Abstract
Hepcidin, a circulating regulatory hormone peptide produced by hepatocytes, functions as the master regulator of cellular iron export by controlling the amount of ferroportin, an iron exporter present on the basolateral surface of intestinal enterocytes and macrophages. Hepcidin binding to ferroportin induces its internalization and degradation, resulting in cellular iron retention and decreased iron export. Whether hepatocytes express ferroportin that could be targeted by hepcidin has remained a subject of debate. Here, we describe a hepatocyte culture system expressing high levels of ferroportin, and demonstrate that both endogenously secreted and synthetic hepcidin are fully active in down-regulating membrane-associated ferroportin. In agreement with this result, ferroportin is stabilized in liver hepatocytes of hepcidin-deficient mice and accumulates in periportal areas, supporting the centrolobular iron deposition observed in these mice. In conclusion, we show that hepcidin can trigger ferroportin degradation in hepatocytes, which must be taken into account when considering hepcidin therapeutics.
Keywords: iron, hormone, hepcidin, hepatocytes, ferroportin
Introduction
Ferroportin is the only known mammalian iron exporter. High levels of ferroportin are detected in mature enterocytes and tissue macrophages of the spleen and the liver,1–3 consistent with the role that ferroportin plays in transporting iron from the duodenum to the serum, and in iron recycling from senescent red cells back into the circulation, respectively. Quantitatively, the major pathway of iron efflux in the body is the release of iron from phagocytosed erythrocytes by the reticuloendothelial system. The magnitude of the iron flux is proportional to the concentration of ferroportin on the cell surface of these cells, which is partly determined by the circulating levels of ferroportin’s ligand, hepcidin. Hepcidin, the iron-regulatory hormone secreted by the hepatocyte, is indeed able to bind to ferroportin inducing its internalization, ubiquitination and degradation, as shown in transfected cell lines.4, 5 While the effect of hepcidin on endogenous ferroportin was clearly demonstrated in macrophagic cell types,6–10 exposure of intestinal epithelial cells to hepcidin was reported to have no effect on ferroportin levels9 and hepcidin-induced duodenal ferroportin regulation was only shown indirectly in mouse models of iron disorders.11–16
Apart from enterocytes and macrophages, not much is known concerning the presence of the iron exporter ferroportin in hepatocytes. Ferroportin-deficient animals accumulated iron in enterocytes, macrophages and to a lesser extent, hepatocytes, consistent with a possible role for ferroportin in these latter cells.17 However, due to the lack of a hepatocytic cellular system expressing high levels of endogenous ferroportin, the systemic regulation of hepatocytic ferroportin by hepcidin has never been adequately addressed. The aim of this study was to set up an experimental model to address this important issue.
Design and Methods
Primary culture of mouse hepatocytes
Hepatocytes were isolated as described,18 either from 3–4 month old female C57Bl/6 mice, or age-matched WT and hepcidin KO mice backcrossed 6 times on C57Bl/6 background. Hepatocytes were cultured in M199+glutamax medium containing 10% fetal bovine serum, 100 nM insulin, 1 mM dexamethasone and 0.2 mM triiodothyronine for 4h. After cell attachment, the medium was replaced by fresh M199 and hepatocytes were treated as indicated. Hepcidin treatment was performed for 24 h in the presence of 350 nM human synthetic hepcidin from Peptide International. As a positive control for ferroportin expression, the mouse monocyte-macrophage cell line J744 was used as previously described.19
RNA analysis
RNA isolation and real-time quantification were as described.18 Sequences of the primers were: hepc1, forward 5′-CCTATCTCCATCAACAGATG-3′ and reverse 5′-TGCAACAGATACCACACTG-3′; ferroportin forward 5′-CTCTGTCAGCCTGCTGTTTG-3′ and reverse 5′-TCAGGATTTGGGGCCAAGATG-3′; ceruloplasmin forward 5′-ACTTATTTCAGTTGACACGG-3′ and reverse 5′-GCAGCACATACACATACTGT-3′; L ferritin forward 5′-GGGCCTCCTACACCTACCTC-3′ and reverse 5′-CTCCTGGGTTTTACCCCATT-3′. GAPDH forward 5′-GTCGGTGTGAACGGATTTGG-3′ and reverse 5′-GACTCCACGACATACTCAGC-3′.
Protein analysis
Microsomal and cytosolic fractions were prepared as previously reported.12 Anti-ferroportin antibodies were previously described.12 Anti-α-tubulin and anti-L-ferritin antibodies were purchased from Sigma and Santa Cruz, respectively.
