Abstract
The dose-volume-outcome data for RT-associated laryngeal edema, laryngeal dysfunction, and dysphagia, have only recently been addressed, and are summarized. For late dysphagia, a major issue is accurate definition and uncertainty of the relevant anatomical structures. These and other issues are discussed.
Keywords: larynx, pharynx, dysphagia, radiotherapy, dose-effect
A (Larynx) and B (Pharynx) 1. Clinical significance
Radiation therapy (RT) is the primary modality allowing larynx preservation in patients with tumors in the upper aero-digestive tract. RT-induced laryngeal edema (due to inflammation and lymphatic disruption) is a common and expected side effect. Progressive edema and associated fibrosis can lead to long-term problems with phonation and swallowing (1). Since the primary goal of larynx preservation is speech/swallowing retention, RT-induced laryngeal dysfunction may undermine this therapeutic approach. In many instances the larynx and pharynx are target structures and purposefully receive high RT doses.
Dysphagia is common after chemo-irradiation of Head-and-Neck (HN) cancer. For example, patients on RTOG 91-11 were randomized to receive RT +/− concurrent cisplatin. The combined modality arm demonstrated improved tumor control rates (2). However, one year after therapy, 23% of the patients in the chemo-RT arm were unable to eat solid food, vs. with 9% with RT alone. Aspiration pneumonia associated with dysphagia after intensive chemo-RT has recently been appreciated (3). The topics reviewed here are the subjects of current intensive research. This review examined key papers published through June 2008.
A (Larynx).2 Endpoints
A.2.1 Larynx edema
Edema can be assessed through flexible fiberoptic examination. The grade of larynx edema is scored according to the RTOG scale as follows: 0) no edema; 1) slight edema; 2) moderate edema; 3) severe edema; and 4) necrosis. Some degree of uncertainty is intrinsic to the subjectivity on the interpretation of RTOG “slight” and “moderate”. Grade 1 edema would correspond to “minimal” thickening of epiglottis, aryepiglottic folds, arytenoids, and false cords. Grade 2 is a more diffuse and evident edema, though still without significant/symptomatic airway obstruction.
A.2.2. Vocal function
Vocal function can be assessed objectively via instruments (e.g. videostroboscopy for direct visualization to assess supraglottic activity, vocal fold edge, amplitude, mucosal wave, phase symmetry and glottic closure (4), aerodynamic measurements of phonation time (5), or via human observes (6)). Subjective assessments can be made with validated patient-focused questionnaires to assess various combinations of voice, eating, speech, and social functions.
A.3 Challenges Defining Volumes
Identification of the most important anatomic sites whose dose-volume parameters would primarily effect vocal function remains controversial. Dornfeld (7) considered dose points in various structures (e.g. base of tongue, epiglottis, lateral pharyngeal walls pre-epiglottic space, aryepiglottic folds, false vocal cords, and upper esophageal sphincter) to be related to vocal injury . Sanguineti (8) considered the larynx from the tip of the epiglottis superiorly to the bottom of the cricoid inferiorly; the external cartilage framework was excluded from the laryngeal volume. Due to the small size and close proximity of these structures, high resolution contrast-enhanced CT is suggested to facilitate accurate (sub)-structure definition.
A.4 Review Of Dose/Volume Data
A.4.1 Laryngeal edema
Sanguineti (8) found that neck stage, nodal diameter , mean laryngeal dose and laryngeal V30Gy →V70Gy were all significantly associated with edema grade ≥2 on univariate analysis. On multivariate analysis, mean laryngeal dose/V50Gy and neck stage were the only independent predictors. The authors suggest that V50Gy and mean laryngeal dose should be kept as low as possible, ideally below 27% and below 43.5 Gy, respectively, to minimize the edema (i.e.<20% actuarial incidence at 1 yr, compared with 45% in patients receiving 44-57 Gy and >80% in patients receiving >57 Gy). Only a minority of their patients received concurrent chemotherapy, which may have affected dose-response relationships.
