Abstract
Background
Transfusion-dependency affects the natural history of myelodysplastic syndromes. Secondary iron overload may concur to this effect. The relative impact of these factors on the outcome of patients with myelodysplastic syndrome receiving allogeneic stem-cell transplantation remains to be clarified.
Design and Methods
We retrospectively evaluated the prognostic effect of transfusion history and iron overload on the post-transplantation outcome of 357 patients with myelodysplastic syndrome reported to the Gruppo Italiano Trapianto di Midollo Osseo (GITMO) registry between 1997 and 2007.
Results
Transfusion-dependency was independently associated with reduced overall survival (hazard ratio=1.48, P=0.017) and increased non-relapse mortality (hazard ratio=1.68, P=0.024). The impact of transfusion-dependency was noted only in patients receiving myeloablative conditioning (overall survival: hazard ratio=1.76, P=0.003; non-relapse mortality: hazard ratio=1.70, P=0.02). There was an inverse relationship between transfusion burden and overall survival after transplantation (P=0.022); the outcome was significantly worse in subjects receiving more than 20 red cell units. In multivariate analysis, transfusion-dependency was found to be a risk factor for acute graft-versus-host disease (P=0.04). Among transfusion-dependent patients undergoing myeloablative allogeneic stem cell transplantation, pre-transplantation serum ferritin level had a significant effect on overall survival (P=0.01) and non-relapse mortality (P=0.03). This effect was maintained after adjusting for transfusion burden and duration, suggesting that the negative effect of transfusion history on outcome might be determined at least in part by iron overload.
Conclusions
Pre-transplantation transfusion history and serum ferritin have significant prognostic value in patients with myelodysplastic syndrome undergoing myeloablative allogeneic stem cell transplantation, inducing a significant increase of non-relapse mortality. These results indicate that transfusion history should be considered in transplantation decision-making in patients with myelodysplastic syndrome.
Keywords: transfusion-dependency, myelodysplastic syndromes, secondary iron overload
Introduction
Myelodysplastic syndromes (MDS) are a heterogeneous group of disorders characterized by peripheral cytopenia and an increased risk of evolution into acute myeloid leukemia (AML).1–3 The natural history of these syndromes, which range from indolent conditions to forms that rapidly progress to leukemia, complicates therapeutic choices and timing of interventions.4
The only curative treatment for patients with MDS is allogeneic stem cell transplantation (SCT).5–8 However, given the considerable morbidity and mortality associated with this approach,9–11 candidate patients must be carefully selected.12,13
All patients with MDS are likely to receive red blood cell transfusions at some point during their disease.2 As a consequence, many subjects with low-risk disease at diagnosis have a long history of transfusion if they eventually undergo transplantation. The onset of transfusion requirement has been found to affect the outcome of MDS patients and is now considered an independent indicator of disease severity.2,4,14 We observed, in addition, that transfusion-dependent patients may have a reduced survival after transplantation, and that the WHO classification-based Prognostic Scoring System (WPSS),14 which includes transfusion as a prognostic variable, improves post-transplantation outcome stratification in MDS.15
Iron-related tissue damage is an important adverse prognostic factor in transfusion-dependent patients with thalassemia undergoing allogeneic SCT.16,17 Hepatic iron accumulation, assessed by magnetic resonance imaging, was recently found in MDS patients who had received 20 or more red cell units.18 Moreover, Armand et al. found that a high pre-transplantation level of serum ferritin is associated with poor outcome after transplantation.19
In this study we retrospectively evaluated the prognostic significance of pre-transplantation transfusion history and secondary iron overload in a cohort of MDS patients who underwent allogeneic SCT between 1997 and 2007.
Design and Methods
Patients’ characteristics and transplant procedures
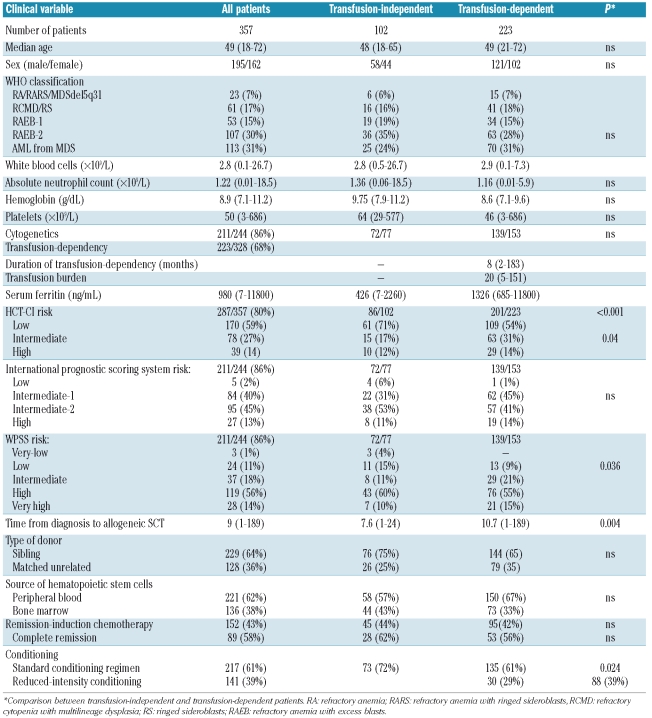
We studied 357 patients undergoing allogeneic SCT for primary MDS between 1997 and 2007 whose data were reported to the GITMO registry. The procedures followed were in accordance with the ethical standards of the Institutional Committee on Human Experimentation and GITMO, as well as with the Declaration of Helsinki. All clinical variables were analyzed at the time of transplantation in patients undergoing upfront allogeneic SCT and at the time of remission-induction chemotherapy in those receiving treatment before transplantation.
