Abstract
Background
Thalidomide and its analogs are effective agents in the treatment of multiple myeloma. Since gelatinases (matrix metalloproteinases-2 and -9) play a crucial role in tumor progression, we explored the effect of thalidomide on gelatinase production by malignant B lymphoid cell lines.
Design and Methods
We investigated the effect of therapeutic doses of thalidomide on integrin-mediated production of gelatinases by malignant B lymphoid cell lines by gelatin zymography, western-blot, reverse transcriptase polymerase chain reaction and invasive capacity through Matrigel-coated Boyden chambers. We also explored the effect of thalidomide on the activation status of the main signaling pathways involved in this process.
Results
Thalidomide strongly inhibited gelatinase production by B-cell lines and primary myeloma cells in response to fibronectin, the most efficient gelatinase inducer identified in lymphoid cells. Thalidomide disrupted integrin-mediated signaling pathways involved in gelatinase induction and release, such as Src and MAP-kinase ERK activation, resulting in decreased cell motility and invasiveness. Unexpectedly, treatment with thalidomide elicited an increase in fibronectin-induced Akt phosphorylation through phosphoinositide 3-kinase-independent pathways since thalidomide decreased fibronectin-induced phosphoinositide 3-kinase phosphorylation and reversed the inhibition of Akt phosphorylation achieved by the phosphoinositide 3-kinase inhibitors wortmannin and LY294002.
Conclusions
Disruption of integrin-mediated signaling may be an important mechanism through which thalidomide and its analogs impair tumor cell interactions with the microenvironment. The unexpected effects of thalidomide on Akt activation indicate the need for further studies to elucidate whether the interference with Akt downstream effects would synergize with the anti-tumor activity of thalidomide.
Keywords: matrix metalloproteinases, thalidomide, B-cell malignancies, integrins
Introduction
Thalidomide has received a great deal of attention in recent years due to its remarkable therapeutic efficacy in the treatment of multiple myeloma (MM).1,2 Thalidomide and its analogs are also being investigated in other hematologic malignancies and hematologic disorders for which there is no effective treatment such as mantle cell lymphoma, chronic lymphocytic leukemia, and myelodysplastic syndromes.3 Thalidomide is not directly cytotoxic and the mechanisms through which it exerts its therapeutic benefits are not well defined and appear to be highly complex. Understanding the molecular basis of the effects of thalidomide is crucial in order to be able to design more efficient and less harmful analogs.4
Survival and progression of myeloma cells are highly dependent on stimuli from the bone marrow microenvironment.5,6 These include soluble factors, contact-dependent interactions with surrounding cells, and angiogenesis. Interleukin-6, tumor necrosis factor-α and insulin-like growth factor are pivotal in promoting proliferation and survival of myeloma cells.6 Contact-dependent interactions with bone marrow stromal cells, endothelial cells, and matrix proteins, including fibronectin, transduce survival signals, which may confer resistance to apoptosis induced by radiation, dexamethasone, or traditional chemotherapy.5 Angiogenesis is a crucial requirement for myeloma progression and the extent of angiogenesis is inversely correlated with survival in patients with disseminated MM7 and also in patients with solitary plasmacytomas.8
Thalidomide was tried in MM on the basis of its ability to inhibit angiogenesis.9 Thalidomide inhibits proliferation of endothelial cells and decreases fibroblast growth factor-2 and vascular endothelium growth factor-induced angiogenesis in several models.10 In addition, it reduces the production of angiogenic factors vascular endothelial growth factor, hepatic growth factor and fibroblast growth factor-2 by endothelial cells obtained from bone marrow biopsies of MM patients.11 However, a correlation between the extent of bone marrow angiogenesis and response to thalidomide has not been consistently demonstrated12 and extramedullary plasmacytomas are resistant to thalidomide in spite of being highly vascularized.13 These observations suggest that, besides inhibiting angiogenesis, thalidomide has additional therapeutic effects probably targeting interactions between tumor cells and the bone marrow microenvironment.1,13,14
Gelatinases (the matrix metalloproteinases MMP2 and MMP9) have a crucial function in the progression of lymphoproliferative disorders.15–18 These proteinases facilitate tumor progression, not only by breaking natural barriers such as basement membranes or interstitial matrix but through many additional mechanisms including activation of cytokines and growth factors by proteolytic cleavage, release of matrix-bound growth factors and exposure of cryptic sites or release of active fragments from large matrix proteins.19 It has been recently shown that integrin-mediated cell interaction with matrix molecules, particularly fibronectin, is the strongest inducer of gelatinase production and activation in cells of lymphoid origin.20,21 Integrin engagement not only induces gelatinase production but also gelatinase activation through coordinated induction of the MMP2 activator MMP14, and down-regulation of tissue inhibitor of metalloproteinases (TIMP)-2. Moreover, integrin-mediated signaling drives a rapid post-transcriptional release of gelatinases through pathways related to cell migration.21
Given the relevance of gelatinases in cell interactions with the microenvironment and ultimately in tumor progression,19 we investigated the effects of thalidomide on matrix-induced MMP production by malignant lymphoid B-cell lines, in search for direct effects on malignant cells with relevant impact on their relationship with the surrounding milieu. We found that thalidomide decreases gelatinase production in response to fibronectin by interfering with multiple integrin-mediated signaling pathways which are also involved in the regulation of cell motility and survival. Thalidomide-mediated disruption of integrin signaling may then impair multiple contact-dependent tumor cell interactions with the bone marrow microenvironment which are though to be crucial for malignant cell survival.5
Design and Methods
Reagents
Thalidomide (Chemie Grünenthal, Germany) was dissolved in dimethylsulfoxide to give a stock solution of 10 mg/mL, and stored at −20° C. Wortmannin was purchased from Sigma-Aldrich (St Louis, MO, USA). LY294002 was obtained from Calbiochem (EMD Chemicals, Inc., Gibbstown, NJ, USA). Lenalidomide was a generous gift from Dr Dolors Colomer (Hematopathology Unit, Dept. of Hematology, Hospital Clínic, Barcelona, Spain).