A.4.2 Vocal dysfunction
Many studies have shown a good voice outcome following RT for T1 laryngeal cancer (typically 60-66 Gy without chemotherapy). In the locally advanced setting, there is less information on voice quality following treatment. Dornfeld and colleagues (7) found a strong correlation between speech and doses delivered to the aryepiglottic folds, pre-epiglottic space, false vocal cords, and lateral pharyngeal walls at the level of the false vocal cords. In particular they noticed a steep drop off in function after 66 Gy to these structures. Their study was limited by not having full three-dimensional (3D) dose metrics. Fung (5)evaluated subjective and objective parameters of vocal function. Changes in voice were related to doses to the larynx and pharynx, and oral cavity. This suggests that saliva, pharyngeal lubrication, and soft tissue/structural changes within the surrounding musculature play an important role on voice function.
A.5 Factors Affecting Risk
Locally advanced laryngeal cancer frequently causes voice dysfunction that may not improve even if cancer is eradicated. This is one of the reasons patients presenting with marked laryngeal dysfunction may be advised to undergo laryngectomy, rather than a trial of chemo-RT. The addition of concurrent chemotherapy to high-dose RT at least doubles the risk of laryngeal edema and dysfunction, while RT without chemotherapy, delivered to small fields for T1 glottic larynx cancer, usually results in excellent voice quality (9).
A.6 Mathematical/Biological Models
Rancati (10) studied the same study population analysed by Sanguineti (8). Using G2-G3 edema within 15 months post-RT as an endpoint, 38/66 patients were available for this purpose and 21/38 experienced G2-G3 edema. Two NTCP models were fitted using a maximum likelihood analysis: (a) LKB model and (b) the Logit model with DVH reduced to the equivalent uniform dose (EUD). A significant volume effect was found for edema, consistent with a prevalent parallel architecture of the larynx for this endpoint. Both NTCP models fit well the clinical data: the relationship between EUD and NTCP can be described with n=0.47±0.3, TD50=46.0±1.85Gy and a steepness parameter k=9.95±3.46Gy. Best fit parameters for LKB are n=0.45±0.28, m=0.16±0.05 and TD50=46.3±1.8Gy (Table 1). Based on these findings, the authors suggest an EUD<30-35 Gy in order to reduce the risk of G2-G3 edema.
Table 1.
Larynx edema: estimated parameter values for various NTCP models with their 1D-68% Confidence Intervals.
A.7-8 Special Situations and Recommended Dose/Volume Limits
The exact correlation between voice abnormalities and the degree of laryngeal edema has not been assessed. Also, most studies do not consider pre-RT voice abnormalities (common with advanced lesions), and thus may overestimate the degree of RT-related damage. Nevertheless, to minimize the risks of laryngeal edema, it is recommended that the larynx V50Gy be ≤27% and the mean laryngeal dose be ≤44 Gy . For model-based predictions, we recommend that EUD be <30-35 Gy, with a volume parameter (n) of ≈ 0.45 (see table 2).
Table 2.
Larynx toxicity: summary of dose-volume relationship and constraints above which toxicity is significantly increased.
| Author (Ref) No. of patients |
Critical Organs | Predictive Dose/Volume Parameter |
Endpoint |
|---|---|---|---|
|
Dornfeld (7) 27 pts* |
aryepiglottic folds pre-epiglottic space false vocal cords lateral pharyngeal walls |
point dose <68 Gy | Vocal function |
|
Sanguineti (8) 66 pts** |
larynx | V50Gy <27% mean dose <43.5 Gy |
Laryngeal edema (fiberoptic examination) |
|
Rancati (10) 38 pts*** |
larynx | EUD1 <30-35 Gy (n=0.45) |
Laryngeal edema (fiberoptic examination) |
22/27 patients who received chemotherapy+RT
12/66 patients received chemotherapy +RT
7/38 patients received chemotherapy +RT
Equivalent uniform dose
Recommendations
RT impacts voice quality in locally advanced head and neck cancer but less so in early stage larynx cancer. An interesting conclusion follows this observation: clinically significant vocal dysfunction requires both the larynx and surrounding supra-laryngeal structures to be effected. The surrounding tissues may indirectly be affected by a reduction in salivary function, or directly by effects on the intrinsic musculature and soft tissue. Based on the data above it seems reasonable to suggest limiting the mean non-involved larynx dose to 40-45 Gy and limiting the maximum dose to less than 63-66 Gy, if possible based on the tumor extent.
