Abstract
Background
Hypogammaglobulinemia is common in Waldenström’s macroglobulinemia. The etiology of this finding remains unclear, but it has been speculated to be based on tumor-induced suppression of the ‘uninvolved’ immunoglobulin production
Design and Methods
We evaluated the incidence of IgA and IgG hypogammaglobulinemia in 207 untreated patients with Waldenström’s macroglobulinemia and investigated the associated clinicopathological findings and impact of therapy. We also sequenced eight genes (AICDA, BTK, CD40, CD154, NEMO, TACI, SH2D1A, UNG) implicated in immunoglobulin deficiency in 19 Waldenström’s macroglobulinemia patients with IgA and/or IgG hypogammaglobulinemia.
Results
At baseline 63.3%, 58.0% and 49.3% of the 207 patients had abnormally low serum levels of IgA, IgG, or both. No association between IgA and IgG hypogammaglobulinemia and disease burden, serum IgM levels, β2-microglobulin, International Prognostic Scoring System score, or incidence of recurrent infections was observed, although the presence of adenopathy and/or splenomegaly was associated with a lower incidence of hypogammaglobulinemia. Lower IgA and IgG levels were associated with disease progression in patients managed with a ‘watch and wait’ strategy. IgA and/or IgG levels remained abnormally low despite response to treatment, including complete remissions. A missense mutation in the highly conserved catalytic site of UNG was observed in a patient with hypogammaglobulinemia, warranting further study of this pathway in Waldenström’s macroglobulinemia.
Conclusions
IgA and IgG hypogammaglobulinemia is common in Waldenström’s macroglobulinemia and persists despite therapeutic intervention and response. IgA and IgG hypogammaglobulinemia does not predict the risk of recurrent infections in patients with Waldenström’s macroglobulinemia, although lower levels of serum IgA and IgG are associated with disease progression in Waldenström’s macroglobulinemia patients being managed with a ‘watch and wait’ strategy.
Keywords: hypogammaglobulinemia, Waldenström’s macroglobulinemia, serum IgM levels
Introduction
Waldenström’s macroglobulinemia (WM) is a distinct B-cell lymphoproliferative disorder characterized primarily by bone marrow infiltration with lymphoplasmacytic cells, together with an IgM monoclonal gammopathy.1 This condition is considered to be a lymphoplasmacytic lymphoma as defined by the World Health Organization (WHO) and Revised European-American classification of lymphoid neoplasms (REAL) classification systems.2,3 Up to 20% of patients with WM appear to have a first-degree relative with WM or related B-cell disorder suggesting a possible genetic predisposition to this rare disease.4
Recurrent infections, particularly involving the respiratory tract, are commonly observed among patients with WM, and may be related to the presence of IgA and IgG hypogammaglobulinemia.5 The presence of hypogammaglobulinemia of the ‘uninvolved’ immunoglobulin has also been reported among other B-cell malignancies.6,7 The etiology of this finding remains unclear, but has been speculated to be based on tumor-induced immunoparesis and host-mediated homeostatic regulation of ‘uninvolved’ immunoglobulin production.
Little is known about the impact of IgA and IgG immunoglobulin levels on the risk of infections in WM patients, who often present with respiratory tract infections,4 or on the evolution of IgA and IgG levels during the course of disease and following therapy. We, therefore, evaluated the incidence of IgA and IgG hypogammaglobulinemia in 207 untreated WM patients and investigated associated clinicopathological findings and the course of the IgA and IgG hypogammaglobulinemia. Additionally, we evaluated the impact of therapy, including the achievement of clinical remission, on IgA and IgG immunoglobulin levels in 93 serially treated patients with WM.
Lastly, as part of these studies, we sequenced eight genes often implicated in immunoglobulin deficiency disorders, such as common variable immunodeficiency disorder, hyper IgM syndrome, and X-linked agammaglobulinemia, in 19 patients with WM who were found to have IgA and/or IgG deficiency.8–12
Design and Methods
To analyze the incidence of hypogammaglobulinemia in WM, serum immunoglobulin levels, assessed by immunofixation, were serially determined in 207 previously untreated patients at their first clinic visit. This study was conducted in accordance with the ethical requirements of the Dana-Farber/Harvard Cancer Center institutional review board. All participants met the consensus panel definition for WM1 and were considered to have hypogammaglobulinemia if their uninvolved immunoglobulin levels were below the lower limit of our institutional normal range (i.e. <700 mg/dL and <70 mg/dL for IgG and IgA, respectively). As part of this analysis, we assessed the impact of the patients’ age, sex, bone marrow disease infiltration by lymphoplasmacytic cells, adenopathy, splenomegaly, serum IgM levels as determined by immunonephelometry, complete blood counts, absolute lymphocyte count, β2-microglobulin, and prognostic score as assessed by the WM International Prognostic Scoring System13 on the presence or absence of IgA and IgG hypogammaglobulinemia. The impact of IgA and IgG hypogammaglobulinemia on the risk of recurring infections, defined as an infection occurring more than once in a year, was also assessed.
