Abstract
A series of ageladine A analogs that include 2-aminoimidazo[4,5-c]azepines (seven-membered rings) and 2-amino-3H-imidazo[4,5-c]pyridine (six-membered rings) derivatives were synthesized and evaluated for their anticancer effects against several human cancer cell lines and MMP-2 inhibition in vitro. Only compounds possessing the aromatic azepine (seven-membered ring) core showed anticancer activity with IC50 values in the low micromolar range.
Ageladine A (1a) is a unique imidazole-pyrrole-based marine natural product which was isolated in 2003 from the sponge Agelas nakamurai (Fig. 1).1 Bioactivity investigations determined that 1a is an inhibitor of matrix metalloproteinases (MMPs). Its use as a potential pH sensitive membrane permeable dye has also been described.2 Several syntheses of ageladine A and related analogs have been published in recent years.3-8 In an effort to discover new anticancer lead structures for drug development, a collection of agelidine A-related compounds was synthesized and tested against different human cancer cell lines. Herein, we report the results of this study and a novel class of anticancer fused seven-membered ring 2-aminoimidazole azepine heterocycles 2.
Figure 1.
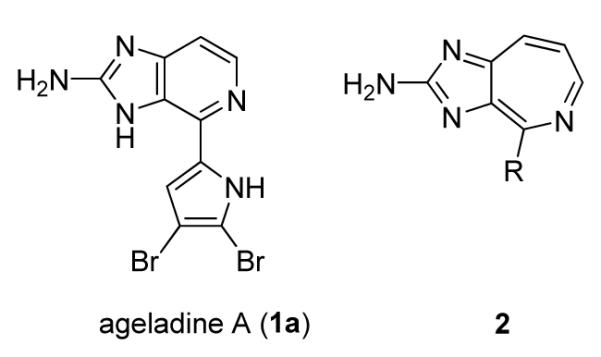
Structure of ageladine A and compound 2.
In 1994, we disclosed that 2-aminoimidazoles such as 3 undergo a facile Pictet-Spengler reaction with aldehydes to yield 2-aminoimidazoazepines 4 in excellent yields (Scheme 1).9 This key reaction enables the construction of the bicyclic core of ageladine A and was independently adapted by Karuso in 2004 for the synthesis of the natural product.8 Due to the important biological activities reported for ageladine A, we recently decided to pursue this reaction in greater depth and extend the methodology for the preparation and biological evaluation of ageladine A analogs.
Scheme 1.
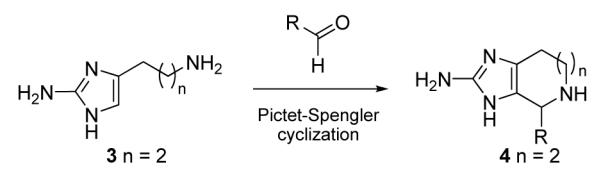
Pictet-Spengler cyclization of 2-aminoimidazoles
Of the 12 initial analogs prepared, eight of them are 3H-imidazo[4,5-c]pyridin-2-amine (six-membered ring) analogs that include the natural product, ageladine A (1a) and four are imidazo[4,5-c]azepin-2-amine (seven-membered ring) derivatives comprising 2 (Fig. 2).
Figure 2.
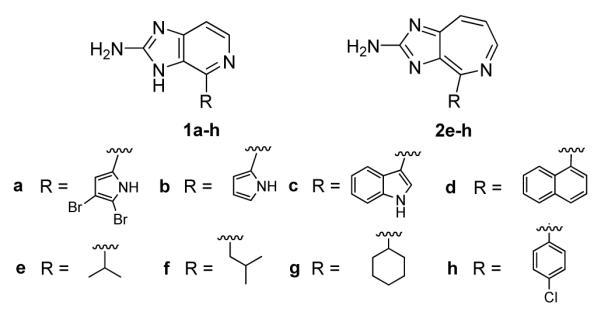
Ageladine A and analogs.
These analogs were prepared using Pictet-Spengler methodology followed by oxidation to the requisite aromatic system.