A.9-10 Future Toxicity Studies and Toxicity Scoring
Longitudinal studies consisting of objective scoring of laryngeal edema, voice quality, and patient-reported measures are necessary in order to assess the inter-correlations among these measures. Such studies should include pre-therapy assessments in order to take into account tumor-related voice abnormalities, and should concentrate on patients receiving concurrent chemo-RT, who are at highest risk of laryngeal toxicity.
B (Dysphagia).2 Endpoints
B.2.1 Objective Evaluation
Instrumental Assessment. Videofluorography (VFG) includes modified barium swallow and esophagogram to visualize the oral, pharyngeal, and esophageal phases of swallowing (11). Additional instrumental assessors include manometry and functional endoscopic evaluation of swallowing (FEES).
B.2.2 Subjective Evaluation: Observer-Assessed
Common Terminology Criteria for Adverse Events (CTCAE) are frequently used to assess acute toxicity, as well as the RTOG/European Organization for Research and Treatment of Cancer (EORTC) criteria and the Subjective Objective Management Analytic (SOMA) scale. None of these tools has been tested for its validity in measuring dysphagia.
Patient-Reported Quality of Life
Various instruments have been developed to assess quality of life (QOL) in patients with head and neck cancer, all of which include questions about swallowing dysfunction. While these instruments all measure some aspects of HN cancer–related QOL, it is not clear which one best applies to the assessment of swallowing dysfunctions. All the HN-specific QOL instruments include domains or few questions related to dysphagia. While each instrument as a whole has been tested for validity, similar tests of the specific dysphagia-related questions have not been performed.
B.3 Challenges Defining Volumes
Swallowing is complex, and involves voluntary and involuntary stages coordinated through several cranial nerves and muscles (12). Due to this complexity, defining the most important anatomic structures whose dose-volume parameters would have a major effect on dysphagia has been difficult and only recently studied. Eisbruch et al. noted anatomic/functional changes in pharyngeal constrictors (PC) and glottic/ supraglottic larynx (GSL) after intensive chemo-RT (13), and explained the post-RT abnormalities in objective swallowing assessments 13, 14. The definition of the pharyngeal constrictors in their study was somewhat different than the definition of the constrictors by Levendag (15). Nevertheless, both groups found significant correlations between constrictor doses and dysphagia endpoints. Other studies demonstrated the importance of specific anatomical points in the glottic (7) and supraglottic larynx (16) or pharynx (7). Fua noted that glottic larynx doses were associated with dysphagia in patients who had received high doses to the larynx (17). Thus, most studies demonstrated relevance to various dysphagia endpoints of the doses to the glottic and supraglottic larynx and to specific points in the pharynx, notably the pharyngeal constrictors.
B.4 Review Of Dose/Volume Data (Table 3)
Table 3.
