Abstract
Background
T follicular helper (TFH) cells reside in the light zone of germinal centers and are considered the cell of origin of angioimmunoblastic T-cell lymphoma. Recently, CXCL13, PD-1 and SAP were described as useful markers for TFH cells and angioimmunoblastic T-cell lymphoma but also reported in some peripheral T-cell lymphomas, not otherwise specified.
Design and Methods
In the present study the expression pattern of ICOS protein was investigated by immunohistochemistry-based techniques in routine sections of normal lymphoid tissues and 633 human lymphomas.
Results
Cells strongly positive for ICOS were restricted to the light zone of germinal centers and co-expressed TFH-associated molecules. In addition, weak to moderate ICOS expression was observed in a small proportion of FOXP3-positive cells. In lymphomas, ICOS expression was confined to angioimmunoblastic T-cell lymphoma (85/86), peripheral T-cell lymphomas of follicular variant (18/18) and a proportion of peripheral T-cell lymphomas, not otherwise specified (24/56) that also expressed other TFH-associated molecules.
Conclusions
ICOS is a useful molecule for identifying TFH cells and its restricted expression to angioimmunoblastic T-cell lymphoma and a proportion of peripheral T-cell lymphomas, not otherwise specified (showing a TFH-like profile) suggests its inclusion in the antibody panel for diagnosing TFH-derived lymphomas. Our findings provide further evidence that the histological spectrum of TFH-derived lymphomas is broader than previously assumed.
Keywords: ICOS, TFH cells, angioimmunoblastic T-cell lymphoma
Introduction
Gene expression profiling studies have proven to be a powerful approach to define the gene signature of distinct subsets of leukocytes and consequently to identify the gene profile associated with specific pools of B and T-cells. T follicular helper (TFH) cells are a specialized subpopulation of T helper cells deputed to help B-cells in immune responses. TFH are located in the apex of the light zone in germinal centers of secondary B-cell follicles and are considered to represent the putative cell of origin of an aggressive type of T-cell neoplasm, namely angioimmunoblastic T-cell lymphoma (AITL).1–3 Compared to other T-cell subsets, TFH cells show a unique transcript signature characterized by the expression of CD4, CD10, CXCR5, BCL-6, and CD57 (which are also present in B-cells and other types of T-cells) and by molecules such as CXCL13, PD-1 and SAP that appear to be more consistently expressed by TFH cells.4,5 Immunohistochemical studies confirmed that CXCL13, PD-1 and SAP label TFH cells in germinal centers6,7 and studies of human lymphomas indicated these molecules as useful markers for the diagnosis of AITL.6–9 However, it was noticed that a proportion of peripheral T-cell lymphomas, not otherwise specified (PTCL, NOS) could also express these TFH-associated proteins.6–10 The results suggest that the histological criteria used to separate AITL from PTCL, NOS need to be reviewed and that a search for more TFH-associated markers in the delineation between the two types of T-cell lymphomas is worthwhile.
A further relevant molecule that appears to be suitable for identifying TFH cells is the inducible T-cell co-stimulator (ICOS) protein. ICOS is a member of the CD28 co-stimulatory receptor family11,12 and its expression has been reported to be restricted to certain subsets of T-cells, with the highest expression being confined to the pool of CD4-positive T-cells.13–15 Functional studies demonstrated that ICOS is either absent or expressed at low levels in naïve T-cells but that its expression is up-regulated upon stimulation, suggesting that ICOS co-stimulatory activity is important for effector and memory T-cells.16–20 Furthermore, moderate levels of ICOS were also found in a subset of T regulatory cells (Treg).21 It has been shown that ICOS favors T-cell proliferation but also the production of cytokines including interleukin (IL)-4 and IL-1012,22–23 that are relevant for the regulation of B-cell response in germinal centers. In keeping with these observations, ICOS is highly expressed in TFH cells and studies in ICOS-deficient mice confirmed the importance of this molecule in the maintenance of B-cell immune response since the animals developed impaired germinal centers, defective isotype switching, decreased numbers of TFH cells and reduced production of IL-4 and IL-10.22,24–27
Although several experimental studies (mainly based upon flow cytometry) reported expression of ICOS associated with TFH cells, to the best of our knowledge the expression of ICOS has not been investigated in human lymphomas. Moreover, its investigation in routine sections has been hampered by the lack of antibodies reacting with formalin-fixation resistant epitopes. Here we describe the reactivity of an antibody raised against the human ICOS protein, which can be used to label routine sections. This antibody was employed with the aim of: (i) studying the distribution and phenotypic characteristics of ICOS-positive cells in vivo by applying multi-immunolabeling techniques in lymphoid tissues, and (ii) assessing the diagnostic relevance of ICOS protein in a survey of human lymphomas focusing in particular on AITL and other subtypes of T-cell lymphomas.
Design and Methods
Tissue samples
A series of normal paraffin-embedded human lymphoid tissues comprising samples of tonsil (n=10), thymus (n=2), spleen (n=3), lymph node (n=3) and bone marrow (n=2) were obtained from the routine diagnostic service of the authors’ institutions. Histologically, the lymph nodes, which were removed as part of surgical resection for benign diseases (e.g., diverticular disease) showed reactive changes without signs of a specific lymphadenitis or viral infection (Epstein-Barr virus infection was investigated by immunohistochemistry for the detection of latent membrane protein-1). Examples of lymph nodes with follicular hyperplasia from patients with human immunodeficiency virus infection and autoimmune disease (n=4) were also included. Cryostat sections of human tonsils (n=3) from the same sources were used for this study which also included a total of 633 human lymphoid neoplasms covering almost all the subcategories of B-, T- and Hodgkin lymphomas. The lymphoma samples analyzed were mainly in the form of 0.6–1 mm core tissue-arrays28 and were constructed under the supervision of expert hematopathologists who are co-authors of this manuscript (YN, SMRP, WK, MLH, SAP, MAP, PG) by choosing representative and tumor cell-rich areas of lymphoma entities. In addition, whole tissue sections were available for cases of MALT lymphoma (n=10), Burkitt lymphoma (n=4), T-cell/histiocyte-rich large B-cell lymphoma (n=5), hairy cell leukemia (n=3), multiple myeloma (n=8), classical Hodgkin lymphoma (n=8), nodular lymphocyte-predominant Hodgkin lymphoma (n=8), AITL (n=20), and PTCL, NOS (n=6) (Table 1). We also examined whole tissue sections from 18 cases of PTCL of follicular variant29 (Table 1).