Immunohistochemistry
Tissues from 3–9 month old mice were fixed in 4% formaldehyde. After methanol deparrafination, sections were processed for immunohistochemistry using the EnVision+ System-HRP (DAB) kit according to the manufacturer’s methods. Antibody against mouse ferroportin (Alpha Diagnostics) was diluted at 1:100.
Results and Discussion
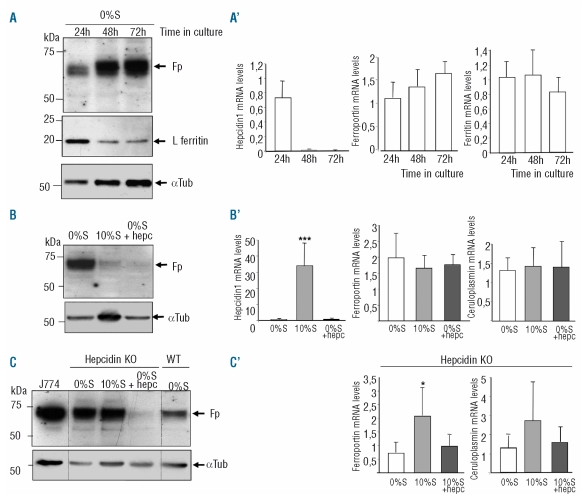
We found that culturing primary mouse hepatocytes without serum for up to 72 h was associated with a dramatic increase in ferroportin and a concomitant decrease of ferritin protein levels (Figure 1A), suggesting a functional increased iron export capacity of the hepatocyte by ferroportin. Hepcidin gene transcription was inversely correlated to ferroportin levels, being undetectable at 72h (Figure 1A′). In contrast, ferritin and ferroportin mRNA levels were unchanged throughout the culture. We took advantage of this culture condition (no hepcidin, high ferroportin) to investigate the role of hepcidin (induced endogenously or added exogenously) on the regulation of hepatocyte ferroportin. Hepatocytes, which had been serum-starved for 48h, were cultured in the presence of 10% serum for 24h, a culture condition associated with a 50-fold increase in hepcidin mRNA levels (Figure 1B′). In the presence of serum, membrane-associated ferroportin protein levels were significantly decreased compared to levels without serum (Figure 1B). This decrease was not transcriptionally related since ferroportin mRNA levels were unchanged (Figure 1B′). To determine whether endogenously synthesized hepcidin was responsible for the down regulation of ferroportin at a post-translational level, the effect of serum on hepatocytes isolated from hepcidin KO animals was assessed. Western blot of Figure 1C indicates clearly that serum treatment had no effect on ferroportin levels (i.e. no degradation) in hepcidin KO hepatocytes likely due to the absence of secreted hepcidin from these hepcidin-deficient hepatocytes. Since the absence of ceruloplasmin was recently shown to be a possible cause for the loss of cell surface ferroportin in various cultured cell lines,20 we measured ceruloplasmin mRNA levels in the hepatocytes and found no change in any culture condition (Figures 1B′, C′). To further investigate the putative role of autocrine hepcidin on ferroportin degradation, the effect of synthetic human hepcidin was tested. Similar to the serum effect, hepcidin treatment led to a complete degradation of ferroportin, both in WT and KO hepatocytes (Figures 1B and C). Importantly, this effect was observed with hepcidin concentration as low as 10 nM (data not shown).
Figure 1.
