Abstract
Objective:
Patients with malignant glioma (MG) must make ongoing medical treatment decisions concerning a progressive disease that erodes cognition. We prospectively assessed medical decision-making capacity (MDC) in patients with MG using a standardized psychometric instrument.
Methods:
Participants were 22 healthy controls and 26 patients with histologically verified MG. Group performance was compared on the Capacity to Consent to Treatment Instrument (CCTI), a psychometric measure of MDC incorporating 4 standards (choice, understanding, reasoning, and appreciation), and on neuropsychological and demographic variables. Capacity outcomes (capable, marginally capable, or incapable) on the CCTI standards were identified for the MG group. Within the MG group, scores on demographic, clinical, and neuropsychological variables were correlated with scores on each CCTI standard, and significant bivariate correlates were subsequently entered into exploratory stepwise regression analyses to identify multivariate cognitive predictors of the CCTI standards.
Results:
Patients with MG performed significantly below controls on consent standards of understanding and reasoning, and showed a trend on appreciation. Relative to controls, more than 50% of the patients with MG demonstrated capacity compromise (marginally capable or incapable outcomes) in MDC. In the MG group, cognitive measures of verbal acquisition/recall and, to a lesser extent, semantic fluency predicted performance on the appreciation, reasoning, and understanding standards. Karnofsky score was also associated with CCTI performance.
Conclusions:
Soon after diagnosis, patients with malignant glioma (MG) have impaired capacity to make treatment decisions relative to controls. Medical decision-making capacity (MDC) impairment in MG seems to be primarily related to the effects of short-term verbal memory deficits. Ongoing assessment of MDC in patients with MG is strongly recommended.
GLOSSARY
- AED
= antiepileptic drug;
- BDI
= Beck Depression Inventory;
- CCTI
= Capacity to Consent to Treatment Instrument;
- GBM
= glioblastoma multiforme;
- HVLT
= Hopkins Verbal Learning Test;
- MDC
= medical decision-making capacity;
- MG
= malignant glioma;
- UAB
= University of Alabama at Birmingham.
Malignant gliomas (MGs), which include glioblastoma multiforme (GBM), anaplastic astrocytoma, and anaplastic oligodendrogliomas, are among the deadliest of the primary CNS cancers. Even with the most intensive treatment, the overall median survival of individuals with GBM is only approximately 14.6 months from time of diagnosis.1 Because of the effects of the brain tumor and associated treatments, patients with MG experience a variety of physical, cognitive, and psychiatric changes that cause substantial disability.2–4
Medical decision-making capacity (MDC), also referred to as consent capacity, is a higher-order functional capacity that is relevant for patients with MG and their families and for clinicians involved in their care. Because of their aggressive, often terminal illness, patients with MG and their families must make ongoing and challenging medical decisions in a disease that progressively and rapidly erodes cognition.5 Clinicians, in turn, are legally and ethically required to ensure that their patients are capable of providing valid consent before initiating treatment.6–10
Given the clinical, legal, and ethical implications of MDC in MG, there is a need for empirical studies examining MDC in patients with brain cancer. This cross-sectional study investigated MDC in a demographically matched sample of healthy controls and patients with MG using a standardized measure of consent capacity. We hypothesized that patients with MG would perform equivalently with controls on a simple consent standard (choice), but would perform below controls on more complex consent standards of understanding, reasoning, and appreciation.11 We also investigated the relationship between cognitive functioning and consent capacity in patients with MG.
METHODS
Participants.
Healthy controls and patients with recently diagnosed MG were prospectively enrolled between July 2001 and January 2005 as part of a pilot study longitudinally investigating decisional capacity in patients with MG. Funding for the project was received internally through the Department of Neurology at University of Alabama at Birmingham (UAB). The number of participants with complete baseline data determined the sample size for this study.