One hundred and ninety-five patients were male and 162 were female. The median age was 49 years (range, 18–72 years). Two hundred and forty-four patients were classified as having MDS according to the WHO criteria,20 while 113 subjects, previously classified as having refractory anemia with excess blasts in transformation according to the French-American-British criteria, were considered to have AML secondary to MDS. Cytogenetic data were available for 211 of the 244 patients with MDS according to the WHO criteria (86%), and the International Prognostic Scoring System21 and WPSS14 scores were assessed in these patients. Data on extra-hematologic co-morbidities, evaluated using the hematopoietic cell transplantation-specific co-morbidity index (HCT- CI),9 were obtained for 287 patients (80%).
Transfusion history was defined in terms of transfusion dependency, burden and duration. Transfusion-dependency was defined, according to the WPSS criteria,14 as requiring at least one red blood cell transfusion every 8 weeks over a period of 4 months before intensive treatment. Data were available for 325 patients (91%). The total number of packed red blood cell (PRBC) units received before intensive treatment was recorded for 203 patients. Serum ferritin levels were assessed before intensive treatment (median time, 1.9 months; range, 0–5.9 months) in 228 patients (64%). In 157 patients data regarding serum albumin, serum iron and transferrin levels were also collected at the same time as serum ferritin was assessed (Table 1). Seventeen patients received chelation therapy before transplantation and were excluded from the analyses.
Table 1.
Clinical characteristics and transplant-related features of patients classified according to the WHO criteria at the time of allogeneic SCT or remission-induction chemotherapy.
Allogeneic SCT was performed at a median of 9 months (range, 1–189) after the diagnosis of MDS. The donors were HLA-matched siblings in 229 cases (64%) and unrelated donors in the other 128 cases (36%). Criteria for the selection of HLA-matched unrelated donors before 2002 included low-resolution typing for HLA class I (A, B) and high-resolution typing for HLA-DRB1, whereas since 2002 the criteria included high-resolution typing for both HLA class I (A, B, C) and class II alleles (DRB1/3/4/5, DQA1, DPB1). The source of hematopoietic stem cells was peripheral blood in 221 patients (62%) and bone marrow in 136 (38%). One hundred and fifty-two patients (43%) received remission-induction chemotherapy prior to their transplant. The conditioning regimen was myeloablative in 217 patients (61%) and of reduced-intensity in 140 patients (39%). The most frequent conditioning regimens included total body irradiation and cyclophosphamide (20% of cases), total body irradiation and fludarabine (8%), busulphan and cyclophosphamide (28%), thiotepa and cyclophosphamide (26%), and thiotepa and fludarabine (11%). For most patients, graft-versus-host disease (GVHD) prophylaxis was a combination of cyclosporine and methotrexate (Table 1).
End-points and statistical analysis
Numerical variables are summarized by their medians and ranges, categorical variables by counts and relative frequencies. The primary end-points were overall survival (OS), non-relapse mortality (NRM) and probability of relapse. OS was defined as the time between transplantation and death (from any cause) or last follow-up (for censored observations). When estimating NRM, any death in the absence of disease relapse was considered an event. The probability of relapse was estimated considering treatment as a failure at the time of hematologic relapse according to standardized criteria.22 Acute GVHD and chronic GVHD were also investigated. The cumulative probabilities of OS, NRM and relapse were estimated using the Kaplan-Meier product limit method. Comparisons between Kaplan-Meier curves were carried out with the Gehan Wilcoxon test. The probabilities of relapse and NRM were analyzed as competing risks.23 Univariate and multivariate survival analyses were performed using Cox proportional hazards regression to identify the most significant independent prognostic factors. To decide which parameterization of the covariates (categorical, with indicator variables, versus continuous, with a single parameter) was preferable, we carried out likelihood ratio tests, none of which was statistically significant. We, therefore, decided to treat all covariates as continuous variables, for simplicity in the presentation of the results. The risk factors associated with the occurrence of acute and chronic GVHD were investigated using multivariate logistic regression models. Analyses were performed using Statistica 7.0 (Statsoft, Tulsa, OK, USA) and Stata 9 (StataCorp, College Station, TX, USA) software.
Results
Post-transplantation outcome according to transfusion-dependency
In the whole cohort, 5-year OS was 39%, the 5-year probability of relapse was 42% and NRM was 41%. The day-100 cumulative incidences of grade I and of grade II to IV acute GVHD were 23% and 39%, respectively. The 5-year cumulative incidences of overall and extensive chronic GVHD were 64% and 34%, respectively.
A regular transfusion need before transplantation was reported in 223 (68%) of 328 evaluable subjects. The median number of PRBC units received was 20 (range, 5–151). Considering that each unit of blood contains approximately 200–250 mg of iron, the median intake of iron due to transfusions was 4 g (range, 1– 30 g). The median duration of transfusion-dependency was 8 months (range, 2–183 months). There was no significant difference in the proportion of transfusion-dependent patients among the WHO categories (P=0.31). The time between diagnosis and transplantation was longer among transfusion-dependent patients than among patients who did not receive transfusion therapy, (10.7 versus 7.6 months; P=0.004). The transfusion-dependent patients had a higher HCT-CI score (P=0.04) and an increased percentage of reduced-intensity conditioning (RIC) (39% versus 28%; P=0.024) (Table 1). An increased occurrence of acute GVHD was noted in transfusion-dependent patients (67% versus 57%; P=0.03), while no significant difference was seen in chronic GVHD.
In univariate analysis, transfusion-dependency significantly affected OS (HR 1.68; P<0.001), NRM (HR 1.72; P=0.001), and probability of relapse (HR 1.61; P=0.011).
