy, indicating a global beneficial effect of lenalidomide on the reserves and clonogenic potential of BM progenitor cells. Previous studies had also shown significant changes in the multipotent and BFU-E colony numbers in MDS patients responding to lenalidomide therapy compared to the numbers in non-responders.7 However, a beneficial effect of the drug on the clonogenic potential of CFU-Meg progenitor cells had not been reported. The positive action of lenalidomide on the clonogenic potential of BM progenitor cells is in agreement with previous observations suggesting that the drug has beneficial effects on the expansion and proliferation rate of normal CD34+ cells.11
It has been hypothesized that lenalidomide may indirectly affect the functional characteristics of BM hematopoietic cells in MDS patients with del(5q) by altering the structures, the cellular components and the humoral compounds of the BM microenvironment. Specifically, it has been shown that treatment with lenalidomide decreases BM microvessel density, indicating an anti-angiogenic activity which has been associated with a reduction in the levels of pro-angiogenic cytokines7,10,43 It has also been shown that lenalidomide may decrease the production of pro-inflammatory mediators in the BM microenvironment and may alter cell-to-cell interactions through modification of expression of adhesion molecules and stimulation of responses of cytotoxic T and NK cells.39 To probe the effect of lenalidomide on the hematopoiesis-supporting potential of BM stroma, we used the LTBMC system which has long been considered as a representative in vitro model mimicking the BM microenvironment.35 We observed a substantial improvement in the capacity of the adherent layers of patients’ LTBMC to sustain autologous and normal hematopoietic progenitor cell growth following lenalidomide treatment. To gain insight into the mechanisms underlying the beneficial effect of lenalidomide on BM stromal cell function, we evaluated the levels of soluble adhesion molecules, pro-inflammatory and pro-angiogenic cytokines in LTBMC supernatants. In accordance with previously reported data showing minimal changes in VEGF and FGF levels in BM plasma following lenalidomide therapy,7 we also found insignificant alterations in the levels of these molecules in LTBMC supernatant after treatment. Minor changes were observed in the levels of the cytokines TNF-α and TGF-β1 and the soluble adhesion molecules VCAM-1 and E-selectin following lenalidomide treatment. However, there were marked increases in the levels of supernatant SDF-1α and ICAM-1 after therapy and these were associated with significant increases in the expression of the respective membrane ligands CXCR4 and CD11a on CD34+ cells. These data suggest that lenalidomide favors the maintenance of CD34+ in the BM by inducing CXCR4/SDF-1α and CD11a/ICAM-1 interactions between hematopoietic and stromal cells. The increased expression of ICAM-1 (CD54) and CD49d on CD34+ cells after therapy corroborates this assumption. Furthermore, it has been shown that the del(5q) early hematopoietic stem cells are unable to repopulate non-obese diabetic/severe combined immunodeficiency mice in standard transplantation models suggesting a defect in cell homing that might be associated with a defective SDF-1α/CXCR4 axis.44 Based on these studies suggesting that the early hematopoietic stem cell compartment in patients with del(5q) is dominated by the clonal cells with possible defective homing properties,44 we may hypothesize that the post-treatment increase in CXCR4 expression in patients may reflect a lenalidomide-induced inhibition of the abnormal clone and expansion of the normal clone with normal homing properties.
A significant increase was observed in the expression of CD48 on CD34+ cells after therapy compared to at baseline. This antigen belongs to the SLAM family of stem/progenitor cell surface receptors and is mainly expressed on committed progenitors rather than on primitive stem cells.33,34 Accordingly, the increased expression of CD48 within the CD34+ cell population following therapy may simply reflect the increased number of clonogenic progenitor cells, which normally express CD48, obtained by lenalidomide treatment. However, CD48 is also a co-stimulatory receptor for CD2 and 2B4 molecules normally expressed on T and NK cells45 and its ligation has been reported to prolong cellular interactions and to facilitate T-and NK-cell-mediated signaling.46,47 The increased expression of CD48 on patients’ CD34+ cells may, therefore, indicate a lenalidomide-inducible effect that could contribute to hematopoietic progenitor cell apoptosis through T-and/or NK-cell mediated effects. Finally, we found a significant increase in the percentage of T cells following lenalidomide therapy which was associated with an activated profile, as demonstrated by the increased proportions of T cells expressing the CD69, CD38, CD25, CD95, and CD71 markers of activation.