Twenty-six patients with histologically verified MG (22 patients with GBM World Health Organization [WHO] grade IV and 4 patients with anaplastic WHO grade III) were recruited for the study from the UAB Neuro-Oncology Clinic. All patients with MG were diagnostically characterized by a board-certified neuro-oncologist (L.B.N.). Patients were selected for the study if they met the following criteria: age 19 years or older, presence of a supratentorial lesion, Karnofsky score between 60 and 100, absence of a serious psychiatric illness, history of substance abuse, or coexisting medical illness adversely affecting cognition. Patients with mild depression were not excluded. Mean time from date of brain cancer diagnosis to study evaluation was 6.9 months (SD 7.3 months), with a median of 4 months (range 1–33 months).
The median Karnofsky performance score of patients with MG was 90 (range 80–100). Twenty-three patients with MG (88.5%) had undergone surgical resection, and 3 (11.5%) had biopsy only. Nineteen patients (73.1%) were treated with radiation and chemotherapy, 5 (19.0%) were treated with radiation only, 1 (3.8%) was treated with chemotherapy only, and 3 (11.5%) were treated with interstitial chemotherapy with carmustine-loaded polymers. Radiation therapy was delivered at a standard regimen of 60 Gy in 30 fractions over 6 weeks. There were 13 patients using both antiepileptic drugs (AEDs) and corticosteroids, 5 using AEDs only, 3 using corticosteroids only, and 5 not using AEDs or corticosteroids.
Twenty-two healthy controls aged 19 years or older also participated in the study. All controls were assessed with neurologic and neuropsychological examinations to ensure the absence of medical and psychiatric conditions that could impair cognition. Controls were individually recruited to match patients with MG on age, education level, gender, and race.
Procedures.
All participants completed the study's consent capacity measure and a comprehensive battery of neuropsychological and personality measures described below.
Standard protocol approvals, registrations, and patient consents.
Written informed consent was obtained from each participant and, in some cases, their legal representative. The UAB institutional review board approved this study.
Measures.
Consent capacity measure.
Consent capacity was assessed with vignette B of the Capacity to Consent to Treatment Instrument (CCTI).12 The CCTI is a conceptually based, reliable, and valid instrument for the assessment of medical decision-making ability in adults.8,12,13 The CCTI comprises 2 clinical vignettes (vignettes A and B). Vignette B presents a hypothetical medical problem and symptoms (cardiovascular disease), and 2 treatment alternatives with associated risks and benefits. Participants then answer standardized questions designed to test each of the following 4 core consent standards7,12,13:
S1: simply expressing a treatment choice (expressing choice);
S3: appreciating the personal consequences of a treatment choice (appreciation);
S4: providing rational reasons for a treatment choice (reasoning); and
S5: understanding the treatment situation, treatment choices, and respective risks/benefits (understanding).
A fifth experimental standard (S2) measures the ability to make a reasonable treatment choice but is not available for CCTI vignette B12.
CCTI vignette A, which presents a hypothetical situation about brain cancer symptoms and treatment, was not administered to participants in this study because of concerns about the participants with MG possibly confusing and confounding personal medical treatment factors and issues with those presented in vignette A.
CCTI administration procedures.
Vignette B of the CCTI was presented in both oral and written formats to all participants. After presentation of the vignette, the written information was removed, and participants responded to questions tapping the 4 core CCTI standards. CCTI administration and scoring were performed by trained research assistants according to detailed and well-operationalized criteria.12 Each participant's responses to the CCTI questions were audio-taped and subsequently transcribed to ensure a high level of scoring accuracy. Research assistants were blinded to participants' group status, and the study investigators were not involved in either administration or scoring of the CCTI.
Neuropsychological test battery.