Cells and cell culture
Human B lymphoblastoid cell lines Raji (derived from an Epstein-Barr virus-positive Burkitt’s lymphoma) and IM9 (derived from a plasma cell leukemia) were obtained from the European Collection of Cell Cultures (Salisbury, UK). RPMI 8226 and KMM1 myeloma cell lines, Hbl-2 (diffuse B-cell lymphoma) and MEC-1 (B-chronic lymphocytic leukemia) cell lines were kindly provided by Dr Dolors Colomer. Cells were maintained in RPMI 1640 medium (GIBCO, Grand Island, NY, USA) supplemented with 10% fetal calf serum (Biological Industries, Israel), 2mM L-glutamine and 50 μg/mL gentamycin at 37°C in 5% CO2.
Primary MM cells were obtained from remaining bone marrow aspirates obtained for diagnostic purposes from patients with MM. The use of this tissue was approved by the Ethics Committee and patients signed informed consent. Mononuclear cells were isolated by Ficoll-Histopaque density centrifugation. Adherent cells were removed by allowing cells to adhere to a 0.1% gelatin-coated dish at 37°C in 5% CO2 for 24 h. Non-adherent cells were collected, cultured in RPMI 1640 with 10% fetal calf serum and used for experiments. Unless otherwise indicated, cells were exposed to chemicals for 4 h and cell viability was confirmed by trypan blue exclusion.
Gelatin zymography
Cells were resuspended in serum-free RPMI 1640 medium at 0.5×106 cells/mL. For each condition, 5×106 cells were pre-incubated with chemicals at the indicated concentrations for 30 min at 37°C before the addition of fibronectin at 10 μg/mL. The supernatant fluid was collected 4 h later and concentrated 200-fold with Urifil-10 concentrator devices (Millipore, Molsheim, France). Concentrated samples were subjected to gelatin zymography as previously described.20,21
Reverse transcriptase polymerase chain reaction
RNA was extracted from 5×106 cells using TRIzol® Reagent (GIBCO). One microgram of RNA was used for cDNA synthesis with the Superscript™ First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA), using oligo d(T)s priming. MMP2, MMP9 and MMP14 cDNA was amplified as previously described.20,21 Thirty-five reaction cycles were run, each consisting of three steps of 45 s at 94°C, 57 °C (for MMP2 and MMP9) or 60°C (MMP14), and 72°C respectively, followed by an elongation period of 10 min at 72°C. Polymerase chain reaction products were analyzed in 1.2% agarose gels (Invitrogen). Multiplex amplification of β2-microglobulin was used as an internal control.
Adhesion assay
Ninety-six-well plates (Nalgene Nunc International, Denmark) were coated with fibronectin (50 μg/well) overnight at 4°C. Cells were suspended in serum-free medium, plated on fibronectin-coated wells at a density of 0.15×106 cells/well and incubated at 37°C for 1 h. Non-adherent cells were aspirated and the remaining cells were fixed and stained with 0.2% crystal violet (Sigma) in 20% methanol, washed with distilled H2O and air-dried. The dye was solubilized with 1% sodium dodecyl sulfate (SDS) and optical density was read with a spectrophotometer at 600 nm wavelength. Conditions were tested in quadruplicate wells.
Matrigel invasion assay
Ten micrometer-pore polycarbonate filters (Nucleopore, Toronto, Canada) were coated with Matrigel (kindly provided by Dr Hynda K Kleinman, NIH, Bethesda, MD, USA) diluted in RPMI 1640 at 1.25 mg/mL, and placed between the lower and upper compartments of 48-well Boyden chambers (Neuro Probe Inc, Gaithersburg, MD, USA). The lower compartments were filled with 25 μL RPMI 1640 with 10% fetal calf serum and 0.1×106 cells in serum-free medium with the indicated concentrations of thalidomide loaded onto the upper chambers. After incubation for 6 h at 37°C, the filter was removed, fixed with methanol and stained with hematoxylin. Cells on the upper side were swept and the number of cells/field on the lower side was counted under an inverted microscope in six randomly selected fields/well. Experiments were performed in quadruplicate wells.
Flow cytometry analysis
For each condition 0.5×106 cells were incubated for 30 min at 4°C with 2 μg of the following monoclonal antibodies diluted in 100 μL of RPMI-1% bovine serum albumin: anti-integrin α4 chain (clone HP2/1) (Immunotech, Marseille, France), anti-integrin α5 chain (clone SAM1) (Immunotech), anti-αvβ3 integrin (clone LM609) (Chemicon International, Inc., Temecula, CA, USA), anti-integrin β1 chain (clone K20) (Immunotech) or HUTS-21 (β1 activated epitope), a generous gift from Dr F Sánchez-Madrid (Hospital La Princesa, Madrid, Spain). Cells were subsequently were washed with cold phosphate-buffered saline-1% bovine serum albumin and incubated for 30 min at 4°C with goat anti-mouse IgG Alexa Fluor® 488 secondary antibody (Molecular Probes, Leiden, The Netherlands) at a 1:200 dilution. After two washes with cold phosphate-buffered saline-1% bovine serum albumin, cells were fixed in 200 μL of 5% formaldehyde in phosphate-buffered saline and fluorescence measured using a FACScan® flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). The expression of HUTS-21 was analyzed in the absence and in the presence of 200μM of Mn2+ divalent cations.22
Western blot
For each condition, 5×106 cells were incubated with the indicated concentrations of chemicals and then exposed or not to fibronectin at 10 μg/mL in serum-free RPMI 1640 medium for 4 h. Cells were lysed in 0.5 mL of modified RIPA buffer (50 mM HEPES, 150 mM NaCl, 10% glycerol, 1% Triton-X-100, 2 mM EDTA, 1% sodium deoxycholate, 0.1% SDS) supplemented with freshly added protease inhibitors (Complete™, Boehringer Mannheim, Germany) and Na3VO4 at 200 μM. The protein content of the lysates was measured with the BCA protein assay (Pierce, Rockford, IL, USA).