Starting from readily available 2-amino-4-(3-aminopropyl)-1H-imidazole (3) and 2-aminohistamine (5),10,11 the corresponding Pictet-Spengler cyclization products 4 and 6 were produced in good yields from reaction with aldehyde substrates 7 (Scheme 2).
Scheme 2.
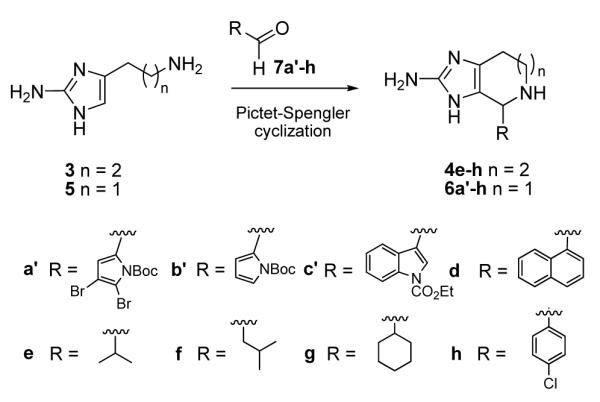
Reagents and conditions: 3·2HCl, 7e-h, H2O/EtOH, Na2CO3, 1 d, 23 °C: products 4e (86%), 4f (70%), 4g (85%), and 4h (60%). 5, 7a′ or 7b′, Sc(OTf)3, EtOH, 23 °C, 1 d: products 6a′ (21%) or 6b′ (22%); 5, 7c′-h, EtOH, 23 °C, 1 d: products 6c′ (32%), 6d (40%), 6e (63%), 6f (51%), 6g (36%), 6h (38%).
With tetrahydro ring products 4 and 6 in hand, oxidation to the corresponding aromatic system was investigated. This was not as straightforward as anticipated. While Karuso describes the use of chloranil for the synthesis of ageladine A, the use of this oxidant could not be generally applied to the azepine series. For instance, attempts to use chloranil in oxidizing pyrrole-azepines 4, no aromatic products corresponding to 2 could be isolated. After experimenting with several different reagents and reaction conditions, moderate success was achieved from a two-step procedure utilizing elemental bromine in methanesulfonic acid followed by treatment with potassium tert-butoxide in air (Scheme 3).
Scheme 3.
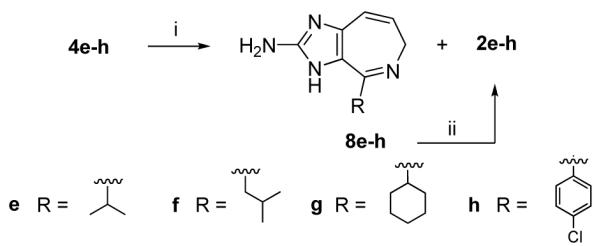
Reagents and conditions: (i) Br2 (2 equiv), MeSO3H, 110°C, 16 h: products 8e (25%) and 2e (11%), 8f (30%) and 2f (8%), 8g (33%) and 2g (6%), 8h (41%) and 2h (5%); (ii) t-BuOK (1 equiv), THF, 16 h: products 2e (26%), 2f (47%), 2g (37%), and 2h (25%).
Initial oxidation with bromine at elevated temperatures (110 °C, sealed tube) produced a mixture of products which primarily consisted of dehydro intermediate 8. This intermediate can be isolated and characterized. Only small amounts of the desired aromatic system 2 were obtained under these conditions. Upon exposure of 8 to potassium tert-butoxide in air, modest but sufficient amounts of 2 were produced and used for bioactivity studies.
The preparation of ageladine A (1a) and analogs 1b-d was achieved by treatment of precursor 6 with chloranil which afforded the desired aromatic derivatives after deprotection with either trifluoroacetic acid or potassium carbonate. (Scheme 4)
Scheme 4.