A standardized neuropsychological test battery relevant to cognitive impairment in MG and associated with consent capacity8,14 was administered to all participants (table 3 and appendix e-1 on the Neurology® Web site at www.neurology.org). Depression symptoms were assessed with the Beck Depression Inventory (BDI).15
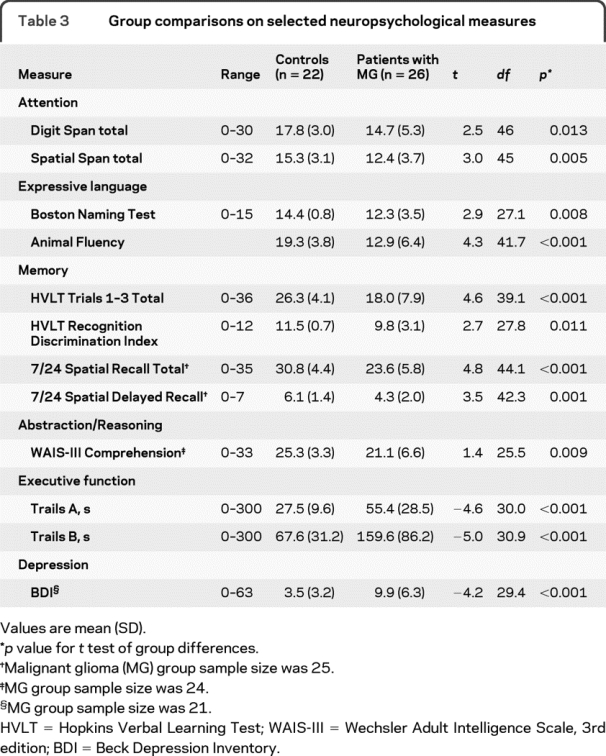
Table 3 Group comparisons on selected neuropsychological measures
Data analyses.
Demographic variables were analyzed using independent sample t tests (age, education) and χ2 tests (gender). Group comparisons on the neuropsychological tests were conducted using independent sample t tests. Group comparisons on the consent standards were performed using independent sample t tests (S3, S4, and S5) or χ2 (S1) tests.
To identify CCTI outcome status (capable, marginally capable, or incapable), we used psychometric cutoff scores derived from control performance. For S3, S4, and S5, a capable outcome was defined as a score >1.5 SD below the control group mean on that standard, a marginally capable outcome was defined as a score ≤1.5 SD but >2.5 SD below the control group mean, and an incapable outcome was defined as a score ≤2.5 SD below the control group mean. For S1, a capable outcome was defined as a score of 2, marginally capable was defined as a score of 1, and incapable was defined as a score of 0. Assignment of psychometrically derived capacity outcomes is useful in categorizing level of decisional impairment and has been used successfully in prior capacity studies.8,11,12 Although these outcomes have scientific and clinical value, they are not representative of participants' legal competency status.
In the MG group, bivariate and multivariate analyses were conducted to identify key neurocognitive predictors of CCTI performance. As a data reduction step, bivariate correlations were computed to identify candidate neuropsychological predictors of CCTI performance using the Pearson test for S3–S5 and Spearman rho test for S1. The top 3 neuropsychological correlates reaching the 0.05 significance level were entered into separate multivariate stepwise linear regression models for each standard. Neuropsychological variables were retained in each model if the change in R2 achieved by their entry was significant at the 0.05 significance level.
Exploratory correlational analyses were also conducted examining the relationship between performance on the CCTI standards and demographic factors (age, education, and gender) and clinical variables (Karnofsky score, time since diagnosis, and BDI scores).
All statistical analyses were performed using SPSS 13.0 (SPSS Inc., Chicago, IL). The significance level for all analyses was p < 0.05.
RESULTS
Demographic.
There were 8 (36.4%) females in the control group and 7 (26.9%) females in the MG group [χ2(2, n = 48) = 0.49, p = 0.543]. The mean ages of controls (mean 51.6 years, SD 11.9 years) and patients with MG (mean 52.7 years, SD 12.2 years) were equivalent [t(46) = −0.31, p = 0.599]. Years of education was similar for controls (mean 15.1 years, SD 2.0 years) and patients with MG (mean 14.7 years, SD 3.0 years) [t(46) = 0.53, p = 0.755]. Although the study was open to participants of all race/ethnic backgrounds, all participants were white.
Comparison of controls and patients with MG on the CCTI standards.
Table 1 presents the CCTI performance of the 2 groups. Controls performed significantly better than patients with MG on the reasoning (S4) and understanding (S5) standards. There was also a strong trend on appreciation (S3) (p = 0.06). There was no group difference on expressing choice (S1).