To delineate the impact of disease progression on uninvolved immunoglobulin levels over time, we monitored the IgA and IgG levels in 102/207 patients who were placed under observation at their first visit, and who continued to be followed-up at our Institution. Sixty of these 102 patients remained progression-free, while the other 42 eventually progressed during the follow-up period. To understand the impact of therapeutic intervention on uninvolved immunoglobulin levels, we also analyzed changes in IgA and IgG levels in a separate cohort of 93 patients who underwent treatment for WM and had immunoglobulin levels tested pre-therapy, post-therapy, and at least 1 month following completion of all therapy. The outcomes of these patients were determined using consensus panel response criteria from the Third International Workshop on WM.14
Sequencing of genes associated with common variable immunodeficiency disorder
DNA from peripheral blood mononuclear cells was obtained from 19 WM patients who had IgA and/or IgG hypogammaglobulinemia. Sequence analysis of the promoter, all exonic, and flanking intronic regions was performed for AICDA, BTK, CD40, CD154, NEMO, TACI, SH2D1A, and UNG which are associated with common variable immunodeficiency disorder, hyper IgM syndrome, and X-linked agammaglobulinemia. All sequencing studies were performed by Correlagen Diagnostics Inc. (Waltham, Massachusetts, USA) using CLIA approved diagnostic assays.
Statistical analyses
Clinical data were analyzed using logistic regression analysis, ANOVA, and Spearman’s rank correlation analysis. All analyses were performed using SAS (SAS Institute, Cary NC, USA). Non-parametric testing was conducted using a two-tailed Fisher’s exact probability test (VassarStats). A P value of 0.05 or less was considered to be statistically significant in all studies.
Results
IgA and IgG hypogammaglobulinemia in Waldenström’s macroglobulinemia
We first analyzed the prevalence of IgA and IgG hypogammaglobulinemia in newly diagnosed patients with WM. The median age of these patients was 60 years (range, 32–83 years) and the male to female ratio was 1.4. The median IgM was 2,910 mg/dL (range, 179–12,400 mg/dL) and the median bone marrow involvement with disease was 40% (range, 5–95%). Of these patients, 131 (63.3%) and 120 (58.0%) patients had low levels of serum IgA and IgG levels, respectively, while 102 (49.3%) of these patients had abnormally low levels of both. The median serum IgA and IgG levels for this cohort were 50 mg/dL (range, 7–597 mg/dL) and 635 mg/dL (range, 127–3,130 mg/dL), respectively. No correlation was found between bone marrow infiltration and uninvolved immunoglobulin levels (P=0.351 and P=0.141 for IgG and IgA, respectively). Additionally, by logistic regression analysis, age, sex, serum IgM levels, white blood count, hematocrit, platelet count, absolute lymphocyte count, β2-microglobulin level, and the WM International Prognostic Scoring System13 score had no impact on the odds ratio of having IgA or IgG or both IgA and IgG hypogammaglobulinemia (data not shown). Interestingly, a higher incidence of adenopathy was observed among those patients who presented with normal range IgA (13.16%) and IgG (11.63%) than among patients presenting with IgA (5.04%) and IgG (4.65%) hypogammaglobulinemia (P=0.03 and P=0.08, respectively). Similarly, the incidence of splenomegaly was higher in those patients who presented with normal range IgA (11.84%) and IgG (11.63%) than among the patients who presented with IgA (3.88%) and IgG (3.36%) hypogammaglobulinemia (P=0.02 and P=0.03, respectively).