In conclusion, this study provides new information on the mechanism of action of lenalidomide in patients with lower risk MDS and del(5q) while confirming the beneficial effect of lenalidomide on the induction of erythroid and cytogenetic responses. According to our data, the clinical effect of lenalidomide is associated with significant increases in the numbers of erythroid, myeloid and megakaryocytic colony-forming cells and a substantial improvement in the hematopoiesis-supporting capacity of BM stroma. Lenalidomide induces significant alterations in the adhesion profile of hematopoietic progenitor cells, including over-expression of membrane CXCR4, CD54, CD11a and CD49d and overproduction of SDF-1α and ICAM-1 in the BM microenvironment. The lenalidomide-mediated induction of the SLAM antigen CD48 on patients’ CD34+ cells may be associated with the drug’s apoptosis-inducing effect through co-stimulatory interactions between CD34+ cells and cytototoxic lymphocytes in the BM microenvironment.
Footnotes
Authorship and Disclosures
MX, MK, AP, KG, MP, PK, CS and CP performed laboratory work and contributed to the analysis and interpretation of data. AG, AS, VP, ZK, DL, EH, SK recruited the patients and contributed to the conception of the study. HAP was the principal investigator, designed and supervised the research, analyzed the data, wrote the paper and takes primary responsibility for the paper.
The authors reported no potential conflicts of interest.
Funding: this study was supported by a grant from the Hellenic General Secretary of Research and Technology (PENED # 03EΔ72).
References
- 1.Mufti GJ. Pathobiology, classification, and diagnosis of myelodysplastic syndrome. Best Pract Res Clin Haematol. 2004;17(4):543–57. doi: 10.1016/j.beha.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17(12):3835–49. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 3.Galili N, Cerny J, Raza A. Current treatment options: impact of cytogenetics on the course of myelodysplasia. Curr Treat Options Oncol. 2007;8(2):117–28. doi: 10.1007/s11864-007-0017-1. [DOI] [PubMed] [Google Scholar]
- 4.Haase D. Cytogenetic features in myelodysplastic syndromes. Ann Hematol. 2008;87(7):515–26. doi: 10.1007/s00277-008-0483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giagounidis AA, Germing U, Aul C. Biological and prognostic significance of chromosome 5q deletions in myeloid malignancies. Clin Cancer Res. 2006;12(1):5–10. doi: 10.1158/1078-0432.CCR-05-1437. [DOI] [PubMed] [Google Scholar]
- 6.List A, Kurtin S, Roe DJ, Buresh A, Mahadevan D, Fuchs D, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352(6):549–57. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- 7.List AF, Baker AF, Green S, Bellamy W. Lenalidomide: targeted anemia therapy for myelodysplastic syndromes. Cancer Control. 2006;13(Supplement):4–11. doi: 10.1177/107327480601304s02. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–88. [PubMed] [Google Scholar]
- 9.List A, Dewald GW, Bennett JM, Giagounidis A, Raza A, Feldman EJ, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355(14):1456–65. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 10.Pellagatti A, Jadersten M, Forsblom AM, Cattan H, Christensson B, Emanuelsson EK, et al. Lenalidomide inhibits the malignant clone and up-regulates the SPARC gene mapping to the commonly deleted region in 5q- syndrome patients. Proc Natl Acad Sci USA. 2007;104(27):11406–11. doi: 10.1073/pnas.0610477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verhelle D, Corral LG, Wong K, Mueller JH, Parseval LM, Jensen-Pergakes K, et al. Lenalidomide and CC-4047 inhibit the proliferation of malignant B cells while expanding normal CD34+ progenitor cells. Cancer Res. 2007;67(2):746–55. doi: 10.1158/0008-5472.CAN-06-2317. [DOI] [PubMed] [Google Scholar]
- 12.Ebert BL, Galili N, Tamayo P, Bosco J, Mak R, Pretz J, et al. An erythroid differentiation signature predicts response to lenalidomide in myelodysplastic syndrome. PLoS Med. 2008;5(2):e35. doi: 10.1371/journal.pmed.0050035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melchert M, Kale V, List A. The role of lenalidomide in the treatment of patients with chromosome 5q deletion and other myelodysplastic syndromes. Curr Opin Hematol. 2007;14(2):123–9. doi: 10.1097/MOH.0b013e328016847a. [DOI] [PubMed] [Google Scholar]