Table 1 Comparisons between controls and patients with malignant glioma on CCTI consent standards
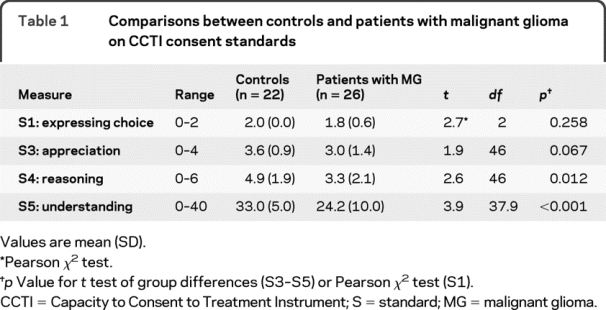
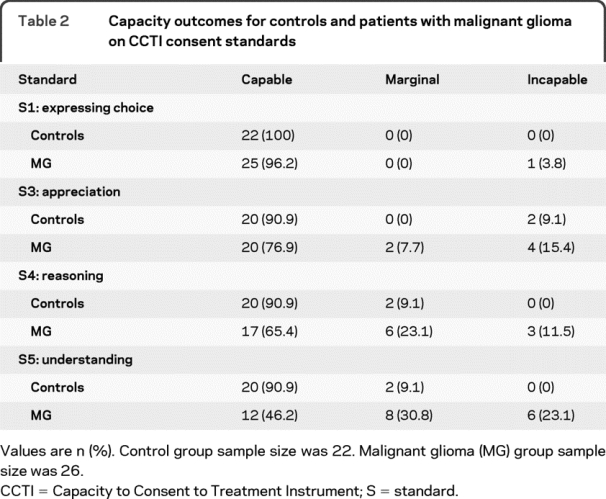
Table 2 displays capacity outcomes by group. A significant proportion of the MG group showed capacity compromise (defined as the combination of marginal and incapable outcomes) on S3–S5. Specifically, patients with MG showed 23.1% compromise on S3 (appreciation), 34.6% compromise on S4 (reasoning), and 53.9% compromise on S5 (understanding). The proportion of patients with capacity compromise increased as the level of complexity of the standard increased (e.g., more patients were impaired on S5 compared with S4 and S3). Most of the patients had capable outcomes on S1, a simple consent standard. All of the controls were capable on S1, and only 9.1% of controls showed capacity compromise on S3, S4, and S5.
Table 2 Capacity outcomes for controls and patients with malignant glioma on CCTI consent standards
Performance on neuropsychological measures.
As expected, the control group performed significantly better than the MG group on all of the neuropsychological tests administered (table 3). The MG group obtained significantly higher scores than the control group on the self-report depression measure, reflecting a greater level of depressive symptoms reported by the MG group.
Cognitive predictors of CCTI consent abilities.
We analyzed the relationship between performance on neuropsychological testing and CCTI performance in the MG group by conducting bivariate correlations (table 4) and subsequent linear regression analyses (table 5). For expressing choice (S1), no neuropsychological variables correlated significantly at the univariate level. Accordingly, no multivariate model was developed for S1.