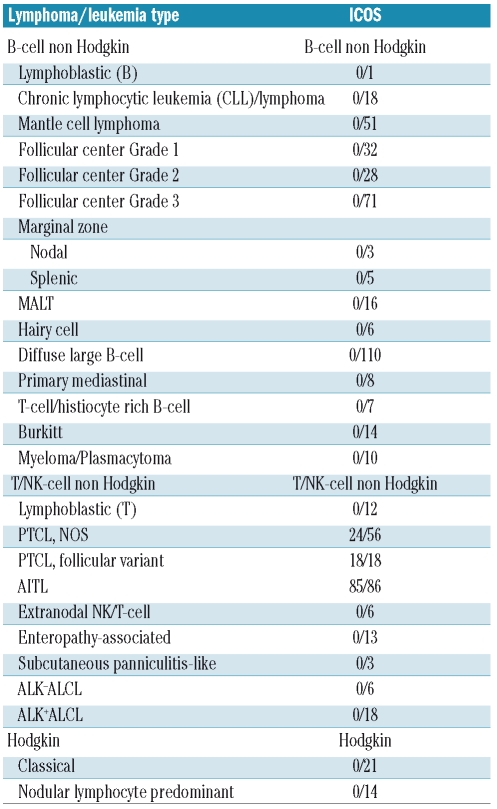
Table 1.
ICOS expression in human lymphomas.
The diagnosis of all lymphoid and myeloid neoplasms was based on the criteria of the World Health Organization (WHO) classification.30 Approval for this study was obtained from the Oxford Research Ethics Committee B (Research Ethics Committee reference number: C02.162).
Peripheral blood samples
Peripheral blood mononuclear cells obtained from a healthy donor after informed consent were isolated from 10 mL of human ethylenediaminetetraacetic acid (EDTA)-anticoagulated peripheral blood by a conventional gradient centrifugation technique using Lymphoprep (Axis-Shield UK, Kimbolton, Cambridgeshire, UK). The isolated peripheral blood mononuclear cells were added to 100 μL of the original sample and used to prepare lymphocyte-enriched peripheral blood smears in the conventional manner.31,32 Cytospin preparations were also made from whole peripheral blood of three additional healthy donors.
Flow cytometry was performed on samples from five healthy donors and two patients with AITL using an FC500 cytometer (Beckman-Coulter, Paris, France), run by CXP software (Beckman-Coulter, Paris, France). Expression of ICOS was assessed on fresh CD4-positive T cells using a whole-blood staining technique. Briefly, 100 μL of whole blood were incubated with a combination of directly conjugated monoclonal antibodies including anti-CD3-ECD (Beckman-Coulter, Paris, France), -CD4-TRI (Caltag, Paris, France), and -ICOS-FITC (R&D Systems, Paris, France) for 15 min at 4°C, then lysed and fixed using the MultiQPrep Beckman-Coulter lysis device.
Details regarding cell lines, antibodies, immunostaining and image acquisition are given in the Online Supplementary Appendix.
Results
ICOS and normal lymphoid samples
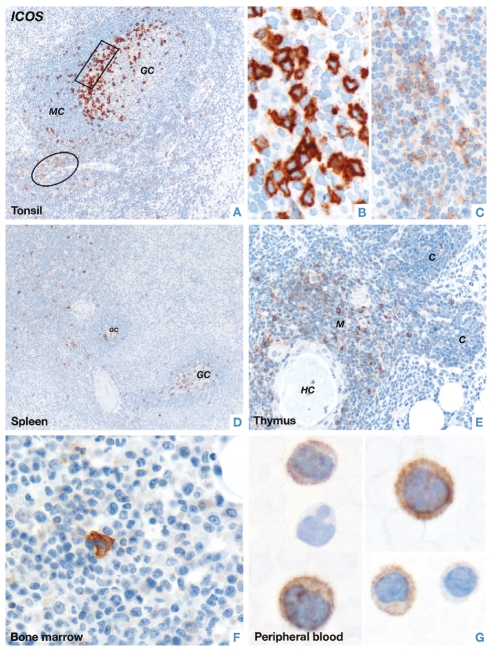
Strong membrane-associated staining was found in lymphoid cells lying at the periphery of the light zone of follicular germinal centers of tonsils (Figure 1), contrasting with the weak labeling of a proportion of lymphocytes in the interfollicular area (Figure 1). Similar findings were reported for lymph node samples with florid follicular hyperplasia (including autoimmune diseases and human immunodeficiency virus-associated lymphadenopathy) showing strong ICOS staining in scattered cells in germinal centers and weak staining in occasional T-cells in the interfollicular compartments.33 In sections of human thymus, the majority of ICOS-positive cells were localized in the medulla (whereas only rare positive cells were scattered in the cortex) (Figure 1). Rare scattered small to medium-sized ICOS-positive cells were found in both the red and white pulp of spleen, in the bone marrow and in cytospins and smears of peripheral blood obtained from three healthy donors (Figure 1). Endothelial cells were variably ICOS-positive.
Figure 1.