Effect of serum and hepcidin treatments on membrane-associated ferroportin and iron-related gene expression in isolated hepatocytes. Hepatocytes were isolated from 3–4 month old female C57Bl/6 mice (A, B), or 2 month old WT and hepcidin KO mice from the same littermate (C) as reported.18 After plating in presence of 10% fetal bovine serum for 4h, hepatocytes were cultured without serum for different time periods (from 24, 48 or 72h in A) or treated, as indicated, either with serum (B, C) or with synthetic hepcidin (B, C). For the Western blots, membrane proteins (50 μg/lane) were separated on SDS-PAGE, electro-transferred onto nitrocellulose membrane and analyzed with anti-ferroportin antibody as described.7 Molecular weight markers are indicated in kDa. Anti-α-tubulin was used as loading controls in blots B, C, and D. A typical experiment, representative of at least three independent experiments, is shown. For gene expression (A′, B′, and C′), relative changes in mRNA levels were quantified by real-time qRT-PCR performed using cDNA synthetized from 2 μg total RNA (GAPDH normalized, arbitrary units). Mean ± SD for n=3 independent samples in one experiment. The experiment was performed at least twice and a representative result is shown. Statistical analysis was performed using Student’s t test (unpaired, two-tailed). (A) Membrane-associated ferroportin levels were studied in hepatocytes cultured 24, 48 or 72h in serum-free medium. Anti-L-ferritin antibodies were used for assessing the hepatocytic iron content in cytosolic protein extracts (25 μg/lane). (A′) Relative changes in hepcidin, ferroportin and ferritin mRNA levels. Results are expressed relative to hepatocytes cultured without serum for 24h. (B) Membrane-associated ferroportin levels were studied in WT hepatocytes cultured 72h in serum-free medium (0%S), 48h in serum-free medium + 24h in the presence of 10% serum (10%S) or 48h in serum free medium + 24h in presence of 350 nM human synthetic hepcidin (0%S+hepc). (B′) Relative changes in hepcidin, ferroportin, and ceruloplasmin mRNA levels. Results are expressed relative to hepatocytes cultured without serum for 72h. ***P<0.0001 as compared to untreated hepatocytes. (C) Membrane-associated ferroportin levels were studied in hepcidin KO hepatocytes cultured 72h in serum-free medium (0%S), 48h in serum-free medium + 24h in the presence of 10% serum (10%S) or 48h in serum free medium + 24h in presence of 350 nM human synthetic hepcidin from Peptide International (0%S+hepc). Membrane proteins (50 μg) from iron-treated monocyte-macrophage cell line J774 were used as a positive control. (C′) Relative changes in ferroportin, and ceruloplasmin mRNA levels. Hepcidin mRNAs were undetectable (not shown). Results are expressed relative to hepatocytes cultured without serum for 72h. *P=0.02 as compared to untreated hepatocytes.
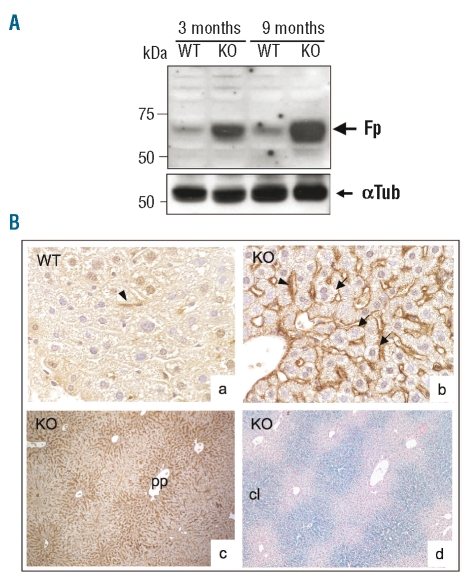
Confirming our hypothesis of hepcidin targeting ferroportin degradation in hepatocytes, we found that hepatocytes isolated from hepcidin KO mice displayed increased levels of ferroportin as compared to WT mice (Figure 1C, last lane). This increase was found not only in isolated hepatocytes but also in vivo as shown in total liver membrane extracts of hepcidin KO mice as compared to WT mice (Figure 2A). The cell type responsible for ferroportin expression in vivo was assessed by immunohistochemistry on liver sections (Figure 2B). In the liver of KO mice, the immunostaining conditions allowed detection of ferroportin not only in the Kupffer cells, as expected due to hepcidin deficiency, but also clearly at the sinusoidal borders of the hepatocytes (Figure 2Bb). Interestingly, ferroportin plasma membrane staining was found much more prominent in the periportal areas (Figure 2Bc). This result is in agreement with a previous study which reported, by in situ hybridization, that ferroportin was expressed predominantly in the hepatocytes around the portal veins in the rat liver.21 Collectively, our results permit us to propose a mechanism explaining the unique feature of the centrolobular iron deposit present in the hepcidin KO mice.22 In the setting of experimental iron overload, increased periportal iron load induces hepcidin secretion, which in turn leads to ferroportin degradation around the periportal veins, leading to a specific periportal iron accumulation. In hepcidin KO mice, hepcidin deficiency induces periportal ferroportin stabilization, and thus iron is exported along the peri-central axis and progressively accumulated in the centrolobular region where it is trapped due to the absence of ferroportin in this area. Figure 2Bd shows the liver Perls staining, a serial section of which was stained for ferroportin (Figure 2Bc). Interestingly, a similar zonal distribution of iron in centrolobular areas was recently reported in the liver of BMP6 KO mice.23 In classical forms of hereditary hemochromatosis related to inappropriate hepcidin levels, it could be anticipated that hepcidin levels are sufficient to degrade periportal ferroportin leading to periportal iron accumulation as reported in HFE and TfR2 mouse models.24,25 In the other form of juvenile hemochromatosis due to HJV mutation, a periportal accumulation of iron was also reported both in KO models11 and in human patients.26 In agreement with these findings, liver ferroportin was detected only in the Kupffer cells and not in the hepatocytes.11 The absence of ferroportin accumulation in the hepatocytes of HJV KO mice is difficult to explain because hepcidin levels were demonstrated to be barely detectable in these mice.11 Further investigations are needed to determine the threshold of hepcidin levels required for ferroportin degradation and whether other iron-related partners might be involved in hepatic iron zonation as recently proposed.27
Figure 2.