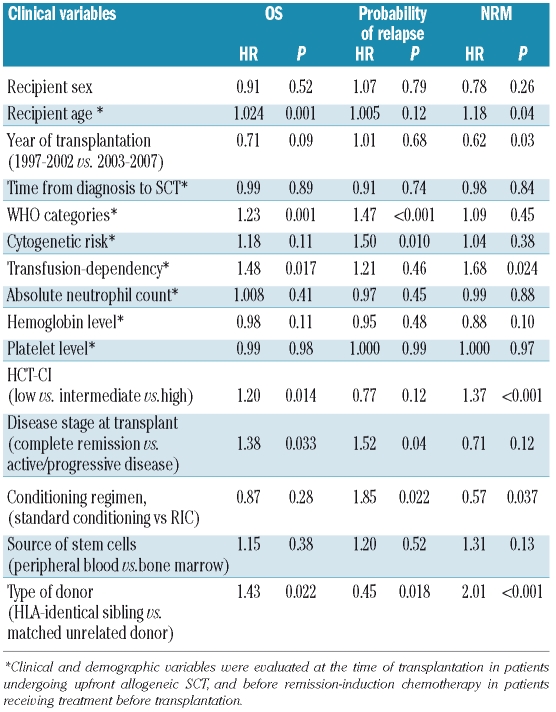
We performed a multivariate Cox survival analysis with the following covariates: WHO category, cytogenetic risk (scored according to the International Prognostic Scoring System), transfusion-dependency, absolute neutrophil count, hemoglobin and platelet levels, presence of extra-hematologic co-morbidities (according to the HCT-CI), age of recipient, time between diagnosis and transplantation, year of transplantation (1997–2002 versus 2003–2007), disease stage at transplantation (complete remission or marrow blasts <5% versus not complete remission or blasts ≥5%), remission-induction chemotherapy (received versus not received), source of hematopoietic stem cells (peripheral blood versus bone marrow), type of donor (HLA-identical sibling versus matched unrelated donor) and type of conditioning (myeloablative conditioning regimen versus RIC).
Recipient age, WHO category, disease stage at transplantation, HCT-CI and type of donor had significant effects on OS. WHO category with excess blasts, unfavorable cytogenetics, active/progressive disease at transplantation, RIC, and the use of an HLA-identical sibling donor were associated with a higher probability of relapse. Recipient age, transplantation prior to 2003, use of myeloablative conditioning, HLA-matched unrelated donor and high HCT-CI score were significant risk factors for NRM (Table 2).
Table 2.
Prognostic factors for post-transplantation outcome in the whole study population (multivariate analysis).
To clarify the prognostic effect of disease status at transplantation we considered patients stratified according to WHO category and type of conditioning regimen. In patients with AML from MDS, there were significant advantages of undergoing transplantation in complete remission in both the groups given standard conditioning and RIC (OS: HR=0.48, P=0.02 and HR=0.37, P=0.039, respectively; probability of relapse: HR=0.41, P=0.017 and HR=0.19, P=0.009, respectively). Considering patients affected with refractory anemia with excess blasts-1 and -2, a borderline effect on the probability of relapse was noted only among patients receiving RIC (HR=0.44, P=0.06).
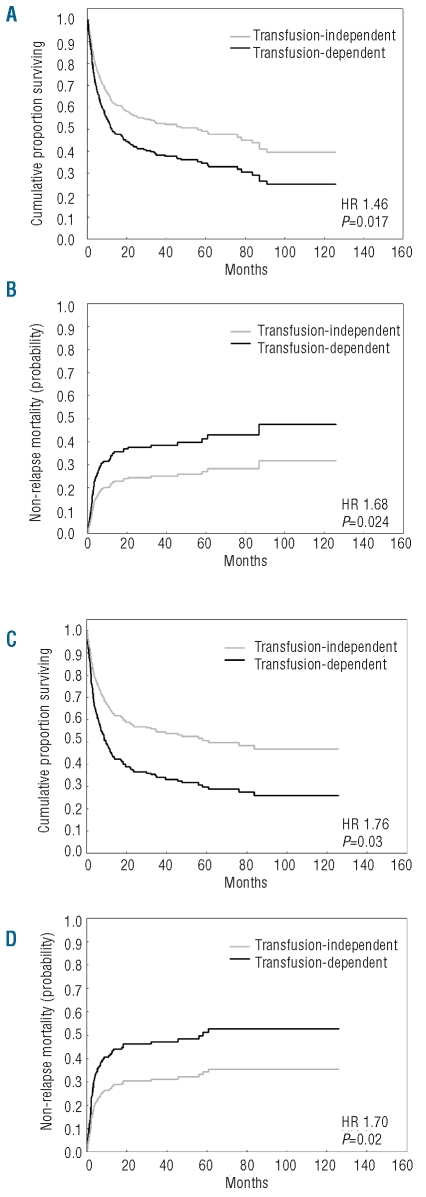
Transfusion-dependency had a significant effect on both OS (HR=1.48, P=0.017) and NRM (HR=1.68, P=0.024), whereas no significant effect was found for the probability of relapse (HR=1.21, P=0.46) (Table 2, Figure 1A). The prognostic effect of transfusion-dependency was maintained when the analysis was focused on the 244 patients with a diagnosis of MDS according to the WHO criteria (OS: HR=1.64, P=0.016; NRM: HR=1.76, P=0.042).
Figure 1.
Post-transplantation outcome according to the presence of transfusion-dependency. Probability of OS and NRM after allogeneic hematopoietic SCT in the whole MDS population (A, B, respectively) and in patients receiving myeloablative conditioning (C, D, respectively). The curves were estimated from multivariable Cox regression analysis adjusted for the patients’ age and sex, WHO category, cytogenetics, disease status at transplant, presence of comorbidities, source of stem cells, type of donor and type of conditioning.
In order to verify whether the introduction of transfusion-dependency may improve the prognostic stratification of MDS patients undergoing allogeneic SCT, we fitted two separate multivariate Cox analyses including the same covariates as detailed above with and without transfusion-dependency, and compared them with the likelihood ratio test. This resulted in a significant P value (P=0.004), confirming the importance of accounting for transfusions in the prognostic model.