For each condition, 20 μg of lysate were subjected to SDS-polyacrylamide gel electrophoresis (PAGE) and transferred onto nitro-cellulose. Membranes were incubated overnight at 4°C with the appropriate primary antibody: ERK MAP kinase activation state was evaluated using anti-phospho-p44/42 MAPK. The nitrocellulose membrane was then stripped and re-probed with p44/42 MAPK rabbit polyclonal IgG antibodies (Cell Signaling, Beverly, MA, USA) at 1:1000 dilution. Src activation was evaluated with anti-phospho-Src at Y416 (activated form), then re-probed with anti-phospho-Src at Y527 (inactivated form) and finally re-probed with anti-Src rabbit polyclonal IgG antibodies (Cell Signaling) at 1:1000 dilution. To assess Akt activation state, a combination of two anti-phospho-Akt (Ser473 and Thr308) was used and the membrane re-probed with Akt rabbit polyclonal IgG antibodies (Cell Signaling) at a dilution of 1:1000. The activation state of phosphatase and tensin homolog (PTEN) was evaluated with anti-phospho-PTEN rabbit polyclonal IgG antibody (Cell Signaling) at a dilution of 1:1000. Anti-phospho-mammalian target of rapamycin (mTOR) and anti-mTOR antibodies were also obtained from Cell Signaling. Monoclonal mouse anti-human β-actin antibody (clone AC-15) (Sigma) was used at a 1:2000 dilution. MMP14 was detected with a polyclonal rabbit anti-human MMP14 (Chemicon) at a 1:1000 dilution.
Immunodetection was performed by incubating membranes with horseradish peroxidase (HRP)-conjugated secondary antibodies (anti-rabbit HRP-conjugated, Cell Signaling or anti-mouse HRP-conjugated, Transduction Laboratories, Lexington, KY, USA) at a 1:2000 dilution. Chemiluminescence signals were detected with LUMIGLO® reagent (Cell Signaling). Membranes were exposed to a LAS3000 chemiluminiscence detector (Fuji Photo Film, Düsseldorf, Germany) and the data collected analyzed with Image Gauge V4.0 software.
Results
Thalidomide strongly decreases fibronectin-induced matrix metalloproteinase production by malignant B lymphoid cells
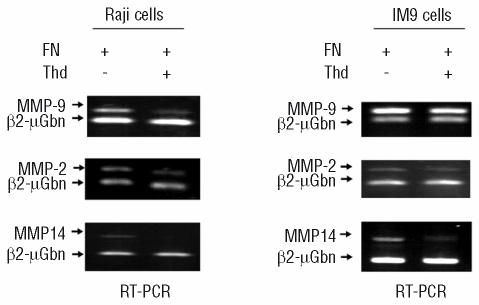
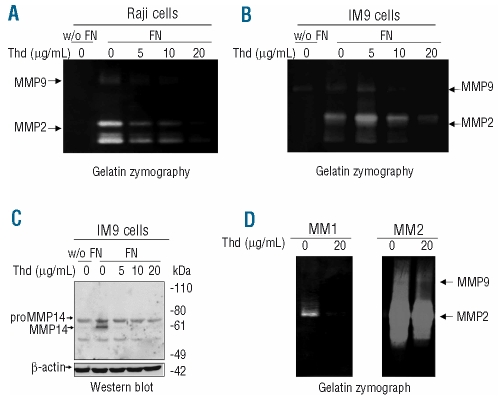
Fibronectin is a matrix protein present in the bone marrow microenvironment which is crucial in promoting tumorigenesis through several mechanisms including stimulation of cell migration, invasiveness and survival.23 These responses are mainly mediated by integrin engagement and signaling. We used two B lymphoid cells, IM9 and Raji, to study gelatinase production induced by fibronectin. As we have previously demonstrated in T-cell lines,20,21 exposure to fibronectin strongly induced metalloproteinase (MMP9, MMP2 and MMP14) expression and release by both IM9 and Raji cells (Figures 1 and 2). Interestingly, thalidomide decreased fibronectin-induced gelatinase production in a dose-dependent manner (Figures 1 and 2A and 2B). Production of the MMP2 activator, MMP14, which is also up-regulated by fibronectin in lymphoid cells,20,21 was strongly inhibited by thalidomide, particularly in its activated form (Figures 1 and 2C). Thalidomide, therefore, not only reduced fibronectin-induced MMP expression, but also restrained gelatinase activation and release.
Figure 1.
Thalidomide (Thd) inhibits gelatinase (MMP2 and MMP9) and MMP14 gene expression in response to fibronectin (FN). Reverse transcriptase polymerase chain reaction amplification of MMP2, MMP9 and MMP14 in Raji (A) and IM9 (B) cells cultured in the presence of fibronectin at 10 μg/mL and exposed to thalidomide at 20 μg/mL for 4 h.
Figure 2.