Table 4 Univariate correlates of CCTI standard performance in patients with malignant glioma (n = 26)
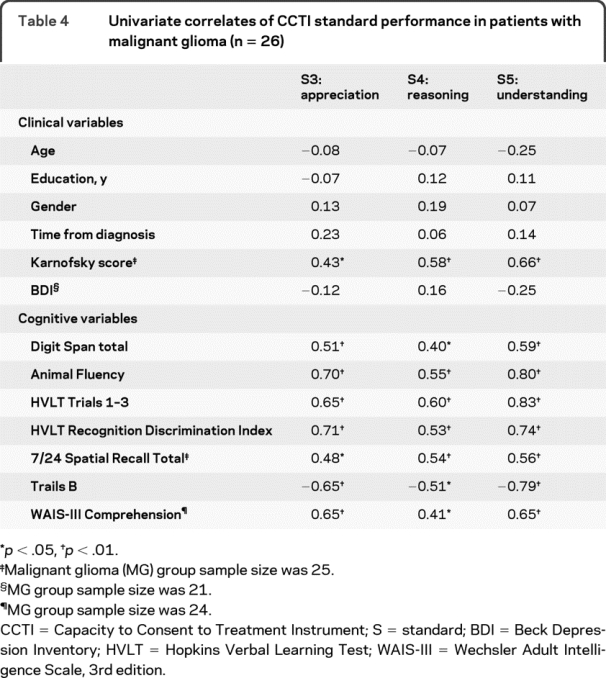
Table 5 Neuropsychological predictors of CCTI standard performance in patients with malignant glioma (n = 26)
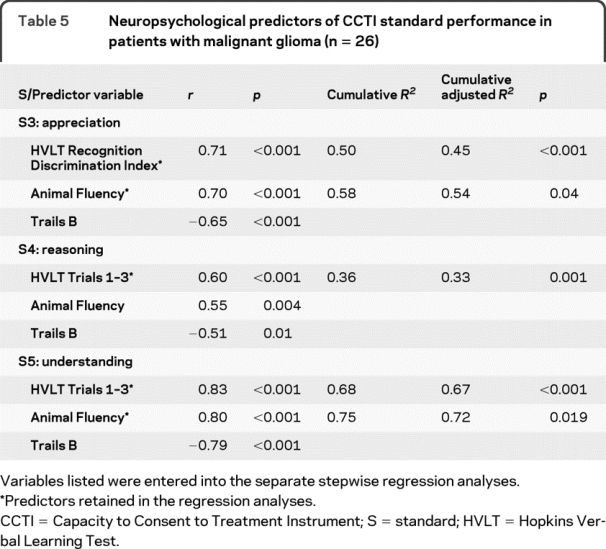
Appreciation (S3) was correlated with measures of verbal memory (Hopkins Verbal Learning Test [HVLT] Recognition Discrimination Index), semantic fluency, and also executive function (Trails B). In the multivariate analysis, measures of verbal memory and semantic fluency together predicted performance on appreciation (S3) and accounted for 54% of the variance in S3 scores.
Reasoning (S4) correlated with measures of verbal acquisition and recall (HVLT Total Score), semantic fluency, and executive function (Trails B). Verbal acquisition and recall was the only multivariate predictor of reasoning (S4), accounting for 33% of the total variance in S4 scores.
Understanding (S5), empirically the most stringent capacity standard in dementia populations,12,16,17 correlated with measures of verbal acquisition and recall (HVLT Total Score), semantic fluency, and executive function (Trails B). Verbal acquisition and recall and semantic fluency were multivariate predictors of understanding (S5) and together accounted for 72% percent of the variance in S5 scores.
Additional analyses.
We also analyzed correlations between CCTI consent standards and demographic and clinical variables (table 4). There were no significant associations between the CCTI consent standards and age, gender, education, or time since diagnosis. Patients with higher Karnofsky scores obtained higher scores on S3–S5, reflecting a strong association between degree of disease disability and MDC. Depressive symptoms were not significantly correlated with CCTI performance, so we did not include this variable as a covariate in the analyses.
DISCUSSION
The present study investigated the ability of patients with MG to make medical treatment decisions. Consistent with our primary study hypothesis, we found that patients with MG demonstrated impairments in MDC relative to demographically matched, healthy controls. On the 2 most stringent and clinically relevant consent standards of understanding (S5) and reasoning (S4), patients with MG performed significantly below controls. There was also a strong trend on appreciation (S3) (p = 0.06). In contrast, patients with MG performed equivalently to controls in expressing choice (S1). The patients with MG demonstrated greater impairment as the complexity and stringency of the CCTI standard increased.