- 14.Ortega J, List A. Immunomodulatory drugs in the treatment of myelodysplastic syndromes. Curr Opin Oncol. 2007;19(6):656–9. doi: 10.1097/CCO.0b013e3282f0e12b. [DOI] [PubMed] [Google Scholar]
- 15.Corral LG, Haslett PA, Muller GW, Chen R, Wong LM, Ocampo CJ, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-α. J Immunol. 1999;163(1):380–6. [PubMed] [Google Scholar]
- 16.Chang DH, Liu N, Klimek V, Hassoun H, Mazumder A, Nimer SD, et al. Enhancement of ligand-dependent activation of human natural killer T cells by lenalidomide: therapeutic implications. Blood. 2006;108(2):618–21. doi: 10.1182/blood-2005-10-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galustian C, Meyer B, Labarthe MC, Dredge K, Klaschka D, Henry J, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother. 2009;58(7):1033–45. doi: 10.1007/s00262-008-0620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004;4(4):314–22. doi: 10.1038/nrc1323. [DOI] [PubMed] [Google Scholar]
- 19.Dredge K, Horsfall R, Robinson SP, Zhang LH, Lu L, Tang Y, et al. Orally administered lenalidomide (CC-5013) is anti-angiogenic in vivo and inhibits endothelial cell migration and Akt phosphorylation in vitro. Microvasc Res. 2005;69(1–2):56–63. doi: 10.1016/j.mvr.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Kurtin S, Sokol L. Practical considerations in the use of lenalidomide therapy for myelodysplastic syndromes. Cancer Control. 2006;13 (Suppl):26–31. doi: 10.1177/107327480601304s05. [DOI] [PubMed] [Google Scholar]
- 21.Giagounidis A, Fenaux P, Mufti GJ, Muus P, Platzbecker U, Sanz G, et al. Practical recommendations on the use of lenalidomide in the management of myelodysplastic syndromes. Ann Hematol. 2008;87(5):345–52. doi: 10.1007/s00277-008-0449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knop S, Einsele H, Bargou R, Cosgrove D, List A. Adjusted dose lenalidomide is safe and effective in patients with deletion (5q) myelodysplastic syndrome and severe renal impairment. Leuk Lymphoma. 2008;49(2):346–9. doi: 10.1080/10428190701799027. [DOI] [PubMed] [Google Scholar]
- 23.Loken MR, Shah VO, Dattilio KL, Civin CI. Flow cytometric analysis of human bone marrow: I. Normal erythroid development. Blood. 1987;69(1):255–63. [PubMed] [Google Scholar]
- 24.Papadaki HA, Kritikos HD, Valatas V, Boumpas DT, Eliopoulos GD. Anemia of chronic disease in rheumatoid arthritis is associated with increased apoptosis of bone marrow erythroid cells: improvement following anti-tumor necrosis factor-alpha antibody therapy. Blood. 2002;100(2):474–82. doi: 10.1182/blood-2002-01-0136. [DOI] [PubMed] [Google Scholar]
- 25.Philpott NJ, Turner AJ, Scopes J, Westby M, Marsh JC, Gordon-Smith EC, et al. The use of 7-amino actinomycin D in identifying apoptosis: simplicity of use and broad spectrum of application compared with other techniques. Blood. 1996;87(6):2244–51. [PubMed] [Google Scholar]
- 26.Papadaki HA, Kritikos HD, Gemetzi C, Koutala H, Marsh JC, Boumpas DT, et al. Bone marrow progenitor cell reserve and function and stromal cell function are defective in rheumatoid arthritis: evidence for a tumor necrosis factor alpha-mediated effect. Blood. 2002;99(5):1610–9. doi: 10.1182/blood.v99.5.1610. [DOI] [PubMed] [Google Scholar]
- 27.Boula A, Voulgarelis M, Giannouli S, Katrinakis G, Psyllaki M, Pontikoglou C, et al. Effect of cA2 anti-tumor necrosis factor-alpha antibody therapy on hematopoiesis of patients with myelodysplastic syndromes. Clin Cancer Res. 2006;12(10):3099–108. doi: 10.1158/1078-0432.CCR-06-0254. [DOI] [PubMed] [Google Scholar]
- 28.Papadaki HA, Eliopoulos AG, Kosteas T, Gemetzi C, Damianaki A, Koutala H, et al. Impaired granulocytopoiesis in patients with chronic idiopathic neutropenia is associated with increased apoptosis of bone marrow myeloid progenitor cells. Blood. 2003;101(7):2591–600. doi: 10.1182/blood-2002-09-2898. [DOI] [PubMed] [Google Scholar]
- 29.Psyllaki M, Damianaki A, Gemetzi C, Pyrovolaki K, Eliopoulos GD, Papadaki HA. Impaired megakaryopoiesis in patients with chronic idiopathic neutropenia is associated with increased transforming growth factor beta1 production in the bone marrow. Br J Haematol. 2006;134(6):624–31. doi: 10.1111/j.1365-2141.2006.06242.x. [DOI] [PubMed] [Google Scholar]