Thalidomide (Thd) inhibits gelatinase (MMP2 and MMP9) and MMP14 production in response to fibronectin (FN). Gelatin zymography of the supernatants of Raji (A) and IM9 (B) cells in the absence or in the presence of fibronectin at 10 μg/mL and exposed to thalidomide at the indicated concentrations for 4 h. (C) Western blot detection of MMP14 in IM9 cell lysates cultured in the same conditions. Similar results were obtained with Raji cells. (D) Gelatin zymography of supernates obtained from primary myeloma cells obtained from bone-marrow aspirates of two different patients and cultured with fibronectin (10 μg/mL) for 4 h in the absence or presence of thalidomide 20 μg/mL.
The Raji and IM9 cells used in this study come from different lymphoid malignancies, suggesting that the effects of thalidomide on integrin signaling are not restricted to a particular cell line. Accordingly, thalidomide’s impairment of gelatinase production in response to fibronectin was also confirmed in primary MM cells obtained from bone marrow aspirates of two patients (Figure 2D). Patient # 1 had 100% plasma cell infiltration whereas patient # 2 had 50% infiltration. The increased gelatinolytic signal observed in cells from patient # 2 may indicate a contribution of additional cells (i.e. from the myelomonocytic lineage) which have greater ability to produce gelatinases than cells of lymphoid origin and suggest that thalidomide may also decrease MMP production by other cell types present in the MM microenvironment.
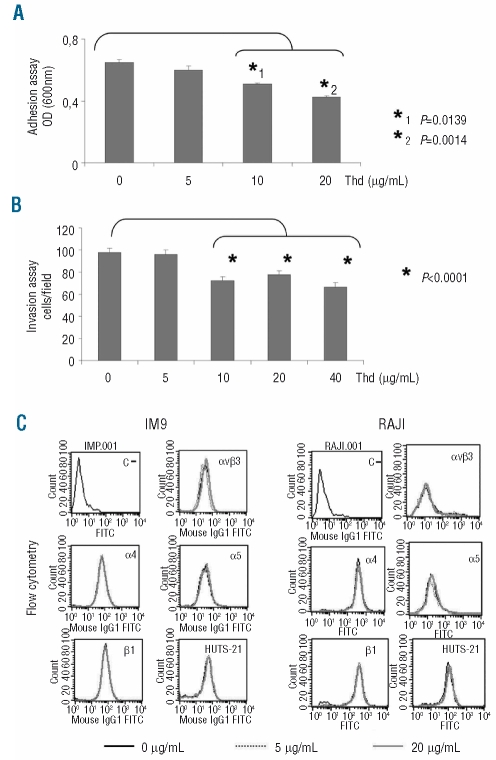
Thalidomide decreases Raji cell adhesion and invasiveness
We have previously shown that fibronectin-induced gelatinase production by lymphoid cells is mediated by integrins α4, α5 and αv.20 Given that thalidomide decreased integrin-mediated induction and release of gelatinases, we assessed its effect on additional integrin-mediated functions, such as cell adhesion to matrix proteins. Preliminary experiments showed that Raji cells significantly adhered to fibronectin whereas adhesion to other matrix proteins such as collagen I, collagen IV or laminin was poor (data not shown). Thalidomide significantly decreased Raji cell adhesion and spreading onto fibronectin in a dose-dependent manner (Figure 3A). Moreover, as shown in Figure 3B, thalidomide significantly reduced Raji cell invasion through the reconstituted basement membrane Matrigel. As reported by others,24 IM9 cells were not naturally adherent to fibronectin. Although IM9 cells interacted with fibronectin and produced gelatinases efficiently in response to fibronectin engagement, these cells were unable to complete additional steps required for cell attachment and migration and were not, therefore, tested in these systems. To confirm that thalidomide analogs elicit similar responses, and to verify activity in other cell lines, we explored the effects of lenalidomide in other cell types including a MM-derived cell line, RPMI8226. It was found that lenalidomide was highly effective in reducing cell adhesion, and gelatinase production in response to fibronectin (Online Supplementary Figure S1).
Figure 3.
Thalidomide (Thd) decreases cell attachment to fibronectin and invasiveness through Matrigel-coated membranes without modifying integrin surface expression. (A) Raji cells treated with the indicated concentrations of thalidomide were incubated on fibronectin-coated wells for 1 h. Bars represent mean in optical density ± SEM. Statistical significance of differences observed was calculated with the Mann-Whitney U test. (B) Invasion of Raji cells treated with various doses of thalidomide through Matrigel-coated polycarbonate membranes over a 6 h period. Bars indicate mean cell number ± SEM on the lower side of the membrane. Statistical significance of differences observed was calculated with the Mann-Whitney U test. (C) Flow cytometry analysis of surface expression of αvβ3 integrin, α4, α5 chain and β1 integrin chains, and β1 activation related epitope recognized by the antibody HUTS21 in IM9 and Raji cells exposed to the indicated concentrations of thalidomide for 1-h. Identical results were obtained after 6-h exposure to thalidomide. Since baseline expression of HUTS21 was weak (data not shown) results represented in the figure were obtained after incubation with Mn2+. Negative control (C-) represents cells incubated with the secondary antibody only.
We next investigated whether the inhibitory effects of thalidomide on integrin-mediated responses were due to a decrease in integrin expression. As illustrated in Figure 3C, both cell lines significantly expressed fibronectin receptors αvβ3, α4 and α5. According to previously published data, these integrins mediate fibronectin-induction of gelatinases.20 In these experimental conditions, thalidomide did not significantly down-regulate integrin surface expression, as detected by flow cytometry (Figure 3C), nor did it modify the expression of β1 integrin chain-activation related epitope recognized by the monoclonal antibody HUTS-2122 which was weakly expressed at baseline but strongly induced by Mn2+. Taken together, these findings indicate that the effects of thalidomide on fibronectin-induced adhesion and gelatinase production do not primarily depend on changes in integrin expression and suggest that thalidomide may influence integrin-mediated signaling pathways.