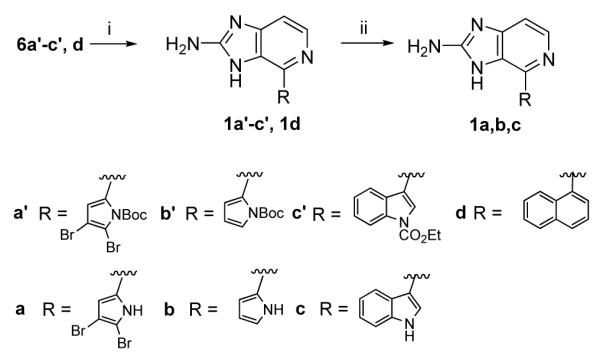
(i) chloranil (3 equiv), CHCl3, 80 °C, 16 h, 1′ (50%), 1b′ (10%), 1c′ (15%), 1d (13%); (ii) TFA/CH2Cl2, 1a (95%), 1b (95%) or K2CO3, MeOH, 1c (80%).
Finally, oxidation of 6e-f was best achieved using bromine in methanesulfonic acid to afford the desired ageladine A analogs 1e-f (Scheme 5).
Scheme 5.
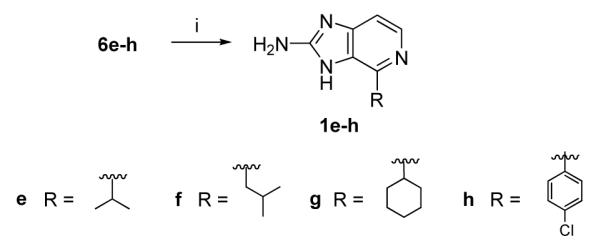
Reagents and conditions: (i) Br2 (2 equiv), MeSO3H, 110 °C, 16 h, 1e (32%), 1f (30%), 1g (31%), 1h (30%).
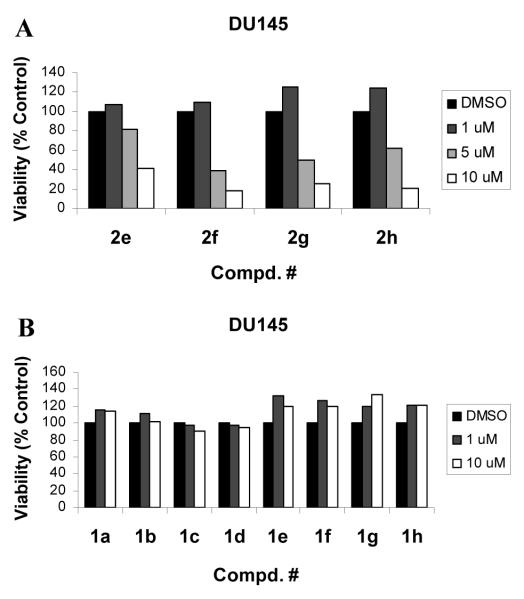
Next, biological assays were performed to determine the effects of these analogs on cancer cell viability and MMP-2 activity. All derivatives were screened for anticancer activities against human DU145 prostate cancer cells (Fig. 3). Aromatic azepines 2e-h dispalyed anticancer activities in a dose-dependent manner. Moreover, the aromatic 2-aminoimidazo[4,5-c]azepine core plays an important role in the anticancer activity since neither the non-aromatic seven-membered azepine derivatives (data not shown) nor the six-membered pyridine (ageladine A) series showed activities.
Figure 3.
Seven-membered ring aromatic analogs inhibit viabilities of DU145 cells (A) but not six-membered analogs (B). Cells were treated with compounds at various concentrations for 48 h. Cell viability was determined by an MTS assay using manufacturer’s (Promega, Madiason, WI) protocol. Values are mean values of three determinations with <10% deviation from the mean value.
Analogs of 1 and 2 were further tested with A2058 melanoma and MDA-MB-435 breast cancer cell lines. Similarly, the seven-membered ring aromatic compounds inhibited cell viabilities as shown in Table 1.
Table 1.