Immunostaining of ICOS in normal human lymphoid tissues. (A) In tonsil, strong ICOS labeling is seen in a population of lymphoid cells lying at the periphery of the light zone of a germinal center (boxed area). In the interfollicular area, many T-cells (circled area) weakly express ICOS (GC: germinal center; MC: mantle cells) (X200). (B) The image illustrates, at higher magnification, strong membrane and cytoplasmic associated ICOS staining of a group of intra-germinal center cells (X600). (C) T-cells in the interfollicular area, shown at higher magnification, are weakly ICOS-positive (X400). (D) In the spleen rare scattered ICOS-positive cells are usually seen in both the red and white pulp. Note the presence of two germinal centers containing many ICOS-positive cells (X100). (E) Many cells in the thymic medulla are ICOS-positive and only rare scattered ICOS-positive cells can be seen in the thymic cortex (M: medulla; C: cortex; HC: Hassal’s corpuscle) (X200). Rare ICOS-positive cells can also be seen in (F) a bone marrow trephine section (X600) and (G) cytospin preparations of peripheral blood from healthy donors (X600). Staining was performed by the immunoperoxidase technique in paraffin-embedded tissue sections or fresh peripheral blood samples using a polyclonal ICOS antibody and hematoxylin counterstain.
Immunoprofiling ICOS-positive cells in normal lymphoid samples
By carrying out double and triple immunoenzymatic labeling we were able to demonstrate the consistent presence of ICOS primarily in cells with the phenotype of TFH cells but also occasionally in other cell subsets (Figures 2 and 3).
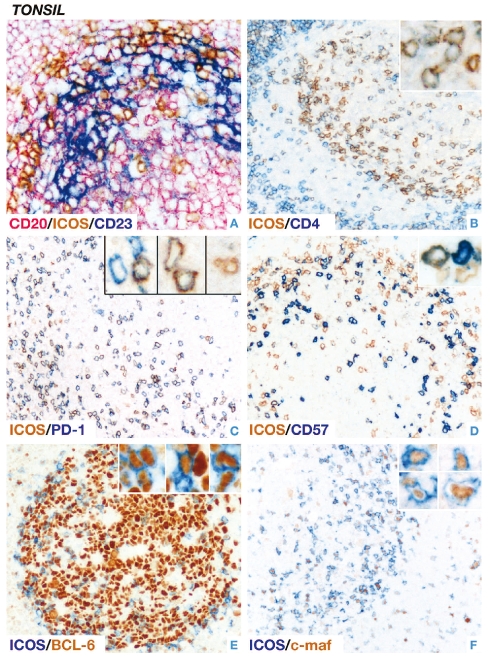
Figure 2.
(left). Immunolabeling of ICOS-positive cells in normal human tonsil. (A) Triple immunoenzymatic labeling shows that ICOS-positive cells (brown) are in close contact with CD23-positive follicular dendritic cells (blue) and CD20-positive B-cells (pink) (X400). Double immunoenzymatic labeling demonstrates that (B) the great majority of ICOS-positive (brown) intra-germinal center cells express the T helper cell marker CD4 (blue) (X200) and (C) the TFH-associated molecule PD-1 (blue) (X200). (D) Only a subset of ICOS-positive (brown) intra-germinal center cells co-express the CD57 antigen (blue) (X200). (E–F) The transcription factors BCL-6 (X400) and c-maf (brown, nuclear) (X200) are found in a proportion of ICOS-positive intra-germinal center cells. The insets show examples of the respective staining at higher magnification (all X600). All staining was performed on paraffin-embedded tissue sections and hematoxylin counterstain was omitted.
Figure 3.
Immunolabeling of ICOS-positive cells in normal human lymphoid tissues. (A) Double immunoenzymatic labeling of tonsil shows, in a germinal center (GC), rare ICOS-positive (brown) cells co-expressing the regulatory T-cell associated transcription factor FOXP3 (blue, nuclear) (x400). (B) In contrast, a higher number of ICOS/FOXP3 double-positive cells are observed in the interfollicular area (X400). (A–B) The insets show examples of double-positive cells at a higher magnification (X600). (C) Triple staining for ICOS (blue), FOXP3 (brown) and the B-cell associated molecule CD79a (pink) in tissue sections of thymus reveals the presence of numerous ICOS/FOXP3 double-positive, CD79a-negative cells (as also shown at higher magnification in the inset, X600) which are mainly localized in the medulla (X200). Double immunoenzymatic labeling in tonsil (D–E) shows that rare ICOS-positive cells (brown) co-express the cytotoxic T-cell molecules CD8 (blue) (X600) and granzyme B (blue) (X600). The arrows indicate double-positive cells; (F) rare CD30-positive (blue) cells (mainly localized in the interfollicular area) co-express ICOS (brown) (X200) as also illustrated at higher magnification in the inset (X600). All staining was performed on paraffin-embedded tissue sections and hematoxylin counterstain was omitted.
T follicular helper cells
We selected a series of TFH-associated molecules and investigated them by their individual combination with ICOS by double staining. The great majority of ICOS-positive intra-germinal center cells co-expressed CD4, PD-1 and SAP (data for SAP not shown) (Figure 2), whereas only a proportion of these cells co-expressed BCL-6, CD57 and c-maf (Figure 2). The results confirmed that our ICOS antibody labeled virtually all germinal center TFH cells (as shown by single immunostaining in Figure 1). Furthermore, we demonstrated by triple immunostaining that the ICOS-positive cells, preferentially localized at the periphery of the light zone, were in intimate contact with CD23-positive follicular dendritic cells and CD20-positive B-cells (Figure 2).
Regulatory T-cells
The expression of ICOS and FOXP3 (a Treg-associated transcription factor) was studied by double immunostaining. In reactive tonsils and lymph nodes, a small proportion (less than 5%) of all FOXP3-positive cells co-expressed ICOS (with its expression in these cells being weaker than in TFH cells): these cells were mainly located in the interfollicular areas and only rarely within germinal centers (Figure 3). The majority of the ICOS/FOXP3-positive cells were also PD-1 positive, as revealed by double immunostaining for PD-1 and FOXP3 on parallel tissue sections of lymph node and tonsil (data not shown).
The greatest number of ICOS/FOXP3 double-positive cells was observed in the thymus (Figure 3), being mainly localized in the medulla, frequently in close vicinity of Hassal’s corpuscles and showing negativity for the B-cell associated molecule CD79a (Figure 3). Only rare scattered double-positive cells were found in the cortex.