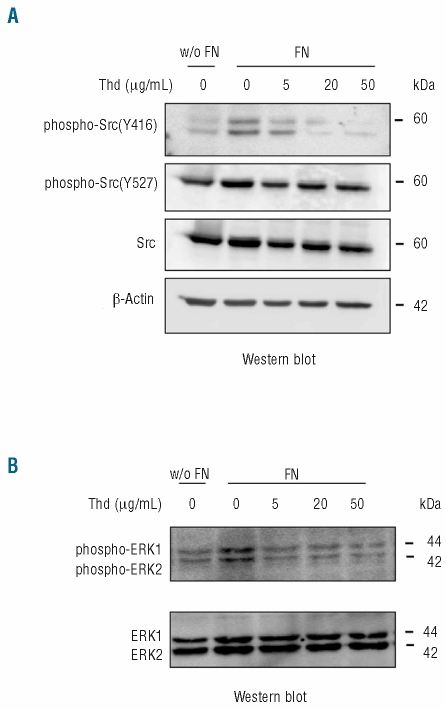
Treatment with thalidomide results in decreased phosphorylation of integrin-activated kinases involved in the regulation of gelatinase production in response to fibronectin
In previous studies, we showed that fibronectin-induced gelatinase expression and release by lymphoid cells is mediated by Src, PI3-kinase and MAP kinases ERK1/2.20,21 However, while inhibition of Src by PP2 also results in decreased gelatinase synthesis and secretion, inhibition of ERK phosphorylation by PD98059 and of PI3-kinase by wortmannin leads to increased fibronectin-induced rapid gelatinase release into the medium followed by a reduction in gelatinase mRNA.20,21 Similar effects were observed with the B-cell lines used in this study (data not shown). We next investigated the effect of thalidomide on these pathways. Fibronectin-induced Src activation was decreased by thalidomide as illustrated by the decrease in Src phosphorylation at Y416 elicited by thalidomide treatment (Figure 4A).25 In contrast, Src phosphorylation at Y527, leading to Src inactivation, remained unmodified (Figure 4A). Thalidomide also resulted in strongly decreased ERK phosphorylation induced by fibronectin (Figure 4B).
Figure 4.
Thalidomide (Thd) inhibits phosphorylation of Src and ERK kinases in response to fibronectin (FN). (A) Western blot detection of Src phosphorylated at Y416 and at Y527 in Raji cells exposed or not to fibronectin (10 μg/mL) and incubated with the indicated concentrations of thalidomide. The membrane was stripped and reprobed with anti p60 Src and with anti β-actin antibody. (B) Western blot detection of phosphorylated p42 and p44 ERK in Raji cells cultured in the same conditions as in A. The blot was subsequently stripped and re-probed with anti-ERK antibody. Similar results were obtained from IM9 cells.
Thalidomide increases integrin-induced Akt phosphorylation in a PI3-kinase-independent pathway
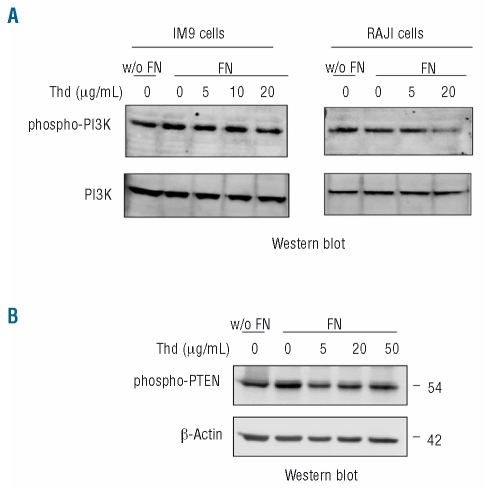
Previous studies have shown that PI3-kinase participates in fibronectin-induced release of MMP.20 Given that thalidomide reduced phosphorylation of several kinases crucial to integrin-signaling, we next explored the effect of thalidomide on PI3-kinase activation and ensuing downstream effects. When activated by tyrosine phosphorylation of its p85 subunit, PI3-kinase catalyzes phosphatidylinositol trisphosphate (PIP3) formation. PIP3 then recruits Akt to the cell membrane where it can be phosphorylated by active phosphoinositide-dependent kinase 1 (PDK1).26,27 Akt activation promotes cell survival by stimulating anti-apoptotic pathways and this mechanism is considered to be a pivotal downstream effect of PI3-kinase function.26,27 Exposure to thalidomide decreased PI3-kinase activation in both cell lines (Figure 5A).
Figure 5.
Thalidomide (Thd) decreases PI3-kinase phosphorylation and increases PTEN activation. (A) Western-blot detection of phosphorylated p85 subunit of PI3-kinase in IM9 and Raji cell lysates after incubation with thalidomide in the presence or absence of fibronectin (FN) (10 μg/mL). (B) Western-blot detection of phosphorylated PTEN and corresponding β-actin in cell lysates obtained from IM9 cells exposed to the indicated concentrations of thalidomide in the absence or presence of fibronectin (10 μg/mL).