Inhibition of cell viability by compounds 2e–h. a
| Compds | IC50 (μM) DU145 |
IC50 (μM) A2058 |
IC50 (μM) MDA-MB-435 |
|---|---|---|---|
| 2e | 7.5 | 9.5 | 6.7 |
| 2f | 4.3 | 4.1 | 2.9 |
| 2g | 5 | 4.5 | 3.4 |
| 2h | 6.2 | 5.1 | 3.6 |
IC50 values were determined with an MTS assay after 48 h treatment. Values are mean values of three determinations with <10% deviation from the mean value.
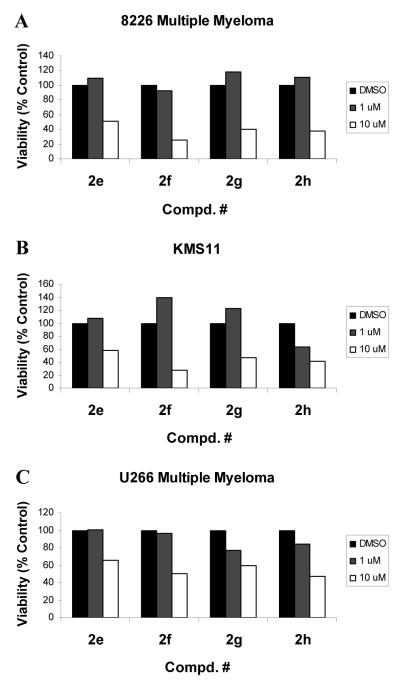
In addition, compounds 2e-h substantially reduced viabilities of human multiple myeloma cells, including 8226, KMS11 and U266 (Fig. 4).
Figure 4.
Compounds 2e-h inhibit viabilities of multiple myeloma cells. Cells were treated with compounds at 1μM or 10 μM for 48 h. Cell viability was determined by an MTS assay. Values are mean values of three determinations with <10% deviation from the mean value.
Previous studies have reported that ageladine A (1a) inhibits MMP-2 activities, which are involved in tumor angiogenesis. Consistently, we observed potent inhibition of MMP-2 activity by ageladine A (1a) (IC50 = 1.7 ± 0.2 μM) among all derivatives (1a-h and 2e-h). All other compounds showed only weak inhibition of MMP-2 activities at higher concentrations (Table 2).
Table 2.
MMP-2 inhibitory activity of compounds 1a-h and 2e-f. a
| Compds | %Inhibition @ 5 μM | %Inhibition @ 50 μM |
|---|---|---|
| 1b | N/Ab | N/Ab |
| 1c | 25 | 26 |
| 1d | 20 | N/Ab |
| 1e | 25 | 17 |
| 1f | 31 | 22 |
| 1g | 9 | 40 |
| 1h | N/Ab | N/Ab |
| 2e | 28 | 52 |
| 2f | 27 | 60 |
| 2g | 23 | 53 |
| 2h | 27 | 54 |
MMP-2 inhibition assay was performed using the manufacturer’s (Enzo Life Sciences AK-409) protocol. Values are mean values of two determinations with <10% deviation from the mean value.
Strong fluorescence of the compound interfered with the assay measurement.
In conclusion, six- and seven-membered ring ageladine A analogs have been synthesized via a two-step process that centers on a Pictet-Spengler cyclocondensation followed by oxidative aromatization. The choice of oxidant and reaction conditions is highly dependent on the desired ring system. While these two classes of heterocycles have structural similarities, the bioactivities of the two systems are dramatically different. The seven-membered analogs 2e-h represent a new pharmacophore that possesses in vitro anticancer activity. This is contrast to the six-membered pyridine series found in ageladine A. Further studies expanding the medicinal chemistry of these derivatives along with investigations directed at the biological mechanism of action are under investigation.