The observation of cells positive only for FOXP3 in the examined normal lymphoid tissues prompted us to investigate the expression pattern of ICOS, FOXP3 and CD25 by triple immunostaining. Besides the presence of cells co-expressing FOXP3 and CD25 only (representing the classical pool of Treg), we also detected a small population of FOXP3+/ICOS+/CD25− cells located in the interfollicular area of lymph nodes and tonsils as well as in the thymic medulla. The latter findings were in agreement with a recent report describing the presence of ICOS in a subset of FOXP3 Treg.21
Minor subsets of ICOS-positive cells
Following data on variable levels of expression of ICOS in cytotoxic T cells, we carried out double immunostaining for ICOS with CD8 or granzyme B. In normal peripheral lymphoid tissues, only rare CD8- and granzyme B-positive cells showed weak expression of ICOS (Figure 3), but the number of these double-positive cells increased when sections of lymph nodes with histological changes due to Epstein-Barr virus infection were examined (data not shown). Similarly, only rare ICOS (weak) and CD30 double-positive cells were found in normal tonsils and lymph nodes (Figure 3).
B-cells
Double immunostaining for the B-cell associated molecules CD79a and PAX5 revealed that B-cells in normal lymphoid tissues were consistently ICOS-negative (data not shown).
ICOS and human lymphomas
Staining was reviewed independently by four expert hematopathologists who are co-authors of this paper (MRJ, SMRP, PG and TM) and subsequently together by TM and PG. ICOS staining was easy to score since cases that were considered positive showed reactivity in almost all tumor cells. To score cases of AITL, we focused primarily on the atypical cells with clear cytoplasm that were usually lying around vessels and residual follicles. Cases that did not contain a positive internal control (represented by either small lymphocytes or endothelial cells) were excluded. Non-Hodgkin B-cell lymphomas were ICOS-negative (Table 1) (including three cases of diffuse large B-cell lymphoma that we previously demonstrated to be PD-1-positive7) and ICOS expression was restricted to a certain type of T-cell neoplasm (Table 1). Among T-cell lymphomas, the most consistent expression of ICOS was observed in AITL (85/86 samples) (Table 1 and Figure 4) and in PTCL of follicular variant (18/18 cases), a recently described variant of PTCL showing phenotypic features in common with AITL29 (Table 1). ICOS expression was also found in a significant proportion (24/56 cases) of PTCL, NOS (Table 1 and Figure 4). The expression pattern of CXCL13, PD-1 and SAP was retrospectively investigated in the majority of these 24 ICOS-positive cases, and in most instances the neoplastic cells showed expression of at least one other TFH marker in addition to that of BCL-6 and/or CD10 in 16 and 10 cases, respectively (Online Supplementary Table S1). Among the cases the highest correlation was found between ICOS and PD-1 (Online Supplementary Table S1). CXCL13 and SAP staining was only available in a limited number of cases and showed positivity in 63% and 91% respectively. None of the ICOS-positive PTCL, NOS were CD8-positive.
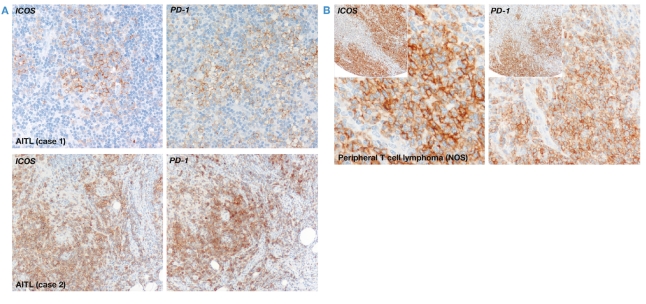
Figure 4.
Immunostaining of ICOS and PD-1 in human T-cell lymphomas. (A) Tumor cells of two cases of angioimmunoblastic T-cell lymphoma (AITL) express the co-stimulatory molecule ICOS and the TFH-associated marker PD-1 (X200). (B) Tumor cells in a case of peripheral T-cell lymphoma not otherwise specified (NOS) show strong expression of ICOS and PD-1 (X400). The insets illustrate, at lower magnification (X100) the staining pattern of ICOS and PD-1. Staining of paraffin-embedded whole or tissue-array sections was performed by the immunoperoxidase technique and counterstained with hematoxylin.
Furthermore, ICOS was revealed to be a useful molecule to distinguish cases of AITL with hyperplastic follicles (ICOS strongly labeled the neoplastic cells highlighting a perifollicular pattern) from cases of reactive hyperplasia where the strongest ICOS staining was found in cells in germinal centers.33
Morphological and clinical data for 11 cases of ICOS-positive peripheral T-cell lymphomas
Morphological and clinical data were available for 11 out of the 24 cases of PTCL showing ICOS positivity. These 11 cases were included in a previous study10 reporting on the presence of PTCL with a TFH phenotype as revealed by their expression of the TFH-associated marker PD-1. Online Supplementary Table S2 summarizes the pathological and clinical characteristics of these 11 cases.
A review of the pathological data showed that in the majority of cases the criteria used for the diagnosis of AITL (i.e. a hyperplastic meshwork of follicular dendritic cells surrounding epithelioid venules, monomorphous infiltrate composed of tumor cells with a clear cytoplasm, presence of vascular proliferation) (Online Supplementary Table S2) were not fulfilled but the phenotype of the tumor cells was that of TFH cells (Online Supplementary Table S1).
The clinical characteristics of the 11 cases were similar to those of PTCL but also presented some features in common with AITL (i.e. polyclonal hypergammaglobulinemia) (Online Supplementary Table S2).
ICOS and lymphoma-derived cell lines
The analysis of ICOS in a series of B- and T-cell lymphoma-derived cell lines showed negative results in almost all lines with the exception of some positive cells found in Karpas 299 (an ALK-positive lymphoma-derived cell line).
ICOS and peripheral blood lymphocytes from patients with angioimmunoblastic T-cell lymphoma
ICOS expression was measured at diagnosis in two patients with AITL. These two patients did not have either lymphocytosis or atypical cells on cytological analysis of peripheral blood smears. They did, however, both have a significant proportion (11% and 26%) of CD4-positive T cells with a CD4+highsCD3−CD7+CD5+CD2+ phenotype expressing ICOS. The analysis of peripheral blood from five healthy donors revealed that the proportion of ICOS-positive cells within the population of sCD3+CD4+ cells was very low, varying from 1% to 2.5%. The rare presence of normal ICOS-positive cells observed by the flow cytometric analysis confirmed the detection of these cells by immunostaining of peripheral blood cytospins from three additional healthy donors (Figure 1).