- 30.Papadaki HA, Gibson FM, Psyllaki M, Gordon-Smith EC, Marsh JC, Eliopoulos GD. Assessment of bone marrow stem cell reserve and function and stromal cell function in patients with severe congenital neutropenia. Eur J Haematol. 2001;67(4):245–51. doi: 10.1034/j.1600-0609.2001.00495.x. [DOI] [PubMed] [Google Scholar]
- 31.Papadaki HA, Gibson FM, Rizzo S, Gordon-Smith EC, Marsh JC. Assessment of bone marrow stem cell reserve and function and stromal cell function in patients with autoimmune cytopenias. Blood. 2000;96(9):3272–5. [PubMed] [Google Scholar]
- 32.Papadaki HA, Stamatopoulos K, Damianaki A, Gemetzi C, Anagnostopoulos A, Papadaki T, et al. Activated T-lymphocytes with myelosuppressive properties in patients with chronic idiopathic neutropenia. Br J Haematol. 2005;128(6):863–76. doi: 10.1111/j.1365-2141.2005.05380.x. [DOI] [PubMed] [Google Scholar]
- 33.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 34.Yilmaz OH, Kiel MJ, Morrison SJ. SLAM family markers are conserved among hematopoietic stem cells from old and reconstituted mice and markedly increase their purity. Blood. 2006;107(3):924–30. doi: 10.1182/blood-2005-05-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coutinho LH, Gilleece MH, De Wynter EA, Will A, Testa NG. Clonal and long-term cultures using human bone marrow. In: Testa NG, Molineux G, editors. Haemopoiesis: A Practical Approach. Oxford, Unired Kingdom: Oxford University Press; 1993. pp. 75–106. [Google Scholar]
- 36.Dunbar CE, Saunthararajah Y. Myelodysplastic syndromes. In: Young NS, editor. Bone Marrow Failure Syndromes. Philadelphia: W.B. Saunders Company; 2000. pp. 69–98. [Google Scholar]
- 37.Cheson BD, Bennett JM, Kantarjian H, Pinto A, Schiffer CA, Nimer SD, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000;96(12):3671–4. [PubMed] [Google Scholar]
- 38.Zhu D, Corral LG, Fleming YW, Stein B. Immunomodulatory drugs Revlimid (lenalidomide) and CC-4047 induce apoptosis of both hematological and solid tumor cells through NK cell activation. Cancer Immunol Immunother. 2008;57(12):1849–59. doi: 10.1007/s00262-008-0512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohamedali A, Mufti GJ. Van-den Berghe’s 5q- syndrome in 2008. Br J Haematol. 2009;144(2):157–68. doi: 10.1111/j.1365-2141.2008.07447.x. [DOI] [PubMed] [Google Scholar]
- 40.Horikawa K, Nakakuma H, Kawaguchi T, Iwamoto N, Nagakura S, Kagimoto T, et al. Apoptosis resistance of blood cells from patients with paroxysmal nocturnal hemoglobinuria, aplastic anemia, and myelodysplastic syndrome. Blood. 1997;90(7):2716–22. [PubMed] [Google Scholar]
- 41.Tang MJ, Tai IT. A novel interaction between procaspase 8 and SPARC enhances apoptosis and potentiates chemotherapy sensitivity in colorectal cancers. J Biol Chem. 2007;282(47):34457–67. doi: 10.1074/jbc.M704459200. [DOI] [PubMed] [Google Scholar]
- 42.Yiu GK, Chan WY, Ng SW, Chan PS, Cheung KK, Berkowitz RS, Mok SC. SPARC (secreted protein acidic and rich in cysteine) induces apoptosis in ovarian cancer cells. Am J Pathol. 2001;159(2):609–22. doi: 10.1016/S0002-9440(10)61732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teo SK. Properties of thalidomide and its analogues: implications for anticancer therapy. AAPS J. 2005;7(1):E14–E19. doi: 10.1208/aapsj070103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nilsson L, strand-Grundstrom I, Arvidsson I, Jacobsson B, Hellstrom-Lindberg E, Hast R, et al. Isolation and characterization of hematopoietic progenitor/stem cells in 5q-deleted myelodysplastic syndromes: evidence for involvement at the hematopoietic stem cell level. Blood. 2000;96(6):2012–21. [PubMed] [Google Scholar]
- 45.Garni-Wagner BA, Purohit A, Mathew PA, Bennett M, Kumar V. A novel function-associated molecule related to non-MHC-restricted cytotoxicity mediated by activated natural killer cells and T cells. J Immunol. 1993;151(1):60–70. [PubMed] [Google Scholar]
- 46.Brown MH, Boles K, van der Merwe PA, Kumar V, Mathew PA, Barclay AN. 2B4, the natural killer and T cell immunoglobulin superfamily surface protein, is a ligand for CD48. J Exp Med. 1998;188(11):2083–90. doi: 10.1084/jem.188.11.2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Assarsson E, Kambayashi T, Persson CM, Chambers BJ, Ljunggren HG. 2B4/CD48-mediated regulation of lymphocyte activation and function. J Immunol. 2005;175(4):2045–9. doi: 10.4049/jimmunol.175.4.2045. [DOI] [PubMed] [Google Scholar]