Acknowledgments
The National Institutes of Health R01GM071985 and Chugai Pharmaceutical Co. are gratefully acknowledged for support of this work. We thank the Irell & Manella Graduate School of Biological Sciences at City of Hope for a merit fellowship to Y.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Fujita M, Nakao Y, Matsunaga S, Seiki M, Itoh Y, Yamashita J, Van Soest RW, Fusetani N. J. Am. Chem. Soc. 2003;125:15700. doi: 10.1021/ja038025w. [DOI] [PubMed] [Google Scholar]
- 2.Bickmeyer U, Grube A, Klings KW, Kock M. Biochem. Biophy. Res. Commun. 2008;373:419. doi: 10.1016/j.bbrc.2008.06.056. [DOI] [PubMed] [Google Scholar]
- 3.Ando N, Terashima S. Bioorg. Med. Chem. Lett. 2007;17:4495. doi: 10.1016/j.bmcl.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Meketa ML, Weinreb SM. Org. Lett. 2006;8:1443. doi: 10.1021/ol0602304. [DOI] [PubMed] [Google Scholar]
- 5.Meketa ML, Weinreb SM. Org. Lett. 2007;9:853. doi: 10.1021/ol063038a. [DOI] [PubMed] [Google Scholar]
- 6.Meketa ML, Weinreb SM. Tetrahedron. 2007;63:9112. [Google Scholar]
- 7.Meketa ML, Weinreb SM, Nakao Y, Fusetani N. J. Org. Chem. 2007;72:4892. doi: 10.1021/jo0707232. [DOI] [PubMed] [Google Scholar]
- 8.Shengule SR, Karuso P. Org. Lett. 2006;8:4083. doi: 10.1021/ol061584y. [DOI] [PubMed] [Google Scholar]
- 9.Horne DA, Yakushijin K. 9,424,136. W.O. Patent. 1994 PCT Int. Appl. (1994); Chem. Abstr. 1994;122:81723. [Google Scholar]; Horne DA, Yakushijin K. 5,834,609 U.S. Patent. 1998
- 10.Lancini GC, Arioli V, Lazzari E, Bellani P. J. Med. Chem. 1969;12:775. doi: 10.1021/jm00305a012. [DOI] [PubMed] [Google Scholar]
- 11.Lancini GC, Lazzari E. J. Heterocycl. Chem. 1966;3:152. [Google Scholar]
- 12.Compound 4e·2HCl: 1H NMR (DMSO-d6, 400 MHz) δ 12 .31 (d, J = 17.0 Hz, 2H), 9.92 (br, 1H), 9.33 (br, 1H), 7.49 (s, 2H), 4.04 (d, J = 9.4 Hz, 1H), 3.38 (br, 2H), 2.67 (br, 2H), 2.26 (m, 1H), 1.92 (br, 2H), 1.02 (d, J = 6.5 Hz, 3H), 0.88 (d, J = 6.5 Hz, 3H), 13C NMR (DMSO-d6, 400 MHz) δ 146.8, 126.1, 124.6, 60.8, 44.4, 29.4, 26.3, 23.3, 19.1, 18.1.
- 13.Compound 8e: 1H NMR (CD3OD, 400 MHz) δ 6.73 (d, J = 9.4 Hz, 1H), 5.66 (m, 1H), 3.62 (d, J = 6.7 Hz, 2H), 3.21 (m, 1H), 1.12 (d, J = 6.8 Hz, 6H), 13C NMR (CD3OD, 400 MHz) δ 167.2, 155.7, 143.6, 130.3, 124.3, 119.4, 47.5, 33.3, 19.8.
- 14.Compound 2e: 1H NMR (CDCl3, 400 MHz) δ 8.79 (d, J = 6.8 Hz, 1H), 8.02 (d, J = 10.0 Hz, 1H), 7.39 (dd, J = 10.0 Hz, J = 6.8 Hz, 1H), 7.29 (s, 2H), 4.24 (m, 1H), 1.34 (d, J = 6.8 Hz, 6H), 13C NMR (CDCl3, 400 MHz) δ 174.0, 164.0, 163.8, 156.9, 149.8, 130.3, 127.3, 34.4, 22.2, HRMS(ESI) m/z calcd for C10H13N4 (M+H): 189.1140; found: 189.1141.