Discussion
T-cell co-stimulatory molecules are important regulators of activation and tolerance in T-cell immunity; they modulate signaling pathways that can positively initiate, increase, maintain or negatively limit, attenuate/terminate T-cell responses. Co-stimulatory proteins do not act on their own favoring amplification or down-regulation of T-cell signaling but are part of a “multisignal integration” process initiated by T-cell receptor engagement.14 In the last decade several new co-stimulatory molecules have been identified and assigned to two major groups: the CD28-superfamily – including BTLA, CD28, CTLA4 and ICOS receptors – and the tumor necrosis factor-family including CD27, CD30, HVEM, OX40 and 4-1BB proteins. The members of each family have been described to have structural and functional similarities, but also unique features.12,34 Some of these molecules are constitutively expressed (e.g. CD28 is present in naïve T cells but also in early activated T cells) whereas others such as ICOS and CTLA4, are more inducible upon activation, linking their expression to a specific stage of T-cell differentiation.14
In the last 10 years we have focused on performing high-throughput screening of signaling molecules in routine sections of human tissue aiming to contribute to the field of diagnostic pathology by identifying new biomarkers. Recently, we have detected an antibody raised against the human ICOS that reacts in routine sections. We describe here the expression pattern of ICOS in human lymphoid samples and its utility in typing and diagnosing some T-cell lymphomas.
ICOS was identified in 1999 by Krocek’s group12 by the generation of monoclonal antibodies against activated human T-cells. The F44 anti-ICOS antibody reacted only in frozen sections of human tonsils and labeled a population of cells located in the apical light zone of germinal centers, suggestive of TFH cells.
The increased scientific interest for ICOS resulted from the generation of ICOS knock-out mice that showed impaired B-cell responses, a deficit in immunoglobulin class switching and absence of germinal centers in secondary lymphoid follicles.22,25,26 It was, consequently, suggested that ICOS had a relevant role in the T-cell-dependent immune response. Functional studies demonstrated that, in T-cells, the appearance of ICOS is associated with T-cell activation and differentiation. T-cells activated through T-cell receptor engagement up-regulate ICOS so favoring T-cell proliferation and the production of cytokines such as IL-4 and IL-10.22,24–27 IL-10 plays an important role in B-cell responses and its presence correlates with ICOS. In both humans and mice, ICOS deficiency is associated with impaired IL-10 production and decreased numbers of TFH cells35 suggesting the crucial role of ICOS for the maintenance of B-cell responses and TFH growth.
In terms of transcript and protein levels, TFH cells (located within the light zone of germinal centers) contain the highest amount of ICOS compared to other subsets of T-cells.4,5 The role of ICOS in supporting TFH cell development and maintenance was further evidenced by molecular studies in Sanroque mice bearing a single recessive mutation in the Roquin gene, a ubiquitin ligase present in many cells.36 As a consequence of the mutated Roquin, Sanroque mice develop severe autoimmune disease and form spontaneous germinal centers enriched in mature T cells that resemble human TFH cells (high levels of ICOS, CD200, CXCR5, and PD-1).36 It has been proposed that Roquin promotes the degradation of Icos mRNA so explaining its repressor activity on ICOS expression in T-cells.37
Using a multi-labeling immunoenzymatic technique (a powerful approach for studying co-expression of several molecules in distinct subsets of cells in routine sections) we confirmed that our anti-ICOS antibody labeled TFH cells in germinal centers since these cells co-expressed the TFH-associated markers BCL-6, CD4, c-maf, PD-1 and SAP. Furthermore, in the interfollicular areas of lymph nodes and tonsils we noted the presence of a proportion of moderately ICOS-positive cells that double immunostaining revealed to co-express FOXP3 and were most likely Treg cells. Many of these FOXP3-positive cells also expressed PD-1 (unpublished data). Similarly, in the medulla of the thymus we found a larger number of ICOS/FOXP3-positive T-cells supporting the view that the thymus is the natural reservoir for generation of Treg cells.38
These findings are in keeping with recent studies reporting the presence of a subset of Treg cells characterized by the expression of ICOS.21,38
Two scenarios can by hypothesized regarding the expression of ICOS in FOXP3-positive cells. One possibility is that expression of ICOS in the FOXP3-positive cells (located in the interfollicular area and regarded to be Treg cells) is induced by the presence of plasmacytoid dendritic cells that are known to express ICOS-ligand. It has been recently demonstrated that plasmacytoid dendritic cells can promote the proliferation of ICOS-expressing Treg cells.38 The second possibility involves the concept of plasticity of lymphoid cells. Recent studies in mice have shown that FOXP3-positive cells have the ability to convert into TFH cells through the modulation of the expression of FOXP3 and some cytokines.39 It is, therefore, possible that the ICOS/FOXP3-positive cells that we observed in the interfollicular area represent “pre-TFH cells” which, depending on specific stimuli, will lose FOXP3 and migrate to germinal centers to carry out their “conventional” function of TFH cells.