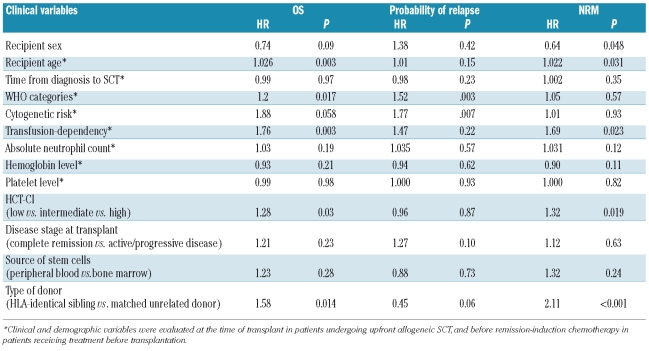
We then carried out separate multivariate analyses to investigate the prognostic effect of transfusion-dependency in selected subgroups of patients. When stratifying according to type of conditioning, transfusion-dependency retained a significant effect in patients receiving myeloablative conditioning (OS: HR=1.76, P=0.003; NRM: HR=1.70, P=0.02; probability of relapse: HR=1.47, P=0.22, Table 3, Figure 1B), whereas no significant effect was seen in patients receiving RIC (OS: HR=0.84, P=0.60; NRM: HR=0.67, P=0.28; probability of relapse: HR 0.81, P=0.63).
Table 3.
Prognostic factors for post-transplantation outcome in 217 MDS patients receiving myeloablative conditioning (multivariate analysis).
When focusing on MDS patients without excess blasts, transfusion-dependency significantly affected post-transplantation OS (HR=3.01, P=0.014) and was associated with increased NRM (HR=3.49, P=0.02). Among MDS patients with excess blasts or MDS-AML, transfusion-dependency retained a significant effect on both OS (HR=1.50, P=0.012) and NRM (HR=1.69, P=0.031).
Finally, we analyzed relapse and NRM as competing risks in these subgroups of patients. Considering patients receiving myeloablative conditioning, the cumulative incidences of relapse and NRM were 23% and 33%, respectively, in transfusion-independent patients and 24% and 45%, respectively, in transfusion-dependent patients. Among the patients receiving RIC, the cumulative incidences of relapse and NRM were 64% and 21%, respectively, in transfusion-independent patients and 52% and 22%, respectively, in transfusion-dependent patients.
Focusing on patients with excess blasts or MDS-AML, the cumulative incidences of relapse and NRM were 35% and 33%, respectively, in transfusion-independent patients and 34% and 43%, respectively, in transfusion-dependent patients. In patients without excess blasts, the cumulative incidences of relapse and NRM were 10% and 26%, respectively, in transfusion-independent patients and 17% and 33%, respectively, in transfusion-dependent patients.
Post-transplantation outcome according to transfusion burden
We focused on 135 transfusion-dependent subjects receiving myeloablative conditioning, with the aim of investigating the prognostic effect of the transfusion burden in these patients. We first performed a multivariate analysis including the number of received PRBC units as a continuous variable, and adjusting for duration of transfusion need. WHO category, cytogenetics, absolute neutrophil count, hemoglobin and platelet levels, age of recipient, disease stage at transplantation, source of hematopoietic stem cells and type of donor were also included as covariates. The number of PRCB units received showed a significant effect on OS (HR=1.029, P=0.022) and NRM (HR=1.034, P=0.021).
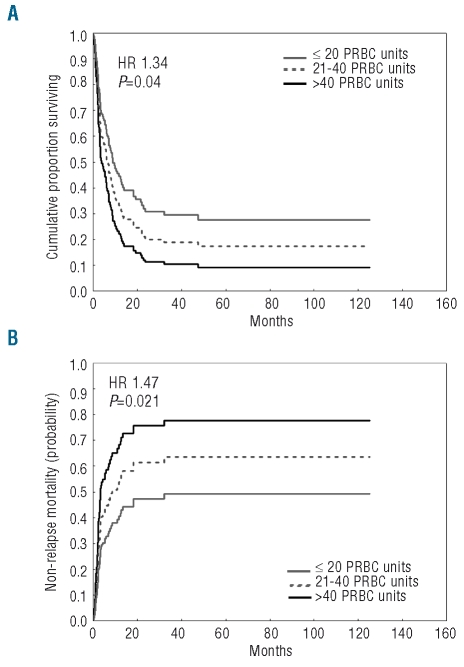
These results were confirmed when transfusion-dependent patients were grouped into three categories according to whether they received 20 or fewer PRBC units (64 patients, 47%), 21–40 PRBC units (45 patients, 33%), or more than 40 PRBC units (26 patients, 19%) prior to transplantation (OS: HR=1.34, P=0.04; NRM: HR=1.47 P=0.021) (Figure 2).
Figure 2.
Post-transplantation outcome of MDS patients receiving standard conditioning according to transfusion burden: probability of overall survival, (A) and non-relapse mortality, (B). Patients were categorized as receiving 20 or fewer, 20–40, or more than 40 PRBC units. The curves were estimated from multivariable Cox regression analysis adjusted for patients’ age and sex, WHO category, cytogenetics, disease status at transplant, presence of comorbidities, duration of transfusion dependency, source of stem cells and type of donor.
With respect to transfusion-independent patients, no significant difference was found between patients receiving 20 or fewer PRBC units before transplantation and transfusion-independent patients (P=0.08 and P=0.24 for OS and NRM, respectively), while post-transplantation outcome was significantly worse in patients receiving 21–40 PRBC units (P=0.002 and P=0.015 for OS and NRM, respectively) and more than 40 PRBC units (P<0.001 and P<0.001 for OS and NRM, respectively).