We also explored the effects of thalidomide on PTEN activation. PTEN is a lipid phosphatase that dephosphorylates PIP3, decreasing Akt activation.28–30 PTEN is considered to be a tumor suppressor gene given that loss of PTEN activity, as a result of deletion or mutation of the gene, is commonly found in solid tumors. However, although PTEN mutations can be found in some cases of MM,28 loss of PTEN function is an uncommon pathway of malignant transformation in lymphoproliferative disorders.6,31 We found that thalidomide reduced fibronectin-induced PTEN phosphorylation in IM9 cells (Figure 5B). Raji cells lacked detectable PTEN protein (data not shown). Since PTEN is inhibited by phosphorylation, this finding indicates that thalidomide increases PTEN activation, which may reduce even more downstream effects of PI3-kinase in IM9 and cells expressing functional PTEN. The increase in PTEN activity could also contribute to the anti-tumor effects of thalidomide since PTEN inhibits cell proliferation through Akt-dependent and independent pathways.32
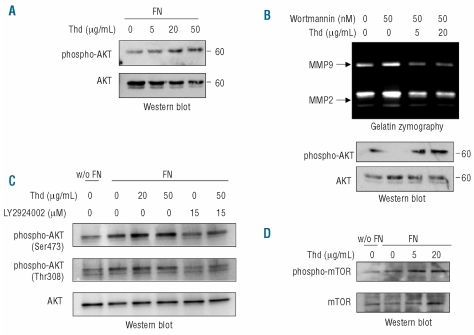
Akt phosphorylation is a crucial downstream effect of PI3-kinase activation. We hypothesized that reduced PI3-kinase activation and increased PTEN activity induced by thalidomide might, therefore, result in decreased Akt activation, in accordance with thalidomide’s anti-tumor effects. Unexpectedly, and in spite of reduced PI3-kinase phosphorylation and increased activity of PTEN in IM9 cells, thalidomide increased fibronectin-induced phosphorylation of Akt in both cell lines (Figure 6A) and was able to overcome the inhibitory effect of the PI3-kinase inhibitor wortmannin on Akt phosphorylation (Figure 6B). This finding was confirmed with the more specific PI3-kinase inhibitor LY294002 (Figure 6C). These findings indicate that thalidomide increases Akt phosphorylation through a PI3-kinase-independent pathway. In support of the functional relevance of the increase in Akt phosphorylation, exposure to thalidomide resulted in increased phosphorylation of the Akt substrate mTOR in both cell lines (Figure 6C).
Figure 6.
Thalidomide (Thd) increases fibronectin (FN)-induced Akt phosphorylation through a PI3-kinase independent pathway. (A) Western-blot detection of phosphorylated Akt of IM9 cell lysates after exposure to the indicated doses of thalidomide for 4 h in the presence of fibronectin (10 μg/mL). (B) Gelatin zymography and western-blot detection of phosphorylated Akt in Raji cell supernates and lysates, respectively, after exposure to thalidomide at the depicted concentrations, in the absence or presence of wortmannin at 50 nM. Similar results were obtained with IM9 cells. (C) Western-blot detection of phosphorylated Akt (Ser473 and Thr308) in Raji cells exposed or not to fibronectin (10 μg/mL) and incubated with the indicated concentrations of thalidomide and PI3-kinase specific inhibitor LY294002 (15 μM). The blot was subsequently stripped and reprobed with anti-Akt antibody. (D) Western-blot detection of mTOR phosphorylation in Raji cell lysates exposed to thalidomide in the presence or absence of fibronectin. Similar results were obtained in IM9 cells.
The increase in Akt phosphorylation induced by thalidomide was confirmed in myeloma cell lines RPMI 8226 and KMM1 (Online Supplementary Figure S2). In these cell lines total Akt content and Akt phosphorylation in response to fibronectin and to thalidomide were less intense than in Raji and IM9 cells, which are derived from more aggressive malignancies.
Discussion
Our data indicate that thalidomide has strong direct effects on tumor cells, leading to disruption of integrin-mediated interactions with the surrounding microenvironment. Interference with integrin-mediated signaling may also contribute to previously identified effects of thalidomide, such as inhibition of angiogenesis and T-cell co-stimulation, since cell-cell and cell-matrix interactions mediated by integrins are crucial in these processes. The signaling pathways disrupted by thalidomide regulate crucial cell functions for tumor progression such as cell motility, cell-cell and cell-matrix interactions, gelatinase production, and cell growth. Src kinases and ERK are key enzymes in promoting tumor cell growth and invasiveness. Increased Src activity by v-Src was one of the first recognized mechanisms of malignant transformation.25,33 Src also plays a crucial role in cell motility and we and others have shown that Src-family kinases are key regulators of integrin-mediated gelatinase production and rapid release through multiple interactions with focal adhesion kinase (FAK) and FAK-associated signaling molecules21,34 Src not only mediates gelatinase production but also post-transcriptional rapid release of gelatinases in response to fibronectin. Src and ERK inhibition may, therefore, both be important mechanisms through which thalidomide decreases cell motility and MMP production in response to matrix proteins. Changes in protein phosphorylation elicited by thalidomide suggest that interference with these and other protein kinase and/or phosphatase activities may lead to additional alterations in cell function which could contribute to the drug’s anti-angiogenic, anti-inflammatory and anti-tumor effects.
However, the increase in Akt activity may seem inconsistent with the anti-tumor activity of thalidomide, given that activated Akt transduces powerful anti-apoptotic signals26,35 and an increase in Akt activation is crucial for the survival of many tumors including MM.35 Recent trials have shown that while thalidomide delays progression in MM, advance is accelerated in some patients when progression occurs, indicating that thalidomide may, indeed, activate some survival pathways.36 Thalidomide-mediated survival signals through Akt activation may be partially overcome by interference with other important pathways stimulating cell growth and migration such as Src or MAP kinases in response to extracellular signals. Interestingly, increased Akt activity renders cells more sensitive to the suppressive effects of mTOR inhibitors on cell survival and on angiogenesis.37 The effect of thalidomide on Akt and mTOR activation suggests that inhibitors of mTOR may potentiate the anti-tumor effects of thalidomide, as indicated by experimental animal models.38,39
In conclusion, functional testing of new thalidomide analogs is currently based on their ability to elicit known effects of thalidomide, such as T-cell co-stimulatory function or inhibition of cytokine production and angiogenesis.4,40 The newly recognized effects of thalidomide reported here may help in the screening of new therapeutic agents.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
EL, MC-B, CV, M-TC and JE performed the laboratory work. MS, NI, JB and MCC participated in the design of the research. MS and MCC analyzed the data and wrote the paper.