It is postulated that TFH cells represent the normal cell counterpart of AITL, one of the most common T-cell lymphomas accounting for approximately 20% of cases.40,41 In the last few years, three molecules: CXCL13, PD-1 and SAP6,7,9 have been proposed to be relevant markers for the diagnosis of AITL. However, these proteins have also been reported to be present in other T-cell lymphomas.7,10 Similar results were observed at the level of gene expression profiling level,42 with the detection of a few PTCL, NOS with an AITL-like gene signature. In our study, the expression of ICOS was restricted to T-cell lymphomas, specifically to AITL and PTCL of follicular variant. This latter variant has only recently been described43,44 and shows some morphological and many phenotypic features in common with AITL.29,31 Overall, ICOS expression showed a close correlation with PD-1, but in contrast to PD-1, which was detected in some cases of B-cell lymphomas,45 none of the investigated cases of B-cell neoplasms was ICOS-positive (Table 1) including three cases of diffuse large B-cell lymphoma that we previously reported to express PD-1.7 However, as for the recently described TFH-associated molecules, ICOS was found in a number of T-cell lymphomas (24/56) whose primary diagnosis was that of PTCL, NOS (Table 1). Re-investigating these ICOS-positive cases and analyzing them for CXCL13, PD-1 and SAP expression, we found that the majority had some features in common with AITL (i.e. the tumor cells expressed at least one of the three investigated TFH-associated proteins (Online Supplementary Table S1) and in addition, over half of the cases showed BCL-6, CD10, CD57 expression and expanded follicular dendtritic cells (Online Supplementary Table S1). The morphological and clinical data obtained for 11 out of the 24 cases were not fully suggestive of AITL, although in some instances there were some clinical features in common (e.g. polyclonal hypergammaglobulinemia) (Online Supplementary Table 2). The hypothesis of aberrant expression of TFH markers appears very unlikely in view of the overlapping expression of several TFH markers in every case. It is more likely that T-cell lymphomas derived from TFH cells can present with three different histological patterns, i.e. in the form of AITL, PTCL of follicular variant or PTCL, NOS. Studies are now needed to clarify whether PTCL of follicular variant and PTCL, NOS with a signature of TFH cells share the same clinical syndrome as AITL.
In conclusion, the screening of antibodies in routine sections has once again proven to be a powerful approach for identifying diagnostic biomarkers such as ICOS, which has been revealed to be a useful new marker (superior to PD-1 that can also be detected in some B-cell lymphomas45 and CXCL13 that shows staining variability depending on tissue material) for the diagnosis of AITL and cases of PTCL, NOS with borderline AITL features. In addition, the immunohistochemical detection of rare ICOS-positive cells in peripheral blood smears from healthy donors suggests that this molecule could form the basis of a quick and efficient test to monitor the level of ICOS-positive cells as a sentinel of the immune-response status in transplant patients (several studies have suggested that ICOS blockade is an important factor in the prevention of bone marrow and solid organ graft rejection.46–49 Finally, since peripheral blood dissemination is a common feature in AITL patients at diagnosis (even in the absence of lymphocytosis and/or cytological atypia on smears)50,51 our finding of a significant proportion of circulating CD4/ICOS-positive/sCD3-negative T-cells in two AITL patients (contrasting with the presence of rare CD4/ICOS/sCD3-positive T-cells in the peripheral blood of five healthy donors) may suggest that ICOS detection by flow cytometry could be useful for tracking minimal residual disease and corroborating the diagnosis of AITL.
Acknowledgments
The authors thank Mr. Ralf Lieberz and Mme. Nadine Martin-Garcia for their technical assistance. The authors are also grateful to Dr. G. Roncador for her generosity in providing aliquots of PD-1 antibody.
Footnotes
Dedication: the authors dedicate this work to the memory of David York Mason, an inspiration to us all.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
TM was the principal investigator, takes primary responsibility for this study and made the figures; JCP and EB performed the laboratory work; TM and PG co-ordinated the research, reviewed the cases and wrote the paper; JCP helped in the revision of the text; HS contributed to the final version of the paper; AP performed the flow cytometry analysis; AC, YN, MR-J, SMR-P, WK, M-LH, SAP, HS, PGI and MAP diagnosed and provided the lymphoma cases; DYM was involved in the early phases of this study.
The authors reported no potential conflicts of interest.
Funding: this work was supported by a Project Grant (No. 0382) from Leukaemia Research and carried out in the Leukaemia Research Immunodiagnostics Unit, Nuffield Department of Clinical Laboratory Sciences, John Radcliffe Hospital, Oxford.
References
- 1.Attygalle A, Al-Jehani R, Diss TC, Munson P, Liu H, Du MQ, et al. Neoplastic T cells in angioimmunoblastic T-cell lymphoma express CD10. Blood. 2002;99(5):627–33. doi: 10.1182/blood.v99.2.627. [DOI] [PubMed] [Google Scholar]
- 2.Dogan A, Attygalle AD, Kyriakou C. Angioimmunoblastic T-cell lymphoma. Br J Haematol. 2003;121(5):681–91. doi: 10.1046/j.1365-2141.2003.04335.x. [DOI] [PubMed] [Google Scholar]
- 3.Yuan CM, Vergilio JA, Zhao XF, Smith TK, Harris NL, Bagg A. CD10 and BCL6 expression in the diagnosis of angioimmunoblastic T-cell lymphoma: utility of detecting CD10+ T cells by flow cytometry. Human Pathol. 2005;36(7):784–91. doi: 10.1016/j.humpath.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol. 2004;173(1):68–78. doi: 10.4049/jimmunol.173.1.68. [DOI] [PubMed] [Google Scholar]