An analysis of the 96 patients with a diagnosis of MDS according to the WHO criteria showed that transfusion burden has a significant effect on NRM (HR=1.56, P=0.023) and a borderline effect on OS (HR=1.39, P=0.063).
Finally, we carried out multivariate logistic regression analyses for the presence of acute and chronic GVHD using recipient age, presence of transfusion-dependency, source of hematopoietic stem cell and type of donor as covariates. Transfusion-dependency was found to be an independent risk factor for developing acute GVHD grades II to IV (P=0.04), while no significant effect on the occurrence of chronic GVHD was noted.
Post-transplantation outcome according to serum ferritin level
We evaluated the impact of secondary iron overload, as assessed by serum ferritin levels, on the outcome of transfusion-dependent MDS patients undergoing myeloablative allogeneic SCT. Pre-transplantation serum ferritin values were available for 129 out of 135 patients (median value, 1270 ng/mL; range, 547–11800 ng/mL). A significant correlation was seen between the number of PRBC units received before transplantation and serum ferritin level (r=0.46, P<0.001).
In order to verify whether increased ferritin level was an expression of iron overload in transfusion-dependent patients, we analyzed the percentage of transferrin saturation: the median value was 86% (range, 58–100%).
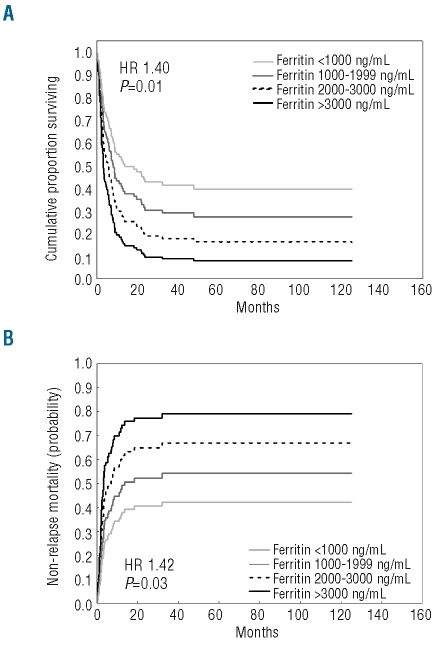
Transfusion-dependent patients were grouped on the basis of serum ferritin concentration as follows: serum ferritin less than 1000 ng/mL (47 patients, 36%), 1000–1999 ng/mL (55 patients, 43%), 2000–3000 ng/mL (17 patients, 13%) and more than 3000 ng/mL (10 patients, 8%). Pre-transplantation ferritin level showed a significant effect on OS (HR=1.40, P=0.01) and NRM (HR=1.42 P=0.03) in a multivariate analysis including WHO category, cytogenetics, absolute neutrophil count, hemoglobin and platelet levels, age of recipient, disease stage at transplantation, source of hematopoietic stem cells and type of donor as co-variates (Figure 3). No significant effect on probability of relapse was found.
Figure 3.
Post-transplantation outcome of transfusion-dependent patients receiving standard conditioning according to serum ferritin level: probability of overall survival, (A) and non-relapse mortality, (B) Patients were categorized as follows: serum ferrtin <1000 ng/mL, 1000–1999 ng/mL, 2000–3000 ng/mL and >3000 mg/mL. The curves were estimated from multivariable Cox regression analysis adjusted for patients’ age and sex, WHO category, cytogenetics, disease status at transplant, presence of comorbidities, source of stem cells and type of donor.
After adding transfusion burden (number of PRBC units) and transfusion duration to the model, the effect of serum ferritin was maintained on both OS and NRM (HR=1.47 P=0.038 and HR=1.54 P=0.04, respectively). A trend towards transfusion burden having a significant effect on post-transplantation outcome was also detected (OS: HR=1.34 P=0.10; NRM: HR=1.43, P=0.09). Limiting the analysis to transfusion-dependent patients with a diagnosis of MDS according to the WHO criteria, serum ferritin level showed a negative effect on NRM (HR=1.44, P=0.04) and a borderline effect on OS (HR=1.39, P=0.07).
In order to account for the possible role of ferritin as an acute-phase reactant, we included pre-transplantation serum albumin in the model, and we found that the impact of ferritin on outcome was unchanged (data not shown).
Finally, applying the same multivariate analysis to transfusion-dependent patients receiving RIC, no significant effect of pre-transplantation serum ferritin on OS (HR=1.03, P=0.61) or NRM (HR=1.11 P=0.28) was observed.
Discussion
We previously showed that transfusion-dependent MDS patients may have a reduced survival after transplantation, and that the WPSS score, which includes transfusion as a prognostic variable, is able to stratify post-transplantation outcome in patients with MDS.15 The present study had a wider scope. The analysis was focused on a more recent and restricted period, in which transplant procedures have become more homogeneous and data registration more complete and accurate. Moreover, the clinical effects of additional parameters such as pre-transplantation transfusion burden and duration and serum ferritin level were assessed.
We clarified that the negative impact of transfusion-dependency is associated with an increased risk of NRM, while no significant effect was seen on the probability of relapse. The effect of transfusion-dependency was restricted to MDS patients who received a myeloablative conditioning regimen and are, therefore, at higher risk of developing transplant-related toxicity; no significant effect was noted in patients receiving RIC. An inverse relationship was seen between transfusion burden and probability of surviving after transplantation: post-transplantation outcome was comparable between patients receiving 20 or fewer PRBC units and transfusion-independent patients, but was significantly worse in subjects with a long history of transfusion-dependency. We also found that the negative effect of transfusion history on post-transplantation outcome might be related at least in part to secondary iron overload.