The authors reported no potential conflicts of interest.
Funding: this work was supported by grants from Fondo de Investigación Sanitaria (FIS 00/0683), Marató TV3 (00/2615), and Ministerio de Ciencia e Innovación and Fondo Europeo de Desarrollo Regional (FEDER) (SAF 08/04328).
References
- 1.Morgan GJ, Krishnan B, Jenner M, Davies FE. Advances in oral therapy for multiple myeloma. Lancet Oncol. 2006;7(4):316–25. doi: 10.1016/S1470-2045(06)70657-X. [DOI] [PubMed] [Google Scholar]
- 2.Palumbo A, Facon T, Sonneveld P, Blade J, Offidani M, Gay F, et al. Thalidomide for treatment of multiple myeloma: 10 years later. Blood. 2008;111(8):3968–77. doi: 10.1182/blood-2007-10-117457. [DOI] [PubMed] [Google Scholar]
- 3.Blade J, Rosinol L. Advances in therapy of multiple myeloma. Curr Opin Oncol. 2008;20(6):697–704. doi: 10.1097/CCO.0b013e3283136984. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004;4(4):314–22. doi: 10.1038/nrc1323. [DOI] [PubMed] [Google Scholar]
- 5.Hideshima T, Mitsiades C, Tonon G, Richardson PG, Anderson KC. Understanding multiple myeloma pathogenesis in the bone marrow to identify new therapeutic targets. Nat Rev Cancer. 2007;7(8):585–98. doi: 10.1038/nrc2189. [DOI] [PubMed] [Google Scholar]
- 6.Hallek M, Bergsagel PL, Anderson KC. Multiple myeloma: increasing evidence for a multistep transformation process. Blood. 1998;91(1):3–21. [PMC free article] [PubMed] [Google Scholar]
- 7.Rajkumar S, Witzig T. A review of angiogenesis and antiangiogenic therapy with thalidomide in multiple myeloma. Cancer Treat Rev. 2000;26(5):351–62. doi: 10.1053/ctrv.2000.0188. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S, Fonseca R, Dispenzieri A, Lacy MQ, Lust JA, Wellik L, et al. Prognostic value of angiogenesis in solitary bone plasmacytoma. Blood. 2003;101(5):1715–7. doi: 10.1182/blood-2002-08-2441. [DOI] [PubMed] [Google Scholar]
- 9.D’Amato R, Loughnan M, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA. 1994;91(9):4082–5. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertolini F, Mingrone W, Alietti A, Ferrucci PF, Cocorocchio E, Peccatori F, et al. Thalidomide in multiple myeloma, myelo-dysplastic syndromes and histiocytosis. Analysis of clinical results and of surrogate angiogenesis markers. Ann Oncol. 2001;12(7):987–90. doi: 10.1023/a:1011141009812. [DOI] [PubMed] [Google Scholar]
- 11.Vacca A, Scavelli C, Montefusco V, Di Pietro G, Neri A, Mattioli M, et al. Thalidomide downregulates angiogenic genes in bone marrow endothelial cells of patients with active multiple myeloma. J Clin Oncol. 2005;23(23):5334–46. doi: 10.1200/JCO.2005.03.723. [DOI] [PubMed] [Google Scholar]
- 12.Rosiñol L, Cibeira M, Segarra M, Cid M, Filella X, Aymerich M, et al. Response to thalidomide in multiple myeloma: impact of angiogenic factors. Cytokine. 2004;26(4):145–8. doi: 10.1016/j.cyto.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Rosiñol L, Cibeira M, Bladé J, Esteve J, Aymerich M, Rozman M, et al. Extra-medullary multiple myeloma escapes the effect of thalidomide. Haematologica. 2004;89(7):832–6. [PubMed] [Google Scholar]
- 14.Kumar S, Anderson KC. Drug insight: thalidomide as a treatment for multiple myeloma. Nat Clin Pract Oncol. 2005;2(5):262–70. doi: 10.1038/ncponc0174. [DOI] [PubMed] [Google Scholar]
- 15.Barillé S, Akhoundi C, Collette M, Mellerin M, Rapp M, Harousseau J, et al. Metalloproteinases in multiple myeloma: production of matrix metalloproteinase-9 (MMP-9), activation of proMMP-2, and induction of MMP-1 by myeloma cells. Blood. 1997;90(4):1649–55. [PubMed] [Google Scholar]
- 16.Overall CM, Kleifeld O. Tumour microenvironment-opinion: validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6(3):227–39. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 17.Vacca A, Ribatti D, Presta M, Minischetti M, Iurlaro M, Ria R, et al. Bone marrow neovascularization, plasma cell angiogenic potential, and matrix metalloproteinase-2 secretion parallel progression of human multiple myeloma. Blood. 1999;93(9):3064–73. [PubMed] [Google Scholar]
- 18.Kossakowska A, Edwards D, Prusinkiewicz C, Zhang M, Guo D, Urbanski S, et al. Interleukin-6 regulation of matrix metalloproteinase (MMP-2 and MMP-9) and tissue inhibitor of metalloproteinase (TIMP-1) expression in malignant non-Hodgkin’s lymphomas. Blood. 1999;94(6):2080–9. [PubMed] [Google Scholar]
- 19.Sternlicht M, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esparza J, Vilardell C, Calvo J, Juan M, Vives J, Urbano-Marquez A, et al. Fibronectin upregulates gelatinase B (MMP-9) and induces coordinated expression of gelatinase A (MMP-2) and its activator MT1-MMP (MMP-14) by human T lymphocyte cell lines. A process repressed through RAS/MAP kinase signaling pathways. Blood. 1999;94(8):2754–66. [PubMed] [Google Scholar]