Impact of IgA and IgG hypogammaglobulinemia on infection risk in Waldenström’s macroglobulinemia
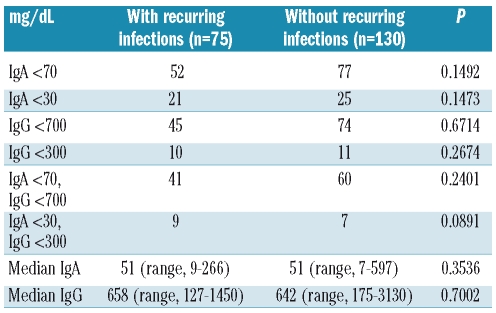
We next assessed the impact of presenting with IgA and IgG hypogammaglobulinemia on the risk of recurrent infections among 205 of the 207 WM patients for whom these data were available. The presence of IgA, IgG or both IgA and IgG hypogammaglobulinemia, even at lower cut-offs (i.e. IgA <30 mg/dL; IgG <300 mg/dL) did not predict for the occurrence of recurring infections, which were nearly all respiratory in nature, mainly grade 1 or 2, and consisted of sinus (n=53; 25.85%), bronchial (n=16; 7.80%), unspecified upper respiratory tract (n=14; 6.83%), and lung (n=7; 3.41%) infections (Table 1).
Table 1.
Impact of IgA and IgG hypogammaglobulinemia on risk of recurrent infection in patients with WM.
Evolution of IgA and IgG hypogammaglobulinemia in Waldenström’s macroglobulinemia
We next evaluated those untreated patients whose disease did not progress during the course of our follow-up. With a median follow-up of 26 months (range, 4–106 months), IgA and IgG levels remained stable in most patients. Twenty-eight of these 60 (47%) patients had low IgA levels at baseline, while the levels were abnormally low at the end of follow-up in 32/60 (53%); similarly 29/60 (48%) of patients had subnormal IgG levels at baseline, which remained unchanged at last follow-up (P=0.47 and P=1.0, respectively). We also evaluated those untreated patients who eventually demonstrated disease progression during the course of the follow-up. With a median follow-up of 13 months (range, 4–144 months), 29/42 (69%) patients had decreased IgA levels at baseline, and 31/42 (74%) at the end of follow-up (P=0.63). Similarly, 27/42 (64%) of patients had decreased IgG levels at baseline, and 30/42 (71%) at the last follow-up (P=0.48). While a comparison of the presence of IgA and/or IgG hypogammaglobulinemia between those patients who remained progression-free and those whose disease progressed did not demonstrate any significant differences (data not shown), the median levels of serum IgA (45 versus 72 mg/dL) and IgG (558 versus 716 mg/dL) were lower among patients whose disease progressed than among those who remained progression-free during the follow-up period, although the difference was only statistically significant for IgG (P=0.018).
Impact of therapy and response on IgA and IgG levels
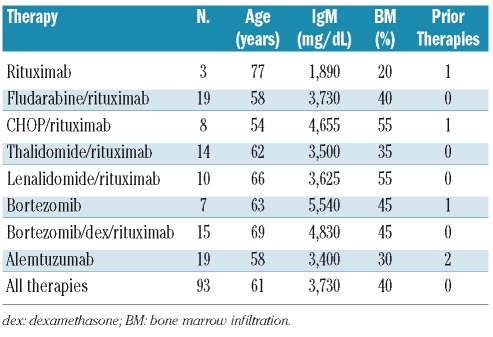
In order to understand the impact of WM-directed therapeutic interventions on uninvolved immunoglobulin levels, we analyzed changes in IgA and IgG levels in a cohort of 93 patients who underwent treatment for WM and whose baseline characteristics are presented in Table 2. The median age of these patients was 61 years (range, 43–86 years), and the median pre-therapy IgM level and bone marrow disease burden were 3,730 mg/dL (range, 458–12,400 mg/dL) and 40% (range, 5–95%), respectively. Therapies for these patients included rituximab (n=3); fludarabine and rituximab (n=19); cyclophosphamide, adriamycin, vincristine, prednisone and rituximab (CHOP-R) (n=8); thalidomide and rituximab (n=14); lenalidomide and rituximab (n=5), bortezomib (n=7), a combination of bortezomib, dexamethasone, and rituximab (n=15), and alemtuzumab (n=19). The median number of prior therapies for patients in this analysis was 0 (range, 0 to 4), and 61% of patients were previously untreated.
Table 2.
Median baseline characteristics for 93 WM patients whose immunoglobulin changes were determined pre- and post-therapy.