In MDS, the presence of transfusion-dependency has already been shown to have a negative prognostic value due to the concomitant effect of more severe anemia, more aggressive disease and secondary iron overload.1,2,14 Recently, an elevated pre-transplantation serum ferritin level was found to be associated with reduced survival in patients with MDS.19,24 Nevertheless, these studies were potentially biased by the fact that they did not take into account transfusion history before transplantation. It is very important to clarify whether high ferritin levels at the time of transplantation were due to transfusions or inflammation: in fact, these two clinical conditions are quite different in terms of biological characteristics and, more importantly, iron metabolism.25
The prognostic effect of transfusion-dependency, the relationship between transfusion burden and transplant-related mortality but not relapse rate, the selective effect in patients receiving myeloablative conditioning, the escalating effect of transfusion burden, the relationship between serum ferritin and transplantation outcome, the fact that there is no difference between non-transfused and lightly transfused patients, all consistently indicate a direct effect of secondary iron overload on post-transplantation outcome.
Our results also suggest that the presence of pre-transplantation transfusion-dependency might be a risk factor for acute GVHD. It is possible to speculate that the presence of parenchymal iron overload, through free iron radical-mediated injury, may increase the susceptibility of organs (in particular the liver) to GVHD.
Data on the role of iron in organ damage in MDS are limited.26 Serum ferritin is the most commonly used indirect estimate of iron overload,27,28 and a direct correlation between serum ferritin and the number of transfusions received has been observed.2 On average, each PRBC unit adds about 200–250 mg of iron to the total iron pool in the body, and iron overload can occur after about 20 transfusions.28 It was recently shown that all MDS patients who had received 20 or more red cell units has iron accumulation in the liver, as detected by magnetic resonance imaging,18 and serum ferritin was found to have a significant impact on survival of patients with refractory anemia.2,4
In this study we observed a significant effect of serum ferritin in transfusion-dependent MDS patients who underwent myeloablative allogeneic SCT. The effect was maintained after adjusting for both transfusion burden and duration. The negative effect of increased serum ferritin levels was mainly an increased NRM, while no effect on the probability of relapse was seen. Secondary iron overload did not have an effect on patients receiving RIC, who were less exposed to the risk of NRM with respect to those receiving a standard conditioning regimen.
A single determination of serum ferritin level as a marker of iron overload should be considered with some caution due to its concomitant role as acute-phase reactant.27,28 However, we found that the prognostic effect of serum ferritin was unlikely to have depended substantially on acute-phase issues. Moreover, when considering transferrin saturation to define the site of iron accumulation25 we observed very high values, suggesting the presence of parenchymal iron loading in transfusion-dependent patients.
There are potential sources of bias in our analysis, inherent to the retrospective nature of a study based on a transplantation registry. However, data about transfusion history and pre-transplantation serum ferritin were available for the great majority of the original population of patients, and the analyses were adjusted for all known potential confounding factors by adopting multivariate techniques and/or performing stratified and subgroup analyses. Therefore, in spite of the limitations, the findings of this study might have relevant clinical implications.
Our results suggest that transfusion history should be considered in MDS transplantation decision-making, as also indicated by the significant impact of WPSS score on post-transplantation outcome.15 For patients who are candidates for allogeneic SCT, it is very important to avoid complications related to the presence of transfusion-dependent anemia (such as secondary iron overload), and this might be particularly relevant for younger patients, who usually receive a myeloablative conditioning. A decision analysis from the International Bone Marrow Transplant Registry demonstrated that life expectancy of patients with low-risk MDS was longer when transplantation was delayed by some period.29 However, the additional risk of disease complications that might increase transplant toxicity or preclude these patients from a later transplantation are to be considered when implementing delayed treatment strategies in clinical practice.
On the other hand, patients with a long history of transfusion and evidence of iron overload at the time of transplantation might benefit for a reduced-intensity conditioning regimen, in order to reduce the probability of NRM.
Finally, the results of the present study support the rationale for direct evaluation of iron overload and iron-mediated tissue damage in MDS patients who are candidates for allogeneic SCT and for clarifying the possible role of chelation therapy in reducing transfusion-related complications in these patients.
Appendix