- 21.Segarra M, Vilardell C, Matsumoto K, Esparza J, Lozano E, Serra-Pages C, et al. Dual function of focal adhesion kinase in regulating integrin-induced MMP-2 and MMP-9 release by human T lymphoid cells. FASEB J. 2005;19(13):1875–7. doi: 10.1096/fj.04-3574fje. [DOI] [PubMed] [Google Scholar]
- 22.Luque A, Gomez M, Puzon W, Takada Y, Sanchez-Madrid F, Cabanas C. Activated conformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region (355–425) of the common beta 1 chain. J Biol Chem. 1996;271(19):11067–75. doi: 10.1074/jbc.271.19.11067. [DOI] [PubMed] [Google Scholar]
- 23.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411(6835):375–9. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 24.Ni H, Li A, Simonsen N, Wilkins JA. Integrin activation by dithiothreitol or Mn2+ induces a ligand-occupied conformation and exposure of a novel NH2-terminal regulatory site on the β1 integrin chain. J Biol Chem. 1998;273(14):7981–7. doi: 10.1074/jbc.273.14.7981. [DOI] [PubMed] [Google Scholar]
- 25.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4(6):470–80. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 26.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24(50):7455–64. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 27.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8(3):179–83. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Ge NL, Rudikoff S. Expression of PTEN in PTEN-deficient multiple myeloma cells abolishes tumor growth in vivo. Oncogene. 2000;19(36):4091–5. doi: 10.1038/sj.onc.1203801. [DOI] [PubMed] [Google Scholar]
- 29.Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22(14):2954–63. doi: 10.1200/JCO.2004.02.141. [DOI] [PubMed] [Google Scholar]
- 30.Yamada KM, Araki M. Tumor suppressor PTEN: modulator of cell signaling, growth, migration and apoptosis. J Cell Sci. 2001;114(Pt 13):2375–82. doi: 10.1242/jcs.114.13.2375. [DOI] [PubMed] [Google Scholar]
- 31.Abbott RT, Tripp S, Perkins SL, Elenitoba-Johnson KS, Lim MS. Analysis of the PI-3-kinase-PTEN-AKT pathway in human lymphoma and leukemia using a cell line microarray. Mod Pathol. 2003;16(6):607–12. doi: 10.1097/01.MP.0000067423.83712.74. [DOI] [PubMed] [Google Scholar]
- 32.Liu JL, Sheng X, Hortobagyi ZK, Mao Z, Gallick GE, Yung WK. Nuclear PTEN-mediated growth suppression is independent of Akt down-regulation. Mol Cell Biol. 2005;25(14):6211–24. doi: 10.1128/MCB.25.14.6211-6224.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Playford MP, Schaller MD. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23(48):7928–46. doi: 10.1038/sj.onc.1208080. [DOI] [PubMed] [Google Scholar]
- 34.Hsia DA, Mitra SK, Hauck CR, Streblow DN, Nelson JA, Ilic D, et al. Differential regulation of cell motility and invasion by FAK. J Cell Biol. 2003;160(5):753–67. doi: 10.1083/jcb.200212114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Choi Y, Mavromatis B, Lichtenstein A, Li W. Preferential killing of PTEN-null myelomas by PI3K inhibitors through Akt pathway. Oncogene. 2003;22(40):6289–95. doi: 10.1038/sj.onc.1206718. [DOI] [PubMed] [Google Scholar]
- 36.Barlogie B, Tricot G, Anaissie E, Shaughnessy J, Rasmussen E, van Rhee F, et al. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. N Engl J Med. 2006;354(10):1021–30. doi: 10.1056/NEJMoa053583. [DOI] [PubMed] [Google Scholar]
- 37.Frost P, Shi Y, Hoang B, Lichtenstein A. AKT activity regulates the ability of mTOR inhibitors to prevent angiogenesis and VEGF expression in multiple myeloma cells. Oncogene. 2007;26(16):2255–62. doi: 10.1038/sj.onc.1210019. [DOI] [PubMed] [Google Scholar]
- 38.Frost P, Moatamed F, Hoang B, Shi Y, Gera J, Yan H, et al. In vivo antitumor effects of the mTOR inhibitor CCI-779 against human multiple myeloma cells in a xenograft model. Blood. 2004;104(13):4181–7. doi: 10.1182/blood-2004-03-1153. [DOI] [PubMed] [Google Scholar]
- 39.Raje N, Kumar S, Hideshima T, Ishitsuka K, Chauhan D, Mitsiades C, et al. Combination of the mTOR inhibitor rapamycin and CC-5013 has synergistic activity in multiple myeloma. Blood. 2004;104(13):4188–93. doi: 10.1182/blood-2004-06-2281. [DOI] [PubMed] [Google Scholar]
- 40.Aragon-Chin JB, Li H, Gardner ER, Figg WD. Thalidomide analogs as anti-cancer drugs. Recent Pat Anticancer Drug Discov. 2007;2(2):167–74. doi: 10.2174/157489207780832478. [DOI] [PMC free article] [PubMed] [Google Scholar]