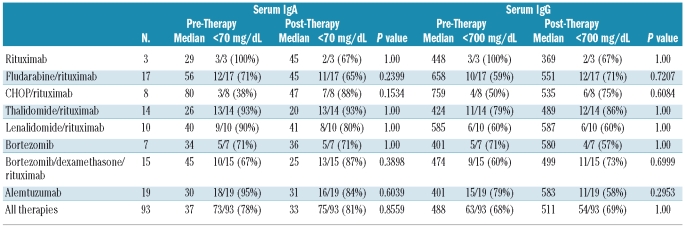
At baseline, 73 (78.5%), 63 (67.7%) and 59 (63.4%) of the patients in this cohort had low levels of IgA, IgG and both IgA and IgG, respectively. The median baseline IgA and IgG levels for these patients were 37 mg/dL (range, 6–597 mg/dL) and 488 mg/dL (range, 50–2,800 mg/dL), respectively. With a median follow-up of 12 months (range, 1–61 months), 72 (77.4%), 64 (68.8%) and 58 (62.4%) demonstrated decreased levels of IgA, IgG or both (P=0.86, P=0.88, and P=0.88, respectively). No significant recovery in the median IgA and IgG levels was observed with any therapy during the course of follow-up (Table 3), including in those patients whose follow-up exceeded 1 (n=46), 2 (n=25), and 3 (n=8) or more years post-therapy (P=NS). Median serum IgM levels during this period declined from 3,730 mg/dL (range, 458–12,400 mg/dL) to 1,320 mg/dL (range, 23–5,650 mg/dL) at best response.
Table 3.
Changes in IgA and IgG levels following treatment in 93 patients with WM. Post-therapy values reflect each patient’s best immunoglobulin level during follow-up.
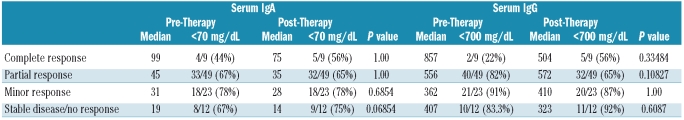
Lastly, we analyzed the impact of response quality on recovery of IgA and/or IgG immunoglobulin levels in this cohort. Eighty-one (87%) of the 93 treated patients achieved at least a minor response. In detail, 9 had a complete response, 49 had a partial response (≥ 50% decrease in IgM), and 23 had a minor response (≥ 25% decrease in IgM). No significant recovery in IgA and IgG levels was observed in any therapeutic response category during the course of follow-up, including among patients who attained a complete response (Table 4).
Table 4.
Changes in IgA and IgG levels according to response category in 93 patients with WM. Post-therapy values reflect each patient’s best immunoglobulin level during follow-up.
Sequencing of genes associated with common variable immunodeficiency disorder
Sequence analysis of the promoter, all exonic, and flanking intronic regions for each gene was performed in 19 patients with WM who had IgA (n=5), or both IgA and IgG (n=14) hypogammaglobulinemia at baseline. Sequencing studies demonstrated no novel variants in the promoter, flanking introns, or exons of AICDA, BTK, CD40, CD154, TACI, and SH2D1A in any of the 19 patients. With regards to NEMO, we observed an intronic variation at position c.1056-6T>C in two patients, and a hemizygous missense mutation at c.337G>A resulting in a change from aspartic acid to asparagine at amino acid position 113 in one other patient. Lastly, a heterozygous missense mutation at c.425A>T, resulting in a change of aspartic acid to valine at amino acid position 142, was observed in UNG in one patient who had both IgA and IgG deficiency.
Discussion
In these studies, we demonstrate that hypogammaglobulinemia of the “uninvolved” immunoglobulins (IgA, IgG) is a common finding in patients with WM. The incidence of IgA, IgG, or both IgA and IgG hypogammaglobulinemia in this series of 207 untreated WM patients was 63.3%, 58.0% and 49.3%, respectively, which is similar to the incidence reported among patients with chronic lymphocytic leukemia,6,7 but higher than that reported in other indolent lymphomas in which the incidence of IgA and IgG hypogammaglobulinemia ranges from 10–20%.7 The incidence of IgA and/or IgG hypogammaglobulinemia observed in this study also appears to be higher than that observed in individuals with IgM monoclonal gammopathy of unknown significance (MGUS), although there is great variation in the reported incidences in this latter conidtion (8–35%).15,16 The inclusion of patients with up to 10% bone marrow disease involvement, who are now considered to have WM based on consensus diagnostic criteria, may account for the higher incidence of IgA and/or IgG hypogammaglobulinemia observed in the series reported by Kyle et al.,17 in which up to 35% of IgM MGUS patients had IgA and/or IgG hypogammaglobulinemia.