Capacity outcomes for the patients with MG followed a similar pattern of impairment. More than 50% of the patients with MG demonstrated capacity compromise (combination of marginally capable and incapable outcomes) on understanding, and 35% and 23% compromise, respectively, on reasoning and appreciation. In contrast, patients with MG demonstrated no compromise on expressing a treatment choice. A second aim of the current study was to identify neurocognitive variables associated with MDC in patients with MG. As discussed below, our findings suggest that similar cognitive processes underlie the performance of patients with MG on the 3 complex consent standards (S3, S4, and S5). Specifically, verbal acquisition and retention, and to a lesser extent, semantic knowledge, seem to be associated with MDC impairment in MG.
Understanding, the most stringent and cognitively demanding consent standard in prior studies,7,8,11,18 is factually intensive and successful performance requires the ability to encode, consolidate, and recall relatively complex medical information shortly after presentation.12 The primary cognitive predictor of understanding was a measure of short-term verbal acquisition and recall (HVLT Total Score). The HVLT Total Score measures a person's ability to immediately recall a list of 12 words in 3 semantic categories across 3 trials and is considered a robust measure of short-term verbal memory.
A measure of semantic word fluency (Animal Fluency) was the secondary predictor of understanding. Semantic fluency involves the spontaneous verbal production of words belonging to a specific category within a 1-minute interval.19 This task measures semantic knowledge as well as expressive language abilities. Thus, performance of patients with MG on understanding seems to be related to abilities for short-term verbal memory and semantic knowledge. Together, these measures accounted for 72% of the total shared variance in the performance of patients with MG on the understanding standard.
Reasoning is a fairly stringent consent standard that requires the person to reason and compare relative risks and benefits of various treatment alternatives, and to use that information to arrive at a treatment decision.7,12 On this standard, a measure of verbal acquisition and recall (HVLT Total Score) was the only multivariate predictor of performance of patients with MG. Memory is relevant to performance on reasoning, because individuals who have difficulty recalling the treatment information presented to them in the vignette have less information to consider when reasoning about treatment information. This measure accounted for 33% of the variance in performance of patients with MG on the reasoning standard.
Appreciation is a clinically relevant standard that requires the individual to appreciate the potential short-term and long-term personal consequences of a treatment decision.8 A measure of verbal memory (HVLT Recognition Discrimination Index) emerged as the first predictor in the multivariate analysis. The MG group's memory deficits likely affected their performance on appreciation because of the reduced amount of information available to them in responding to the requirements of this standard. However, the MG group's impairment on this standard was not completely due to short-term memory deficits; semantic fluency, which is a measure of semantic knowledge, was a secondary multivariate predictor of performance on appreciation. This finding is consistent with prior research in Alzheimer disease and Parkinson disease demonstrating a strong association between semantic fluency and performance on appreciation.8,17 Taken together, verbal memory and semantic fluency account for 54% of the variance in performance of patients with MG on appreciation.
The generalized pattern of neuropsychological impairment demonstrated by our MG group is consistent with the scientific literature on cognitive impairment in MG.14,20–23 Generalized cognitive impairment in MG is common and can occur secondary to a variety of interconnected factors: increased intracranial pressure, seizures, altered hormone and endocrine patterns, and current treatments for MG (surgical resection, radiation therapy, chemotherapy, antiepileptics, and corticosteroids).14,20–23 Our multivariate regression analyses also illustrate the importance of specific cognitive abilities to treatment consent capacity, in particular short-term verbal memory and semantic knowledge.13,24,25 However, the degree to which specific vs generalized cognitive impairment is related to decisional capacity in MG still remains an important empirical question.26
We also investigated the relationships between demographic and clinical variables with performance on the CCTI standards. There were no significant associations between age, education, gender, or depressive symptoms with performance on the standards. There was a significant relationship between Karnofsky score and performance on S3–S5, with patients with MG who have greater disability ratings having poorer performance on these consent standards.
Clinically, our findings highlight the need for careful attention to the capacity of a patient with MG to consent to medical treatment. Because of the rapid cognitive decline that occurs in MG, ongoing assessment of MDC is recommended. In cases of suspected MDC impairment, it may be necessary to increase the level of involvement of the patient's family in the treatment decision-making process. Clinicians may also want to provide information to patients through written formats in addition to verbal discussion. Such written approaches may reduce auditory verbal memory demands, thereby supporting MDC in patients with MG.