Organs at Risk and Dose/Volume Relationship above which swallowing dysfunctions increases significantly
| Author (Ref) No. of patients |
Critical Organs | Dose/Volume Data | End point | Evaluation Method |
||||
|---|---|---|---|---|---|---|---|---|
| Mean Dose (Gy) |
Median Dose (Gy) |
V50 | V60 | V65 | ||||
|
Eisbruch (13)/ Feng (14) 36pts • IMRT • + Chemo |
Larynx | 50% | • Aspiration | |||||
| PC | 60 | 80% | 70% | 50% | • Aspiration | VF | ||
| PC | 66 | 85% | 70% | 60% | • Stricture | |||
|
Caglar (19) 96 pts • IMRT • ± Chemo |
Larynx | 48* | 21% | Aspiration & Stricture |
VF | |||
| IC | 54 | 51% | ||||||
|
Doornaert (18) 81 pts RT ± Chemo |
Pharyngeal Mucosa & Constrictors |
45 | QOL | • RTOG • EORTC C-30 & H/N 35 |
||||
|
O'Meara (20) 148 pts 2DRT +Chemo |
Pharyngo- esophageal inlet |
50 | Grade 3 + Pharyngo- esophageal Dysfunction |
• RTOG late toxicity |
||||
|
Levendag (15) 81 pts 3DCRT/IMRT ± Brachy ± Chemo |
Superior and Middle Constrictors |
55 | • Grade ≥3 • EORTC • PSS – H&N • MDADI |
• RTOG • QOL • QOL |
||||
|
Dornfeld (7) 27 pts. • IMRT • ± Chemo |
Aryepiglottic fold False cord Lateral pharyngeal Wall near false cord |
50 | • Diet score • H & N QOL • Weight loss • PEG Tube |
• QOL • Clinical Assessment |
||||
|
Jensen (16) 25 pts. • 3DCRT • Radiation alone |
Larynx/ Upper Esophageal Sphincter |
60 | • Aspiration • QOL |
EORTC QOL FEES |
||||
PC = Pharyngeal Constrictors IC = Inferior Constrictor
No correlation with stricture formation
FEES = Functional Endoscopic Evaluation of Swallowing. VF = Videofluoroscopy
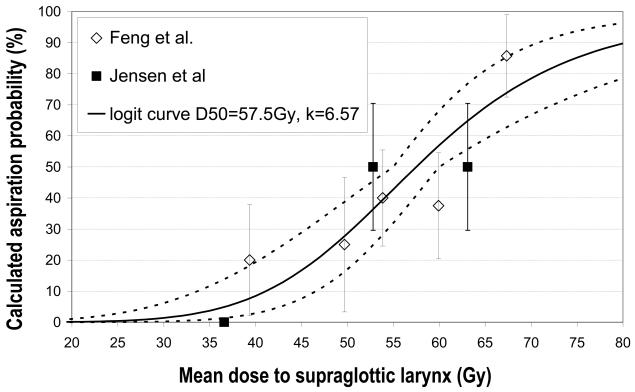
Laryngopharyngeal disorders resulting in late dysphagia and aspiration are not specific and can result from edema and/or fibrosis of various structures. In a prospective study utilizing IMRT to reduce dysphagia, Feng demonstrated dose–volume relationship for swallowing structures in 36 patients treated with chemoradiation (14). A strong correlation was observed between the mean doses and dysphagia endpoints (Figure 1). Aspiration was observed when the PC mean dose exceeded 60 Gy and the dose–volume threshold was V40Gy=90%, V50Gy=80%, V60Gy=70%, and V65Gy>50%. For aspiration to occur, the GSL dose–volume threshold was V50Gy>50% (>50% of volume receiving 50Gy). In a retrospective study, Jensen found that doses <60 Gy to the supraglottic area, larynx, and upper esophageal sphincter resulted in a low risk of aspiration (16). As this study used conventional radiation fields, it is likely that the lack of correlations of pharyngeal doses and dysphagia was related to relative uniformity among the patients in the doses delivered to these structures.
Figure 1.