Ferroportin expression in WT and hepcidin KO mice. (A) Detection of membrane-associated ferroportin in total liver extracts prepared from WT and KO mice of 3 and 9 months of age. Membrane proteins (60 μg/lane) were separated by SDS-PAGE, electro-transferred onto nitrocellulose membrane and analyzed with anti-ferroportin antibody as described(7). Anti-α-tubulin was used as loading control. A typical experiment, representative of at least two independent experiments, is shown. (B) Immunohistochemical staining of livers from WT (a) and hepcidin KO (b–c) mice using anti-mouse ferroportin antibodies (Alpha Diagnostics). A neighboring serial section of the KO liver used in (c) was used for Perls staining and is shown in (d). Original magnification: 20× (c, d) and 40× (a, b). Kupffer cell staining (black arrowheads) and hepatocytes (black arrows) are indicated. PP is for periportal and CL for centrolobular.
In conclusion, our results clearly identify the hepatocyte as a cellular target for hepcidin. This result may provide clues for hepcidin therapeutics. Indeed, while the physiological role of hepatocytes in iron export is marginal as compared to that of the reticuloendothelial macrophages, this observation may be important in pathological conditions associated with iron overload disease due to hepcidin deficiency.
Acknowledgments
The authors would like to thank all the hepcidin team at the Cochin Institute for fruitful discussions and critical reading of the manuscript.
Footnotes
Authorship and Disclosures
GR and JCD designed and performed research, analyzed data and contributed to the writing of the paper; BD performed research; FC-H contributed reagent, analyzed data and contributed to the writing of the paper; GN analyzed data and contributed to the writing of the paper; SV designed research, analyzed data and wrote the paper.
The authors declare to have no conflicts of interest.
Funding: this work was supported in part by grants from the Agence Nationale de la Recherche, the European Comission (LSHM-CT-2006-037296), and the Association de la Recherche contre le Cancer.
References
- 1.Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000;275(26):19906–12. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- 2.McKie AT, Marciani P, Rolfs A, Brennan K, Wehr K, Barrow D, et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5(2):299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- 3.Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403(6771):776–81. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- 4.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–3. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 5.De Domenico I, Ward DM, Langelier C, Vaughn MB, Nemeth E, Sundquist WI, et al. The Molecular Mechanism of Hepcidin-mediated Ferroportin Down-Regulation. Mol Biol Cell. 2007;18(7):2569–78. doi: 10.1091/mbc.E07-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci USA. 2005;102(5):1324–8. doi: 10.1073/pnas.0409409102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delaby C, Pilard N, Goncalves AS, Beaumont C, Canonne-Hergaux F. The presence of the iron exporter ferroportin at the plasma membrane of macrophages is enhanced by iron loading and downregulated by hepcidin. Blood. 2005;106(12):3979–84. doi: 10.1182/blood-2005-06-2398. [DOI] [PubMed] [Google Scholar]
- 8.Andriopoulos B, Pantopoulos K. Hepcidin generated by hepatoma cells inhibits iron export from co-cultured THP1 monocytes. J Hepatol. 2006;44(6):1125–31. doi: 10.1016/j.jhep.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 9.Chaston T, Chung B, Mascarenhas M, Marks J, Patel B, Srai SK, et al. Evidence for differential effects of hepcidin in macrophages and intestinal epithelial cells. Gut. 2008;57(3):374–82. doi: 10.1136/gut.2007.131722. [DOI] [PubMed] [Google Scholar]
- 10.Theurl I, Theurl M, Seifert M, Mair S, Nairz M, Rumpold H, et al. Autocrine formation of hepcidin induces iron retention in human monocytes. Blood. 2008;111(4):2392–9. doi: 10.1182/blood-2007-05-090019. [DOI] [PubMed] [Google Scholar]