Limitations of this study should be mentioned. First, this was an initial and exploratory study in a relatively small sample of white patients and controls, thereby limiting the generalizability and strength of the conclusions. However, confidence in our findings is strengthened by the inclusion of a well-characterized sample of patients with MG and a demographically matched sample of healthy controls. Second, the CCTI outcome ratings were generated for scientific purposes and do not represent actual legal capacity judgments, which are reserved to legal professionals and the courts.18 Third, individuals may respond differently to a hypothetical vignette than to real-life, personal medical situations. For example, the emotional aspects of MDC that are triggered by real-life medical problems are likely not captured by hypothetical vignettes.12 Nonetheless, the CCTI provides a structured and well standardized method for systematically evaluating consent capacity at varying thresholds and can assist clinicians in making clinical capacity judgments. Finally, the regression analyses were not conducted as a test of specific a priori models but were exploratory in nature, which places limits on our interpretation of these analyses. However, the current data are useful for generating and testing new hypotheses about the role of verbal memory and semantic knowledge, and also generalized cognitive impairment, in explaining impaired MDC in patients with MG.
AUTHOR CONTRIBUTIONS
Statistical analyses were performed by Dr. Triebel, Department of Neurology, University of Alabama at Birmingham, Alabama.
ACKNOWLEDGMENT
The authors thank the staff of the Neuropsychology Laboratory in the Department of Neurology for their assistance with data collection.
DISCLOSURE
Dr. Triebel reports no disclosures. Dr. Martin serves on the editorial board of Epilepsy & Behavior; and receives research support from the NIH [NICHD/NCMMR 1R01 HD053074 (Coinvestigator), NIA 1R01 AG021927 (Investigator), and NIA 1P50 AG16582 (Investigator–Educ Core)] and the CDC [MM-1042 (Investigator)]. Dr. Nabors has received research support from the NCI [P50 CA97247 (Coinvestigator)]. Dr. Marson has received honoraria for lectures or educational activities not funded by industry; serves/has served as a consultant to Medivation, Inc., the American Psychological Association, Rush University, and the American Bar Association; receives research support from the NIH [HD053074 (PI), AG021927 (PI), AG16582 (PI), NIA Director's Supplementary Reserve (PI), AG24904 (Site PI), and AG10483 (Site PI)]; receives royalty payments as Coinventor of CCTI assessment instrument, which is owned by UAB Research Foundation (since 1996); and has provided expert consultation and testimony in approximately 30 legal cases.
Supplementary Material
Address correspondence and reprint requests to Dr. Daniel C. Marson, Department of Neurology, University of Alabama at Birmingham, Sparks Center 650, 1720 7th Ave. South, Birmingham, AL 35294-0017 dmarson@uab.edu
Supplemental data at www.neurology.org
Supported by the University of Alabama at Birmingham Department of Neurology, Division of Neuro-oncology.
Disclosure: Author disclosures are provided at the end of the article.
Received June 30, 2009. Accepted in final form October 7, 2009.