The following institutions (GITMO centers) in Italy contributed to the trial: Division of Hematology, Ospedale “S. S. Antonio e Biagio” Alessandria (A. Levis); Division of Hematology, Ospedali Riuniti, Bergamo (A. Rambaldi); Institute of Hematology and Clinical Oncology “L. A. Seragnoli,” Ospedale “S. Orsola-Malpighi,” University of Bologna, Bologna (G. Bandini); Department of Hematology, Ospedale Regionale, Bolzano (M. Casini); Division of Hematology, Spedali Civili, Brescia (G. Rossi); Division of Hematology and Bone Marrow Transplant Center, Ospedale Oncologico “A. Businco,” Cagliari (E. Angelucci, D. Baronciani); Bone Marrow Transplantation Unit, Ospedale “R. Binagli,” University of Cagliari, Cagliari (G. La Nasa); Division of Hematology and Bone Marrow Transplantation, Ospedale “Ferrarotto,” Catania (G. Milone); Division of Hematology, Ospedale “S. Croce e Carlo,” Cuneo (N. Mordini); Department of Hematology, Ospedale “Careggi,” University of Florence, Firenze (S. Guidi, A. Bosi); Division of Hematology, Ospedale “S. Martino,” Genova (A. Bacigalupo, M. T. Van Lint), Hematology–Bone Marrow Transplantation Unit, Istituto Nazionale dei Tumori, University of Milano, Milano (P. Corradini, R. Milani), Division of Hematology Ospedale “Ca‘ Granda” Niguarda, Milano (E. Morra, P. Marenco); Department of Hematology, Fondazione IRCCS Ospedale Maggiore Policlinico, Mangiagalli e Regina Elena, Milano (G. Lambertenghi Deliliers, F. Onida); Hematology and Bone Marrow Transplantation Unit, S. Raffaele Scientific Institute, Milano (F. Ciceri, M. Bernardi); Transplantation Unit Department of Oncology-Hematology, IRCCS Clinica Humanitas, Rozzano (L. Castagna); Department of Oncology and Hematology University of Modena and Reggio Emilia, Modena (F. Narni); Division of Hematology and Transplant Unit, Ospedale “S. Gerardo,” University of Milano-Bicocca, Monza (P. Pioltelli), Division of Hematology, University of Napoli “Federico II” Medical School, Napoli (C. Selleri); Division of Hematology and Transplant Unit, Ospedale “V. Cervello,” Palermo (R. Scime‘); Division of Hematology, University of Palermo, Palermo (E. Iannitto); Department of Oncology, Hematology Unit, Ospedale “La Maddalena,” Palermo (M. Musso), Division of Hematology, University of Pavia, Fondazione IRCCS Policlinico “S. Matteo,” Pavia (E. P. Alessandrino); Pediatric Hematology-Oncology University of Pavia, Fondazione IRCCS Policlinico “S. Matteo,” Pavia (F. Locatelli); Department of Hematology, University of Perugia, Policlinico “Monteluce,” Perugia (F. Martelli); Hematology and Transplant Center, Ospedale “S. Salvatore,” Pesaro (G. Visani); Department of Hematology, Ospedale Civile, Pescara (P. Di Bartolomeo); Oncology and Hematology Department, Ospedale “Guglielmo da Saliceto,” Piacenza (L. Cavanna); Division of Hematology, Univeristy of Pisa, Pisa (F. Papineschi); Transplant Unit “A. Neri,” Ospedale “Bianchi-Melacrino-Morelli,” Reggio Calabria (G. Messina); Hematology Unit, Arcispedale “S. Maria Nuova,” Reggio Emilia (L. Gugliotta); Division of Hematology, Department of Cellular Biotechnologies and Hematology, University “La Sapienza” (A. P. Iori, B. Lucarelli, R. Foa‘); Hematology and Stem Cell Transplantation Unit Ospedale “S. Camillo,” Roma (A. Locasciulli, I. Majolino); Hematology, University “S. Cuore,” Roma (P. Chiusolo, G. Leone); Hemato-Oncology Transplant Unit, University “Tor Vergata,” Roma (W. Arcese, R. Cerretti); Unit of Hematology and Bone Marrow Transplantation, IRCCS, “Casa Sollievo della Sofferenza,” S. Giovanni Rotondo (A. M. Carella, N. Cascavilla); Institute of Hematology, Ospedale Nord, Taranto (P. Mazza); Division of Hematology, Ospedale “S. Giovanni Battista,” Torino (M. Falda); Division of Hematology, University of Torino, Ospedale “S. Giovanni Battista,” Torino (B. Bruno, M. Boccadoro), Division of Hematology and Bone Marrow Transplantation, University of Udine, Udine (R. Fanin, M. Cerno); and Department of Hematology, Ospedale “S. Bortolo,” Vicenza (R. Raimondi).
Footnotes
EPA and MGDP contributed equally to this manuscript.
Authorship and Disclosures
EPA, MGDP, ABa and ABo designed the research; EPA, MGDP, ABa, LM, EA, MTVL, MF, FO, MB, SG, BL, AR, RC, PM, PP, LP, RF and ABo collected data; EPA, MG DP, AB, LM and CP analyzed and interpreted data; CP and RO performed the statistical analyses; EPA, MGDP, ABa, LM and AA wrote the manuscript.
The authors indicated no potential conflicts of interest.
Funding: this study was supported by grants from Fondazione IRCCS Policlinico S. Matteo, Pavia, Italy (EPA), from Piano Regionale Sangue 2006, Regione Lombardia, Milan, Italy (EPA), and from Associazione Italiana per la Ricerca sul Cancro (AIRC), Milan, Italy (MGDP fellowship).
References
- 1.Cazzola M, Malcovati L. Myelodysplastic syndromes – coping with ineffective hematopoiesis. N Engl J Med. 2005;352(6):536–8. doi: 10.1056/NEJMp048266. [DOI] [PubMed] [Google Scholar]
- 2.Malcovati L, Della Porta MG, Pascutto C, Invernizzi R, Boni M, Travaglino E, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23(30):7594–603. doi: 10.1200/JCO.2005.01.7038. [DOI] [PubMed] [Google Scholar]
- 3.Della Porta MG, Malcovati L, Boveri E, Travaglino E, Pietra D, Pascutto C, et al. Clinical relevance of bone marrow fibrosis and CD34-positive cell clusters in primary myelodysplastic syndromes. J Clin Oncol. 2009;27(5):754–62. doi: 10.1200/JCO.2008.18.2246. [DOI] [PubMed] [Google Scholar]
- 4.Malcovati L, Della Porta MG, Cazzola M. Predicting survival and leukemic evolution in patients with myelodysplastic syndrome. Haematologica. 2006;91(12):1588–90. [PubMed] [Google Scholar]
- 5.Appelbaum FR, Barrall J, Storb R, Fisher LD, Schoch G, Ramberg RE, et al. Bone marrow transplantation for patients with myelodysplasia. Pretreatment variables and outcome. Ann Intern Med. 1990;112(8):590–7. doi: 10.7326/0003-4819-112-8-590. [DOI] [PubMed] [Google Scholar]