- 11.Huang FW, Pinkus JL, Pinkus GS, Fleming MD, Andrews NC. A mouse model of juvenile hemochoromatosis. J Clin Invest. 2005;115(8):2187–91. doi: 10.1172/JCI25049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viatte L, Lesbordes-Brion JC, Lou DQ, Bennoun M, Nicolas G, Kahn A, et al. Deregulation of proteins involved in iron metabolism in hepcidin-deficient mice. Blood. 2005;105(12):4861–4. doi: 10.1182/blood-2004-12-4608. [DOI] [PubMed] [Google Scholar]
- 13.Nishina S, Hino K, Korenaga M, Vecchi C, Pietrangelo A, Mizukami Y, et al. Hepatitis C virus-induced reactive oxygen species raise hepatic iron level in mice by reducing hepcidin transcription. Gastroenterology. 2008;134(1):226–38. doi: 10.1053/j.gastro.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Wang RH, Li C, Xu X, Zheng Y, Xiao C, Zerfas P, et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2(6):399–409. doi: 10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Harrison-Findik DD, Schafer D, Klein E, Timchenko NA, Kulaksiz H, Clemens D, et al. Alcohol metabolism-mediated oxidative stress down-regulates hepcidin transcription and leads to increased duodenal iron transporter expression. J Biol Chem. 2006;281(32):22974–82. doi: 10.1074/jbc.M602098200. [DOI] [PubMed] [Google Scholar]
- 16.Folgueras AR, de Lara FM, Pendas AM, Garabaya C, Rodriguez F, Astudillo A, et al. Membrane-bound serine protease matriptase-2 (Tmprss6) is an essential regulator of iron homeostasis. Blood. 2008;112(6):2539–45. doi: 10.1182/blood-2008-04-149773. [DOI] [PubMed] [Google Scholar]
- 17.Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, et al. The iron exporter ferroportin (Slc40a1) is essential for iron homeostasis. Cell Metab. 2005;1(3):191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Ramey G, Deschemin JC, Vaulont S. Crosstalk between the mitogen activated protein kinase and bone morphogenetic protein/hemojuvelin pathways is required for the induction of hepcidin by holotransferrin in primary mouse hepatocytes. Haematologica. 2009;94(6):765–72. doi: 10.3324/haematol.2008.003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gagliardo B, Kubat N, Faye A, Jaouen M, Durel B, Deschemin JC, et al. Pro-hepcidin is unable to degrade the iron exporter ferroportin unless maturated by a furin-dependent process. J Hepatol. 2009;50(2):394–401. doi: 10.1016/j.jhep.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 20.De Domenico I, Ward DM, di Patti MC, Jeong SY, David S, Musci G, et al. Ferroxidase activity is required for the stability of cell surface ferroportin in cells expressing GPI-ceruloplasmin. Embo J. 2007;26(12):2823–31. doi: 10.1038/sj.emboj.7601735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang AS, Xiong S, Tsukamoto H, Enns CA. Localization of iron metabolism-related mRNAs in rat liver indicate that HFE is expressed predominantly in hepatocytes. Blood. 2004;103(4):1509–14. doi: 10.1182/blood-2003-07-2378. [DOI] [PubMed] [Google Scholar]
- 22.Lesbordes-Brion JC, Viatte L, Bennoun M, Lou DQ, Ramey G, Houbron C, et al. Targeted disruption of the hepcidin 1 gene results in severe hemochromatosis. Blood. 2006;108(4):1402–5. doi: 10.1182/blood-2006-02-003376. [DOI] [PubMed] [Google Scholar]
- 23.Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41(4):478–81. doi: 10.1038/ng.320. [DOI] [PubMed] [Google Scholar]
- 24.Fleming RE, Holden CC, Tomatsu S, Waheed A, Brunt EM, Britton RS, et al. Mouse strain differences determine severity of iron accumulation in Hfe knockout model of hereditary hemochromatosis. Proc Natl Acad Sci USA. 2001;98(5):2707–11. doi: 10.1073/pnas.051630898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace DF, Summerville L, Subramaniam VN. Targeted disruption of the hepatic transferrin receptor 2 gene in mice leads to iron overload. Gastroenterology. 2007;132(1):301–10. doi: 10.1053/j.gastro.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 26.Huang FW, Rubio-Aliaga I, Kushner JP, Andrews NC, Fleming MD. Identification of a novel mutation (C321X) in HJV. Blood. 2004;104(7):2176–7. doi: 10.1182/blood-2004-01-0400. [DOI] [PubMed] [Google Scholar]
- 27.Troadec MB, Fautrel A, Drenou B, Leroyer P, Camberlein E, Turlin B, et al. Transcripts of ceruloplasmin but not hepcidin, both major iron metabolism genes, exhibit a decreasing pattern along the portocentral axis of mouse liver. Biochim Biophys Acta. 2008;1782(4):239–49. doi: 10.1016/j.bbadis.2007.12.009. [DOI] [PubMed] [Google Scholar]