An important observation in our series of 207 untreated WM patients was that the presence of IgA and/or IgG hypogammaglobulinemia was unrelated to age, sex, serum IgM levels, degree of bone marrow disease involvement, myelosuppression, absolute lymphocyte count, or prognostic indices including β2-microglobulin levels and the WM International Prognostic Scoring System score. The presence of IgA and IgG hypogammaglobulinemia is, therefore, unlikely to be a consequence of disease burden per se. Surprising, a lower incidence of IgA and IgG hypogammaglobulinemia was observed in the presence of adenopathy and splenomegaly, both features which are seen in less than 20% of WM patients. This finding may signify that the pathogenesis of WM in patients exhibiting adenopathy/and or splenomegaly could reflect a disease process more in keeping with other indolent non-Hodgkin’s lymphomas in which IgA and/or IgG hypogammaglobulinemia is less common, as previously discussed.
The presence of IgA and/or IgG hypogammaglobulinemia was reported to predict for evolution of IgM MGUS to WM in the series of patients studied by Kyle et al.16 While the presence of IgA and IgG hypogammaglobulinemia per se was not associated with disease progression, WM patients in this series who were initially managed with a ‘watch and wait’ strategy but whose disease ultimately progressed had lower median IgA and IgG levels. Another striking observation was that the presence of IgA and/or IgG hypogammaglobulinemia did not predict for recurrent infections. A comparison of the median IgA and IgG levels in patients with and without recurrent infections, as well as the presence of IgA and/or IgG hypogammaglobulinemia using our institutional cut-offs did not show any significant differences. The use of lower IgA (<30 mg/dL) and IgG (IgG <300 mg/dL) cut-offs also did not demonstrate any differences in the incidence of recurrent infections. These results suggest that other immunological and possibly even anatomical differences may contribute to the risk of recurrent infections, particularly for respiratory tract infections. The routine use of intravenous immunoglobulin replacement for WM patients with low IgA and IgG levels should, therefore, be considered prudently, since low IgA and IgG levels per se do not imply an increased risk of infection in WM patients, and such replacement therapy should be considered on a case-by-case basis taking into account each patient’s history of infections, as has been advocated for patients with other B-cell malignancies.17
An important finding in this study was the lack of impact of therapy on IgA and/or IgG levels in WM patients. Despite effective treatment, including the attainment of complete remissions in some patients, no substantial recovery of IgA and/or IgG levels was observed, even among patients who were followed up for more than 3 years after therapy. While most of the 93 patients in this series received rituximab-based therapy, which could have further aggravated IgA and IgG hypogammaglobulinemia through sustained B-cell depletion, lack of IgA and IgG recovery was also observed in this study as well as in another studies in patients who received and responded to bortezomib.18 These findings suggest that the IgA and IgG hypogammaglobulinemia in WM may represent either a constitutional defect in plasma cell or immunoglobulin production, including heavy chain switching, or that other cellular elements may contribute to the continued presence of humoral immunodeficiency. As such, patients should not be treated with the intent of restoring their IgA and IgG levels.19
As part of these studies, we performed extensive sequence analysis of the eight most often observed genes involved in immunoglobulin deficiency disorders such as common variable immunodeficiency disorder, hyper IgM syndrome, and X-linked agammaglobulinemia.8–12 These genes were AICDA, BTK, CD40, CD154, NEMO, TACI, SH2D1A, and UNG. The only novel variant of interest demonstrated by these studies was an amino acid substitution at position 142 of UNG which is present in the highly conserved catalytic domain of uracil-DNA glycosylase.20 Uracil-DNA glycosylase is essential to the generation of somatic hypermutation by generating strand breaks that lead to immunoglobulin heavy chain class switching.10,21 Individuals deficient in uracil-DNA glycosylase exhibit a hyper-IgM syndrome, with increased IgM levels and decreased IgA and IgG levels. Moreover, mice deficient in UNG develop B-cell lymphomas late in life suggesting a tumor suppressor role for uracil-DNA glycosylase.22,23 To our knowledge, however, this is the first report of a patient with a B-cell lymphoma in whom a mutation in UNG has been identified. Further validation of these findings, as well as investigation of UNG, other uracil DNA glycosylases, and other less characterized mutations involved in humoral immunodeficiencies such as ICOS, ICOS ligand, and CD19 are, therefore, warranted to clarify the underlying etiology of IgA and IgG hypogammaglobulinemia in WM.