- 5.Kim CH, Lim HW, Kim JR, Rott L, Hillsamer P, Butcher EC. Unique gene expression program of human germinal center T helper cells. Blood. 2004;104(7):1952–60. doi: 10.1182/blood-2004-03-1206. [DOI] [PubMed] [Google Scholar]
- 6.Grogg KL, Attygalle AD, Macon WR, Remstein ED, Kurtin PJ, Dogan A. Expression of CXCL13, a chemokine highly upregulated in germinal center T-helper cells, distinguishes angioimmunoblastic T-cell lymphoma from peripheral T-cell lymphoma, unspecified. Mod Pathol. 2006;19(8):1101–7. doi: 10.1038/modpathol.3800625. [DOI] [PubMed] [Google Scholar]
- 7.Roncador G, Garcia Verdes-Montenegro JF, Tedoldi S, Paterson JC, Klapper W, Ballabio E, et al. Expression of two markers of germinal center T cells (SAP and PD-1) in angioimmunoblastic T-cell lymphoma. Haematologica. 2007;92(8):1059–66. doi: 10.3324/haematol.10864. [DOI] [PubMed] [Google Scholar]
- 8.Dorfman DM, Brown JA, Shahsafaei A, Freeman GJ. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2006;30(7):802–10. doi: 10.1097/01.pas.0000209855.28282.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dupuis J, Boye K, Martin N, Copie-Bergman C, Plonquet A, Fabiani B, et al. Expression of CXCL13 by neoplastic cells in angioimmunoblastic T-cell lymphoma (AITL): a new diagnostic marker providing evidence that AITL derives from follicular helper T cells. Am J Surg Pathol. 2006;30(4):490–4. doi: 10.1097/00000478-200604000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Pinilla SM, Atienza L, Murillo C, Pérez-Rodriguez A, Montes-Moreno S, Roncador G, et al. Peripheral T-cell lymphoma with follicular T-cell markers. Am J Surg Pathol. 2008;32(12):1787–99. doi: 10.1097/PAS.0b013e31817f123e. [DOI] [PubMed] [Google Scholar]
- 11.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–48. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 12.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397(6716):263–6. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 13.Beier KC, Hutloff A, Dittrich AM, Heuck C, Rauch A, Büchner K, et al. Induction, binding specificity and function of human ICOS. Eur J Immunol. 2000;30(12):3707–17. doi: 10.1002/1521-4141(200012)30:12<3707::AID-IMMU3707>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 14.Kroczek RA, Mages HW, Hutloff A. Emerging paradigms of T-cell co-stimulation. Curr Opin Immunol. 2004;16(3):321–7. doi: 10.1016/j.coi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 15.McAdam AJ, Chang TT, Lumelsky AE, Greenfield EA, Boussiotis VA, Duke-Cohan JS, et al. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J Immunol. 2000;165(9):5035–40. doi: 10.4049/jimmunol.165.9.5035. [DOI] [PubMed] [Google Scholar]
- 16.Bonhagen K, Liesenfeld O, Stadecker MJ, Hutloff A, Erb K, Coyle AJ, et al. ICOS+ Th cells produce distinct cytokines in different mucosal immune responses. Eur J Immunol. 2003;33(2):392–401. doi: 10.1002/immu.200310013. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalo JA, Tian J, Delaney T, Corcoran J, Rottman JB, Lora J, et al. ICOS is critical for T helper cell-mediated lung mucosal inflammatory responses. Nat Immunol. 2001;2(7):597–604. doi: 10.1038/89739. [DOI] [PubMed] [Google Scholar]
- 18.Smith KM, Brewer JM, Webb P, Coyle AJ, Gutierrez-Ramos C, Garside P. Inducible costimulatory molecule-B7-related protein 1 interactions are important for the clonal expansion and B cell helper functions of naive, Th1, and Th2 T cells. J Immunol. 2003;170(5):2310–5. doi: 10.4049/jimmunol.170.5.2310. [DOI] [PubMed] [Google Scholar]
- 19.Smith KM, Garside P, McNeil RC, Brewer JM. Analysis of costimulatory molecule expression on antigen-specific T and B cells during the induction of adjuvant-induced Th1 and Th2 type responses. Vaccine. 2006;24(15):3035–43. doi: 10.1016/j.vaccine.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 20.Sperling AI, Bluestone JA. ICOS costimulation: it’s not just for TH2 cells anymore. Nat Immunol. 2001;2(7):573–4. doi: 10.1038/89709. [DOI] [PubMed] [Google Scholar]
- 21.Burmeister Y, Lischke T, Dahler AC, Mages HW, Lam KP, Coyle AJ, et al. ICOS controls the pool size of effector-memory and regulatory T cells. J Immunol. 2008;180(2):774–82. doi: 10.4049/jimmunol.180.2.774. [DOI] [PubMed] [Google Scholar]
- 22.Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, et al. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409(6816):97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 23.Löhning M, Hutloff A, Kallinich T, Mages HW, Bonhagen K, Radbruch A, et al. Expression of ICOS in vivo defines CD4+ effector T cells with high inflammatory potential and a strong bias for secretion of interleukin 10. J Exp Med. 2003;197(2):181–93. doi: 10.1084/jem.20020632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mak TW, Shahinian A, Yoshinaga SK, Wakeham A, Boucher LM, Pintilie M, et al. Costimulation through the inducible costimulator ligand is essential for both T helper and B cell functions in T cell-dependent B cell responses. Nat Immunol. 2003;4(8):765–72. doi: 10.1038/ni947. [DOI] [PubMed] [Google Scholar]
- 25.McAdam AJ, Greenwald RJ, Levin MA, Chernova T, Malenkovich N, Ling V, et al. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409 (6816):102–5. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- 26.Tafuri A, Shahinian A, Bladt F, Yoshinaga SK, Jordana M, Wakeham A, et al. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409(6816):105–9. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- 27.Wong SC, Oh E, Ng CH, Lam KP. Impaired germinal center formation and recall T-cell-dependent immune responses in mice lacking the costimulatory ligand B7-H2. Blood. 2003;102(4):1381–8. doi: 10.1182/blood-2002-08-2416. [DOI] [PubMed] [Google Scholar]
- 28.Hsu FD, Nielsen TO, Alkushi A, Dupuis B, Huntsman D, Liu CL, et al. Tissue microarrays are an effective quality assurance tool for diagnostic immunohistochemistry. Mod Pathol. 2002;15(12):1374–80. doi: 10.1097/01.MP.0000039571.02827.CE. [DOI] [PubMed] [Google Scholar]