Dose-effect relation for dysphagia based on Feng et al (14) and Jensen et al (16). The solid line is fit to the combined data, along with the dotted 68%-confidence area for the NTCP-logit curve
Dornfeld (7) reported that swallowing difficulties and the type of diet tolerated worsened progressively with radiation doses >50 Gy to the aryepiglottic folds, false vocal cords, and lateral pharyngeal walls near the false cord. Levendag (15) reported on patients with oropharyngeal carcinoma treated with 3D conformal RT or IMRT with or without brachytherapy + chemotherapy. The use of brachytherapy, which reduces doses to some of the pharyngeal tissues, significantly reduced patient-reported dysphagia. A significant correlation was observed between the mean dose to the superior and middle pharyngeal constrictor muscles and patient complaints of severe dysphagia. A median dose of 50 Gy predicted a 20% probability of dysphagia. This probability increased significantly beyond a mean dose of 55 Gy, with an increase of 19% associated with each additional 10 Gy to superior and middle constrictors. Doornaert 18 reported a steep dose–effect relationship beyond 45 Gy to the pharyngeal wall and concluded that a mean dose of 45 Gy is the optimal threshold dose for predicting swallowing difficulties. Similar findings were reported in retrospective series by Caglar et al (19) and O'Meara et al (20).
There is a paucity of dose/volume data about hypopharyngeal/upper esophageal stricture in HN cancer patients treated with RT + chemotherapy. Laurell (21) recommended a mean dose of <65 Gy to the first 2 cm of proximal esophagus and a mean dose of <60 Gy to the first 5 cm of proximal esophagus as tolerance dose below which the incidence of esophageal stricture is low. Caglar et al found that the volume of the larynx or the inferior constrictor receiving >50 Gy was associated with strictures (19).
B.5 Factors Affecting Risk
Supportive measures during RT may impact on long-term dysphagia. Rosenthal (22) and Mekhail (23) suggested that a nasogastric (NG) feeding tube decreases the need for esophageal dilatation vs a percutaneous endoscopic gastrostomy (PEG) tube. They hypothesized that the NG tube serves as a stent to prevent stricture formation. Amifostine (WR 2721) is the most commonly used cytoprotector for reducing the incidence of xerostomia and mucositis (24). However, there is no data to support its role in decreasing late swallowing disorders..
B.6 Mathematical/Biological Models
The relative paucity of dose-volume data relates to the questions regarding the most important anatomical structures whose dysfunction following chemo-RT causes dysphagia. Data indicating that the pharyngeal constrictors and the larynx are the most likely candidates has been very recent and additional data is being gathered (Table 3). At this time, modeling suggests that 50% NTCP is observed at mean doses of 50-60 Gy to these structures (Fig 1). Limitations of these models include treatment variables, the most important being concurrent chemotherapy, and variations in tumor locations and in pre-therapy dysphagia, which have been accounted for in very few studies (14). The need to consider pre-therapy dysphagia is especially important in laryngeal cancers, in which the rates of pre-therapy dysphagia and aspirations are high and tumor regression after chemo-irradiation may actually reduce the rate of frank aspirations (25). This could confound the results of retrospective dose-effect studies which do not take into account pre-therapy findings.
B.7 Special Situations
Much of the data considered in this review concern patients irradiated either with relatively simple techniques or with IMRT approaches that did not explicitely aim at sparing dysphagia-related anatomical structures. As a consequence, high doses were delivered to these structures and drawing strict dose-volume constraints or volume effect parameters is far from trivial. In addition, high doses to the larynx, for example, are expected in cases of laryngeal or hypopharyngeal cancers, which are associated with a high rates of pre-RT dysphagia and /or aspiration, confounding evaluations of post-RT dose-effect relationships.
B.8 Recommended dose-volume limits
The limited available data suggest that minimizing the volume of the pharyngeal constrictors and larynx receiving ≥60 Gy, and reducing when possible the volume receiving ≥50 Gy, is associated with reduced dysphagia/aspiration. In several cases, such sparing can be achieved without compromising target doses (13, 14). A separate question is whether such sparing is safe clinically, taking into account uncertainties in target delineation. This issue is beyond the scope of this paper.