In summary, the results of these studies suggest that most patients with WM have IgA and IgG hypogammaglobulinemia which persists despite therapeutic interventions and response. IgA and IgG hypogammaglobulinemia did not predict the risk of recurrent infections in WM patients, although lower levels of serum IgA and IgG were associated with disease progression in WM patients who were managed with a ‘watch and wait’ strategy. These studies highlight the importance of further investigations into the IgA and IgG hypogammaglobulinemia of WM, as well as the signaling pathways involved in B-cell differentiation and immunoglobulin heavy chain class switching in the pathogenesis of WM.
Footnotes
Funding: this study was supported by the International Waldenström’s Macroglobulinemia Foundation, and the Bing Fund for Waldenström’s Macroglobulinemia, the Bailey Family Fund for Waldenström’s Research, the Linda and Edward Nelson Fund for Waldenström’s Macroglobulinemia, and a gift from the Bauman Family Trust.
Authorship and Disclosures
SPT and ZRH designed the experiments and prepared the manuscript. RMJ, CH, LI, ZRH, CJP, and MCL collected patients’ data and supported the clinical database that made these studies possible. ZRH BTC, HT, PG, CL, YZ, GY, JS, MM, and LX collected and processed samples, and were involved in performing and analyzing data from sequencing analysis. SPT and PS were involved in caring for the patients contributing to these studies. MM is an employee of Correlagen Diagnostics.
The other authors reported no potential conflicts of interest.
References
- 1.Owen RG, Treon SP, Al-Katib A, Fonseca R, Greipp PR, McMaster ML, et al. Clinicopathological definition of Waldenström’s macroglobulinemia: consensus panel recommendations from the Second International Workshop on Waldenström’s Macroglobulinemia. Semin Oncol. 2003;30(2):110–5. doi: 10.1053/sonc.2003.50082. [DOI] [PubMed] [Google Scholar]
- 2.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. The World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues. Report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November, 1997. Ann Oncol. 1999;10(12):1419–32. doi: 10.1023/a:1008375931236. [DOI] [PubMed] [Google Scholar]
- 3.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, et al. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84(5):1361–92. [PubMed] [Google Scholar]
- 4.Treon SP, Hunter ZR, Aggarwal A, Ewen EP, Masota S, Lee C, et al. Characterization of familial Waldenström’s macroglobulinemia. Ann Oncol. 2006;17(3):488–94. doi: 10.1093/annonc/mdj111. [DOI] [PubMed] [Google Scholar]
- 5.Stanley PJ, Corbo G, Cole PJ. Serum IgG subclasses in chronic and recurrent respiratory infections. Clin Exp Immunol. 1984;58(3):703–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Orfao A, Gonzalez M, San Miguel JF, Tomas JF, Canizo MC, Lopez-Berges MC, et al. Surface phenotype and immunoglobulin levels in B-cell chronic lymphocytic leukaemia. Haematologica. 1990;23(1):49–56. [PubMed] [Google Scholar]
- 7.Biggar RJ, Christiansen M, Rostgaard K, Smedby KE, Adami HO, Glimelius B, Hjalgrim H, Melbye M. Immunoglobulin subclass levels in patients with non-Hodgkin lymphoma. Int J Cancer. 2009;124(11):2616–20. doi: 10.1002/ijc.24245. [DOI] [PubMed] [Google Scholar]
- 8.Castigli E, Wilson SA, Garibyan L, Rachid R, Bonilla F, Schneider L, et al. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Genet. 2005;37(8):829–34. doi: 10.1038/ng1601. [DOI] [PubMed] [Google Scholar]
- 9.Conley ME, Dobbs AK, Farmer DM, Kilic S, Paris K, Grigoriadou S, et al. Primary B cell immunodeficiencies: comparisons and contrasts. Annu Rev Immunol. 2009;27:199–227. doi: 10.1146/annurev.immunol.021908.132649. [DOI] [PubMed] [Google Scholar]