- 6.de Witte T, Hermans J, Vossen J, Bacigalupo A, Meloni G, Jacobsen N, et al. Haematopoietic stem cell transplantation for patients with myelodysplastic syndromes and secondary acute myeloid leukaemias: a report on behalf of the Chronic Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Br J Haematol. 2000;110(3):620–30. doi: 10.1046/j.1365-2141.2000.02200.x. [DOI] [PubMed] [Google Scholar]
- 7.Sierra J, Pérez WS, Rozman C, Carreras E, Klein JP, Rizzo JD, et al. Bone marrow transplantation from HLA-identical siblings as treatment for myelodysplasia. Blood. 2002;100(6):1997–2004. [PubMed] [Google Scholar]
- 8.Deeg HJ, Shulman HM, Anderson JE, Bryant EM, Gooley TA, Slattery JT, et al. Allogeneic and syngeneic marrow transplantation for myelodysplastic syndrome in patients 55 to 66 years of age. Blood. 2000;95(4):1188–94. [PubMed] [Google Scholar]
- 9.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–9. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diaconescu R, Flowers CR, Storer B, Sorror ML, Maris MB, Maloney DG, et al. Morbidity and mortality with nonmyeloablative compared with myeloablative conditioning before hematopoietic cell transplantation from HLA-matched related donors. Blood. 2004;104(5):1550–8. doi: 10.1182/blood-2004-03-0804. [DOI] [PubMed] [Google Scholar]
- 11.Martino R, Parody R, Fukuda T, Maertens J, Theunissen K, Ho A, et al. Impact of the intensity of the pretransplantation conditioning regimen in patients with prior invasive aspergillosis undergoing allogeneic hematopoietic stem cell transplantation: a retrospective survey of the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2006;108(9):2928–36. doi: 10.1182/blood-2006-03-008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alessandrino EP, Amadori S, Barosi G, Cazzola M, Grossi A, Liberato LN, et al. Evidence- and consensus-based practice guidelines for the therapy of primary myelodysplastic syndromes. A statement from the Italian Society of Hematology. Haematologica. 2002;87(12):1286–306. [PubMed] [Google Scholar]
- 13.Bowen D, Culligan D, Jowitt S, Kelsey S, Mufti G, Oscier D, et al. Guidelines for the diagnosis and therapy of adult myelodysplastic syndromes. Br J Haematol. 2003;120(2):187–200. doi: 10.1046/j.1365-2141.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- 14.Malcovati L, Germing U, Kuendgen A, Della Porta MG, Pascutto C, Invernizzi R, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25(23):3503–10. doi: 10.1200/JCO.2006.08.5696. [DOI] [PubMed] [Google Scholar]
- 15.Alessandrino EP, Della Porta MG, Bacigalupo A, Van Lint MT, Falda M, Onida F, et al. WHO classification and WPSS predict posttransplantation outcome in patients with myelodysplastic syndrome: a study from the Gruppo Italiano Trapianto di Midollo Osseo (GITMO) Blood. 2008;112(3):895–902. doi: 10.1182/blood-2008-03-143735. [DOI] [PubMed] [Google Scholar]
- 16.Lucarelli G, Galimberti M, Polchi P, Angelucci E, Baronciani D, Giardini C, et al. Bone marrow transplantation in patients with thalassemia. N Engl J Med. 1990;322 (7):417–21. doi: 10.1056/NEJM199002153220701. [DOI] [PubMed] [Google Scholar]
- 17.Lucarelli G, Clift RA, Galimberti M, Angelucci E, Giardini C, Baronciani D, et al. Bone marrow transplantation in adult thalassemic patients. Blood. 1999;93(4):1164–7. [PubMed] [Google Scholar]
- 18.Di Tucci AA, Matta G, Deplano S, Gabbas A, Depau C, Derudas D, et al. Myocardial iron overload assessment by T2* magnetic resonance imaging in adult transfusion dependent patients with acquired anemias. Haematologica. 2008;93(9):1385–8. doi: 10.3324/haematol.12759. [DOI] [PubMed] [Google Scholar]
- 19.Armand P, Kim HT, Cutler CS, Ho VT, Koreth J, Alyea EP, et al. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood. 2007;109(10):4586–8. doi: 10.1182/blood-2006-10-054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100(7):2292–302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 21.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–88. [PubMed] [Google Scholar]
- 22.Cheson BD, Bennett JM, Kantarjian H, Pinto A, Schiffer CA, Nimer SD, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000;96(12):3671–4. [PubMed] [Google Scholar]
- 23.Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res. 2007;13(2 Pt 1):559–65. doi: 10.1158/1078-0432.CCR-06-1210. [DOI] [PubMed] [Google Scholar]
- 24.Platzbecker U, Bornhäuser M, Germing U, Stumpf J, Scott BL, Kröger N, et al. Red blood cell transfusion dependence and outcome after allogeneic peripheral blood stem cell transplantation in patients with de novo myelodysplastic syndrome (MDS) Biol Blood Marrow Transplant. 2008;14(11):1217–25. doi: 10.1016/j.bbmt.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cazzola M, Del la Porta MG, Malcovati L. Clinical relevance of anemia and transfusion iron overload in myelodysplastic syndromes. Hematology Am Soc Hematol Educ Program. 2008:166–75. doi: 10.1182/asheducation-2008.1.166. [DOI] [PubMed] [Google Scholar]
- 26.Schafer AI, Cheron RG, Dluhy R, Cooper B, Gleason RE, Soeldner JS, et al. Clinical consequences of acquired transfusional iron overload in adults. N Engl J Med. 1981;304(6):319–24. doi: 10.1056/NEJM198102053040603. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs A, Worwood M. Ferritin in serum. Clinical and biochemical implications. N Engl J Med. 1975;292(18):951–6. doi: 10.1056/NEJM197505012921805. [DOI] [PubMed] [Google Scholar]
- 28.Porter JB. Practical management of iron overload. Br J Haematol. 2001;115(2):239–52. doi: 10.1046/j.1365-2141.2001.03195.x. [DOI] [PubMed] [Google Scholar]
- 29.Cutler CS, Lee SJ, Greenberg P, Deeg HJ, Pérez WS, Anasetti C, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104(2):579–85. doi: 10.1182/blood-2004-01-0338. [DOI] [PubMed] [Google Scholar]