B.9 Future Toxicity Studies
Late dysphagia is often a consequential effect of acute mucositis. Careful assessment and reporting of the severity of acute mucositis may shed light on the likelihood of late dysphagia and its predictors, and whether successful reduction in acute dysphagia would lead to improvements in late swallowing abnormalities.
Validation of assessors of dysphagia: the most commonly used observer-rated dysphagia grading tool is the CTCAE dysphagia item, which has not been validated formally. Similarly, multiple patient-reported QOL instruments have been used, as detailed above, and few have formally been validated regarding their dysphagia components.
The issue of what are the most important anatomical structures and sub-structures whose damage is the likely cause of dysphagia is the subject of current research by many investigators. An important aspect of this research is the effects of the tumor on pre-therapy swallowing and on the functional results after therapy. In order to capture these effects, prospective studies which include pre-therapy evaluation are essential.
B.10. Toxicity Scoring
As detailed above, prospective evaluation is critical due to tumor-related dysphagia and aspiration, particularly in patients with advanced cancers. While CTCAE-based scoring is simple and commonly applied, the evidence of “silent aspiration” after RT (aspiration not eliciting a cough due to laryngeal sensory deficit) requires objective measurement using imaging and interpretation by professional speech/language pathologists. In addition, objective swallow assessment may help quantify swallowing assessments. Correlating observer-rated scores such as the CTCAE system, patient-reported scores, and objective swallowing dysfunction, is recommended for future focused studies. Until more data regarding this issue are available, we recommend the use of the CTCAE system, as well as a patient-reported QOL instrument, for large-scale clinical studies of chemo-irradiation of HN cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- 1.Fung K, Yoo J, Leeper HA, et al. Effects of head and neck radiation therapy on vocal function. J Otolaryngol. 2001;30:133–139. doi: 10.2310/7070.2001.20192. [DOI] [PubMed] [Google Scholar]
- 2.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. New Eng J Med. 2003;349:2091–8. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 3.Eisbruch A, Lyden T, Bradford CR, et al. Objective assessment of swallowing dysfunction and aspiration after radiation concurrent with chemotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2002;53:23–28. doi: 10.1016/s0360-3016(02)02712-8. [DOI] [PubMed] [Google Scholar]
- 4.Hirano M. Clinical examination of voice. In: Arnold GE, Winkel F, Wyke BD, editors. Disorders of human communication. Springer-Verlag; New York, NY: 1981. pp. 81–84. [Google Scholar]
- 5.Fung K, Yoo J, Leeper A, et al. Vocal function following radiation for non-laryngeal versus laryngeal tumors of the head and neck. Laryngoscope. 2001;111(11):1920–1924. doi: 10.1097/00005537-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Laver J, Wirz S, Mackenzie J, et al. Work in Progress. Vol. 14. University of Edinburgh, Linguistics Department; Edinburgh, Scotland: 1981. A perceptual protocol for the analysis of vocal profiles; pp. 139–155. [Google Scholar]
- 7.Dornfeld K, Simmons JR, Karnell L, et al. Radiation doses to structures within and adjacent to the larynx are correlated with long-term diet– and speech–related quality of life. Int J Radiat Oncol Biol Phys. 2007;68:750–757. doi: 10.1016/j.ijrobp.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 8.Sanguineti G, Adapala P, Endres EJ, et al. Dosimetric predictors of laryngeal edema. Int J Radiat Onc Biol Phys. 2007;68(3):741–749. doi: 10.1016/j.ijrobp.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Fu KK, Woofhouse RJ, Quivey JM, et al. The significance of laryngeal edema following radiotherapy of carcinoma of the vocal cord. Cancer. 1982;49:6555–8. doi: 10.1002/1097-0142(19820215)49:4<655::aid-cncr2820490409>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.Rancati T, Sanguineti G, Fiorino C. NTCP Modeling of Subacute/Late Laryngeal Edema Scored by Fiberoptic Examination: Evidence of a Large Volume Effect. Int J Radiat Onc Biol Phys. 2007;69(S3):S409–S410. doi: 10.1016/j.ijrobp.2009.04.087. [DOI] [PubMed] [Google Scholar]