REFERENCES
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–996. [DOI] [PubMed] [Google Scholar]
- 2.Meyers CA, Hess KR. Multifaceted end points in brain tumor clinical trials: cognitive deterioration precedes MRI progression. Neuro Oncol 2003;5:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brazil L, Thomas R, Laing R, et al. Verbally administered Barthel Index as functional assessment in brain tumour patients. J Neurooncol 1997;34:187–192. [DOI] [PubMed] [Google Scholar]
- 4.Remer S, Murphy ME. The challenges of long-term treatment outcomes in adults with malignant gliomas. Clin J Oncol Nurs Aug 2004;8:368–376. [DOI] [PubMed] [Google Scholar]
- 5.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol 2006;24:1305–1309. [DOI] [PubMed] [Google Scholar]
- 6.Roth L, Meisel A, Lidz C. Tests of competency to consent to treatment. Am J Psychiatry 1977;134:279–284. [DOI] [PubMed] [Google Scholar]
- 7.Appelbaum P, Grisso T. Assessing patients' capacities to consent to treatment. N Engl J Med 1988;319:1635–1638. [DOI] [PubMed] [Google Scholar]
- 8.Marson D, Ingram K. Competency to consent to treatment: a growing field of research. J Ethics Law Aging 1996;2:59–63. [PubMed] [Google Scholar]
- 9.Grisso T, Appelbaum P. Assessing Competence to Consent to Treatment: A Guide for Physicians and Other Health Professionals. New York: Oxford University Press; 1998. [Google Scholar]
- 10.Berg JW, Appelbaum P, Lidz C, Parker L. Informed Consent: Legal Theory and Clinical Practice, 2nd ed. New York: Oxford University Press; 2001. [Google Scholar]
- 11.Okonkwo O, Griffith HR, Belue K, et al. Medical decision-making capacity in patients with mild cognitive impairment. Neurology 2007;69:1528–1535. [DOI] [PubMed] [Google Scholar]
- 12.Marson DC, Ingram KK, Cody HA, Harrell LE. Assessing the competency of patients with Alzheimer's disease under different legal standards. Arch Neurol 1995;52:949–954. [DOI] [PubMed] [Google Scholar]
- 13.Dymek MP, Marson DC, Harrell L. Factor structure of capacity to consent to medical treatment in patients with Alzheimer's disease: an exploratory study. J Forensic Neuropsychol 1999;1:27–48. [Google Scholar]
- 14.Lezak M, Howieson D, Loring D. Neuropsychological Assessment, 4th ed. New York: Oxford University Press; 2004. [Google Scholar]
- 15.Beck A, Ward C, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry 1961;4:561–571. [DOI] [PubMed] [Google Scholar]
- 16.Marson D. Loss of competency in Alzheimer's disease: conceptual and psychometric approaches. Int J Law Psychiatry 2001;8:109–119. [DOI] [PubMed] [Google Scholar]
- 17.Dymek M, Atchison P, Harrell L, Marson D. Competency to consent to treatment in cognitively impaired patients with Parkinson's disease. Neurology 2001;56:17–24. [DOI] [PubMed] [Google Scholar]
- 18.Martin R, Okonkwo O, Hill J, et al. Medical decision-making capacity in cognitively impaired Parkinson's disease patients without dementia. Mov Disord 2008;23:1867–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strauss E, Sherman E, Spreen O. A Compendium of Neuropsychological Tests, 3rd ed. New York: Oxford University Press; 2006. [Google Scholar]
- 20.Meyers CA, Weitzner MA, Valentine AD, Levin VA. Methylphenidate therapy improves cognition, mood, and function of brain tumor patients. J Clin Oncol 1998;16:2522–2527. [DOI] [PubMed] [Google Scholar]
- 21.DeAngelis LM. Brain tumors. N Engl J Med 2001;344:114–123. [DOI] [PubMed] [Google Scholar]
- 22.Anderson-Hanley C, Sherman ML, Riggs R, Agocha VB, Compas BE. Neuropsychological effects of treatments for adults with cancer: a meta-analysis and review of the literature. J Int Neuropsychol Soc 2003;9:967–982. [DOI] [PubMed] [Google Scholar]
- 23.Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol 2004;3:159–168. [DOI] [PubMed] [Google Scholar]
- 24.Dymek MP, Atchison P, Harrell L, Marson DC. Competency to consent to medical treatment in cognitively impaired patients with Parkinson's disease. Neurology 2001;56:17–24. [DOI] [PubMed] [Google Scholar]
- 25.Okonkwo OC, Griffith HR, Belue K, et al. Cognitive models of medical decision-making capacity in patients with mild cognitive impairment. J Int Neuropsychol Soc Mar 2008;14:297–308. [DOI] [PubMed] [Google Scholar]
- 26.Palmer BW, Savla GN. The association of specific neuropsychological deficits with capacity to consent to research or treatment. J Int Neuropsychol Soc Nov 2007;13:1047–1059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