- 29.Huang Y, Moreau A, Dupuis J, Streubel B, Petit B, Le Gouill S, et al. Peripheral T-cell lymphomas with a follicular growth pattern are derived from follicular helper T cells (TFH) and may show overlapping features with angioimmunoblastic T-cell lymphomas. Am J Surg Pathol. 2009;33(5):682–90. doi: 10.1097/PAS.0b013e3181971591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC; 2008. [Google Scholar]
- 31.Bacon CM, Paterson JC, Liu H, Payne K, Munson P, Du MQ, et al. Peripheral T-cell lymphoma with a follicular growth pattern: derivation from follicular helper T cells and relationship to angioimmunoblastic T-cell lymphoma. Br J Haematol. 2008;143(3):439–41. doi: 10.1111/j.1365-2141.2008.07352.x. [DOI] [PubMed] [Google Scholar]
- 32.Marafioti T, Paterson JC, Ballabio E, Reichard KK, Tedoldi S, Hollowood K, et al. Novel markers of normal and neoplastic human plasmacytoid dendritic cells. Blood. 2008;111(7):3778–92. doi: 10.1182/blood-2007-10-117531. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Justo M, Attygalle AD, Munson P, Roncador G, Marafioti T, Piris MA. Angioimmunoblastic T-cell lymphoma with hyperplastic germinal centres: a neoplasia with origin in the outer zone of the germinal centre? Clinicopathological and immunohistochemical study of 10 cases with follicular T-cell markers. Mod Pathol. 2009;22(6):753–61. doi: 10.1038/modpathol.2009.12. [DOI] [PubMed] [Google Scholar]
- 34.Rudd CE, Schneider H. Unifying concepts in CD28, ICOS and CTLA4 co-receptor signalling. Nat Rev Immunol. 2003;3(7):544–56. doi: 10.1038/nri1131. [DOI] [PubMed] [Google Scholar]
- 35.Wang S, Chen L. T lymphocyte co-signaling pathways of the B7-CD28 family. Cell Mol Immunol. 2004;1(1):37–42. [PubMed] [Google Scholar]
- 36.Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435(7041):452–8. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 37.Yu D, Tan AH, Hu X, Athanasopoulos V, Simpson N, Silva DG, et al. Roquin represses autoimmunity by limiting inducible T-cell co-stimulator messenger RNA. Nature. 2007;450(7167):299–303. doi: 10.1038/nature06253. [DOI] [PubMed] [Google Scholar]
- 38.Ito T, Hanabuchi S, Wang YH, Park WR, Arima K, Bover L, et al. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008;28(6):870–80. doi: 10.1016/j.immuni.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T, et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer’s patches. Science. 2009;323(5920):1488–92. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 40.Armitage J, Vose J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–30. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 41.Dogan A, Gaulard P, Jaffe E, Ralfkiaer E, Muller-Hermelink H. Angioimmunoblastic T-cell lymphoma. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC; 2008. [Google Scholar]
- 42.de Leval L, Rickman DS, Thielen C, Reynies A, Huang YL, Delsol G, et al. The gene expression profile of nodal peripheral T-cell lymphoma demonstrates a molecular link between angioimmunoblastic T-cell lymphoma (AITL) and follicular helper T (TFH) cells. Blood. 2007;109(11):4952–63. doi: 10.1182/blood-2006-10-055145. [DOI] [PubMed] [Google Scholar]
- 43.de Leval L, Savilo E, Longtine J, Ferry JA, Harris NL. Peripheral T-cell lymphoma with follicular involvement and a CD4+/bcl-6+ phenotype. Am J Surg Pathol. 2001;25(3):395–400. doi: 10.1097/00000478-200103000-00015. [DOI] [PubMed] [Google Scholar]
- 44.Ikonomou IM, Tierens A, Troen G, Aamot HV, Heim S, Lauritzsen GF, et al. Peripheral T-cell lymphoma with involvement of the expanded mantle zone. Virchows Arch. 2006;449(1):78–87. doi: 10.1007/s00428-005-0123-z. [DOI] [PubMed] [Google Scholar]
- 45.Xerri L, Chetaille B, Seriari N, Attias C, Guillaume Y, Arnoulet C, et al. Programmed death 1 is a marker of angioimmunoblastic T-cell lymphoma and B-cell small lymphocytic lymphoma/chronic lymphocytic leukemia. Human Pathol. 2008;39(7):1050–8. doi: 10.1016/j.humpath.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 46.Guo L, Li XK, Funeshima N, Fujino M, Nagata Y, Kimura H, et al. Prolonged survival in rat liver transplantation with mouse monoclonal antibody against an inducible costimulator (ICOS) Transplantation. 2002;73(7):1027–32. doi: 10.1097/00007890-200204150-00003. [DOI] [PubMed] [Google Scholar]
- 47.Harada H, Salama AD, Sho M, Izawa A, Sandner SE, Ito T, et al. The role of the ICOS-B7h T cell costimulatory pathway in transplantation immunity. J Clin Invest. 2003;112(2):234–43. doi: 10.1172/JCI17008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogawa S, Nagamatsu G, Watanabe M, Watanabe S, Hayashi T, Horita S, et al. Opposing effects of anti-activation-inducible lymphocyte-immunomodulatory molecule/inducible costimulator antibody on the development of acute versus chronic graft-versus-host disease. J Immunol. 2001;167(10):5741–8. doi: 10.4049/jimmunol.167.10.5741. [DOI] [PubMed] [Google Scholar]
- 49.Taylor PA, Panoskaltsis-Mortari A, Freeman GJ, Sharpe AH, Noelle RJ, Rudensky AY, et al. Targeting of inducible costimulator (ICOS) expressed on alloreactive T cells down-regulates graft-versus-host disease (GVHD) and facilitates engraftment of allogeneic bone marrow (BM) Blood. 2005;105(8):3372–80. doi: 10.1182/blood-2004-10-3869. [DOI] [PubMed] [Google Scholar]
- 50.Baseggio L, Berger F, Morel D, Delfau-Larue MH, Goedert G, Salles G, et al. Identification of circulating CD10 positive T cells in angioimmunoblastic T-cell lymphoma. Leukemia. 2006;20(2):296–303. doi: 10.1038/sj.leu.2404013. [DOI] [PubMed] [Google Scholar]
- 51.Serke S, van Lessen A, Hummel M, Szczepek A, Huhn D, Stein H. Circulating CD4+ T lymphocytes with intracellular but no surface CD3 antigen in five of seven patients consecutively diagnosed with angioimmunoblastic T-cell lymphoma. Cytometry. 2000;42(3):180–7. doi: 10.1002/1097-0320(20000615)42:3<180::aid-cyto4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]