- 11.Kendall KA, McKenzie SW, Leonard RJ, et al. Timing of events in normal swallowing: a videofluroscopic study. Dysphagia. 2000;15:74–84. doi: 10.1007/s004550010004. [DOI] [PubMed] [Google Scholar]
- 12.Logemann J. Mechanism of normal and abnormal swallowing. In: Cummings JW, Flint PW, Haughey BA, Richardson MA, Robbins KT, Thomas JR, et al., editors. Otolaryngology Head and Neck Surgery. 5th ed. Mosby; St. Louis: 2008. [Google Scholar]
- 13.Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head and neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60:1425–1439. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 14.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose-effect relationships for the swallowing structures. Int J Radiat Onco Biol Phys. 2007;68:1289–1298. doi: 10.1016/j.ijrobp.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 15.Levandag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose–effect relationship. Radiother Oncol. 2007;85:64–73. doi: 10.1016/j.radonc.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Jensen K, Lambertsen K, Grau C. Late swallowing dysfunction and dysphagia after radiotherapy for pharynx cancer: frequency, intensity, and correlation with dose and volume parameters. Radiother Oncol. 2007;85:74–82. doi: 10.1016/j.radonc.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Fua TF, Corry J, Milner AD, et al. Intensity–modulated radiotherapy for nasopharyngeal carcinoma: clinical correlation of dose to the pharyngoesophageal axis and dysphagia. Int J Radiat Oncol Biol Phys. 2007;67:976–981. doi: 10.1016/j.ijrobp.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Doornaert P, Slotman BJ, Rietveld DHF, et al. The mean radiation dose in pharyngeal structures is a strong predictor of acute and persistent swallowing dysfunction and quality of life in head and neck radiotherapy [Abstract #97] Int J Radiat Oncol Biol Phys. 2007;69(suppl):55. [Google Scholar]
- 19.Caglar HB, Allen AM, Othus M, et al. Dose to the larynx predicts for swallowing complications following IMRT and chemotherapy. Int J Radiat Oncol Bio Phys. 2008;72:1110–8. doi: 10.1016/j.ijrobp.2008.02.048. [DOI] [PubMed] [Google Scholar]
- 20.O'Meara EA, Machtay M, Moughan J, et al. Association between radiation doses to pharyngeal regions and severe late toxicity in head and neck cancer patients treated with concurrent chemoradiotherapy—An RTOG analysis [Abstract #96] Int J Radiat Oncol Biology Phys. 2007;69(suppl):54. [Google Scholar]
- 21.Laurell G, Kraepelien T, Mavroidis P, et al. Stricture of the proximal esophagus in head and neck carcinoma patients after radiotherapy. Cancer. 2003;97:1693–1700. doi: 10.1002/cncr.11236. [DOI] [PubMed] [Google Scholar]
- 22.Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J. Clin Oncol. 2006;24:2636–43. doi: 10.1200/JCO.2006.06.0079. [DOI] [PubMed] [Google Scholar]
- 23.Mekhail TM, Adelstein DJ, Rybicki LA, et al. Enteral nutrition during the treatment of head and neck carcinoma: is percutaneous endoscopic gastrostomy tube prefereable to a nasogastric tube? Cancer. 2001;91:1785–1790. [PubMed] [Google Scholar]
- 24.Brizel DM, Wasserman TH, Henke M, et al. Phase 3 randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18:3339–3345. doi: 10.1200/JCO.2000.18.19.3339. [DOI] [PubMed] [Google Scholar]
- 25.Langerman A, MacCracken E, Kasza K, et al. Aspiration in chemoradiated patients with head and neck cancer. Arch Otolaryngol Head neck Surg. 2007;133:1289–1295. doi: 10.1001/archotol.133.12.1289. [DOI] [PubMed] [Google Scholar]