- 10.Imai K, Slupphaug G, Lee WI, Revy P, Nonoyama S, Catalan N, et al. Human uracil-DNA glycosylase deficiency associated with profoundly impaired immunoglobulin class-switch recombination. Nat Immunol. 2003;4(10):1023–8. doi: 10.1038/ni974. [DOI] [PubMed] [Google Scholar]
- 11.Lee WI, Torgerson TR, Schumacher MJ, Yel L, Zhu Q, Ochs HD. Molecular analysis of a large cohort of patients with the hyper immunoglobulin M (IgM) syndrome. Blood. 2005;105(5):1881–90. doi: 10.1182/blood-2003-12-4420. [DOI] [PubMed] [Google Scholar]
- 12.Salzer U, Chapel HM, Webster AD, Pan-Hammarstrom Q, Schmitt-Graeff A, Schlesier M, et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet. 2005;37(8):820–8. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 13.Morel P, Duhamel A, Gobbi P, Dimopoulos MA, Dhodapkar MV, McCoy J, et al. International Prognostic Scoring System for Waldenstrom macroglobulinemia. Blood. 2009;113(18):4163–70. doi: 10.1182/blood-2008-08-174961. [DOI] [PubMed] [Google Scholar]
- 14.Kimby E, Treon SP, Anagnostopoulos A, Dimopoulos M, Garcia-Sanz R, Gertz MA, et al. Update on recommendations for assessing response from the Third International Workshop on Waldenström’s Macroglobulinemia. Clin Lymphoma Myeloma. 2006;6(5):380–3. doi: 10.3816/CLM.2006.n.013. [DOI] [PubMed] [Google Scholar]
- 15.Morra E, Cesana C, Klersy C, Barbano L, Miqueleiz S, Varettoni M, et al. Prognostic factors for transformation in asymptomatic immunoglobulin M monoclonal gammopathies. Clin Lymphoma Myeloma. 2005;5(4):265–9. doi: 10.3816/clm.2005.n.013. [DOI] [PubMed] [Google Scholar]
- 16.Kyle RA, Benson J, Larson D, Therneau T, Dispenzieri A, Melton JL, III, Rajkumar SV. IgM monoclonal gammopathy for undetermined significance and smoldering Waldenström’s macroglobulinemia. Clin Lymphoma Myeloma. 2009;9(1):17–8. doi: 10.3816/CLM.2009.n.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raanani P, Gafter-Gvili A, Paul M, Ben-Bassat I, Leibovici L, Shpilberg O. Immunoglobulin prophylaxis in chronic lymphocytic leukemia and multiple myeloma: systematic review and meta-analysis. Leuk Lymphoma. 2009;50(5):764–72. doi: 10.1080/10428190902856824. [DOI] [PubMed] [Google Scholar]
- 18.Treon SP, Hunter ZR, Matous J, Joyce RM, Mannion B, Advani R, et al. Multicenter clinical trial of bortezomib in relapsed/refractory Waldenström’s macroglobulinemia: results of WMCTG trial 03-248. Clin Cancer Res. 2007;13(11):3320–5. doi: 10.1158/1078-0432.CCR-06-2511. [DOI] [PubMed] [Google Scholar]
- 19.Treon SP. How I treat Waldenstrom’s macroglobulinemia. Blood. 2009;114(12):2375–85. doi: 10.1182/blood-2009-05-174359. [DOI] [PubMed] [Google Scholar]
- 20.Mol CD, Arvai AS, Slupphaug G, Kavli B, Alseth I, Krokan HE, Tainer JA. Crystal structure and mutational analysis of human uracil-DNA glycosylase: structural basis for specificity and catalysis. Cell. 1995;80(6):869–78. doi: 10.1016/0092-8674(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 21.Sousa MM, Krokan HE, Slupphaug G. DNA-uracil and human pathology. Mol Aspects Med. 2007;28(3–4):276–306. doi: 10.1016/j.mam.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Nilsen H, Stamp G, Andersen S, Hrivnak G, Krokan HE, Lindahl T, Barnes DE. Gene-targeted mice lacking the UNG uracil-DNA glycosylase develop B-cell lymphomas. Oncogene. 2003;22(35):5381–6. doi: 10.1038/sj.onc.1206860. [DOI] [PubMed] [Google Scholar]
- 23.Andersen S, Ericsson M, Dai HY, Peña-Diaz J, Slupphaug G, Nilsen H, et al. Monoclonal B-cell hyperplasia and leukocyte imbalance precede development of B-cell malignancies in uracil-DNA glycosylase mice. DNA Repair. 2005;4(12):1432–41. doi: 10.1016/j.dnarep.2005.08.004. [DOI] [PubMed] [Google Scholar]