Abstract
As a part of a larger study of normal aging and Alzheimer’s disease (AD), which included patients with mild cognitive impairment (MCI), we investigated the response to median nerve stimulation in primary and secondary somatosensory areas. We hypothesized that the somatosensory response would be relatively spared given the reported late involvement of sensory areas in the progression of AD. We applied brief pulses of electric current to left and right median nerves to test the somato-sensory response in normal elderly (NE), MCI, and AD. MEG responses were measured and were analyzed with a semi-automated source localization algorithm to characterize source locations and timecourses. We found an overall difference in the amplitude of the response of the primary somatosensory source (SI) based on diagnosis. Across the first three peaks of the SI response, the MCI patients exhibited a larger amplitude response than the NE and AD groups (P < 0.03). Additional relationships between neuropsychological measures and SI amplitude were also determined. There was no significant difference in amplitude for the contralateral secondary somatosensory source across diagnostic category. These results suggest that somatosensory cortex is affected early in the progression of AD and may have some consequence on behavioral and functional measures.
Keywords: Somatosensory evoked fields, Aging, Alzheimer’s disease, Mild cognitive impairment, Magnetoencephalography
Introduction
The prevalence of Alzheimer’s Disease (AD) is expected to increase as the US population ages (Brumback and Leech 1994). Therefore, it is important to better understand how this disease affects the brain and its functions, in addition to looking for early markers for this disorder. If AD is identified early, then one can improve the quality of life for these individuals as well as potentially decrease the long-term healthcare costs. Markers of brain abnormality that correlate with functional decline also provide opportunities to test pharmacological agents that may more precisely target brain dysfunction.
A few studies have used EEG to look for differences in the somatosensory response to median nerve stimulation in patients with AD relative to normal elderly (NE) controls (Abbruzzese et al. 1984). These results have consistently shown no difference in the median nerve response between NE and patients with AD until late in the course of the disease. However, in an animal model of AD, Suva et al. (1999) found differences present in primary somatosensory cortex at mild to moderate stages of the disease model and found the presence of plaques in the somatosensory cortex at the same time plaques became visible in association cortices. Only hippocampi were affected earlier than the other cortical areas tested. This suggests that while sensory functions appear to remain intact longer than higher cognitive abilities, such as memory, they may still play a role in declining function.
Based on previous literature, we hypothesized that primary somatosensory cortex would be relatively spared in mild cognitive impairment (MCI) and AD (Geula and Mesulam 1996; Teipel et al. 2007), but that secondary somatosensory cortices (SII) may be affected since association cortices have been reported to be affected earlier than primary sensory areas (Uylings and de Brabander 2002; Teipel et al. 2007). Since AD patients show a decline in functional abilities and white matter tract integrity (Teipel et al. 2007), we expected patients with AD to reveal reduced amplitude(s) and/or delayed responses in SII cortex. We tested these hypotheses by performing a standard median nerve task and measuring the responses using magnetoencephalography.
Materials and methods
Patient consents
The Human Research Review Committee at the University of New Mexico and the New Mexico VA Research and Development Committee approved the research; and written informed consent was obtained from all participants.
Participants
Sixteen healthy NE (6 females), four MCI and five patients with probable AD (one female) were recruited from the Albuquerque area to participate in the MEG study. Participants ranged in age from 63 to 83 years with a mean age of 74 years. Potential participants were excluded if they were diagnosed with chronic neurological disorders, such as epilepsy, Parkinson’s disease, focal neurological deficits, head trauma with persistent neurologic abnormalities, or other severe medical illnesses that would prevent cooperation with the study protocol. Participants were excluded if they met criteria for major depression or any other major DSM-IV axis I disorder, including alcohol/substance abuse. Healthy normal participants underwent a neurological exam performed by a Board Certified Neurologist with expertise in geriatric and behavioral neurology (J. Adair or J. Knoefel). This exam was used to confirm the self-reported normal status of the participants. Some potential participants were not studied based on the initial exam or due to metal artifact noted when placed under the MEG helmet due to excessive dental work. These individuals are not included in the reported participant numbers.
The patients with MCI and AD were recruited through cognitive disorders clinics at the Albuquerque VA Medical Center and the University of New Mexico Health Sciences Center. Complete medical and neurological histories were obtained and complete physical examinations were conducted, similar to the normal controls. All participants underwent a standard battery of psychometric tests including an indicator of global cognitive ability (Mini Mental State Exam, MMSE), as well as specific assessments of simple attention, verbal learning, executive function, and confrontation naming. Affective symptoms were evaluated with the Geriatric Depression Scale. Functional status was measured with the Functional Activities Questionnaire completed by a knowledgeable informant who accompanied the patient to clinic. All patients underwent a set of additional studies, including brain magnetic resonance imaging or computed tomography scans and laboratory tests, to exclude other etiologies of cognitive dysfunction.
Individuals were diagnosed as amnestic MCI based on criteria modified from Petersen et al. (2001) as follows. First, patients demonstrated normal general cognitive function, indicated by age- and education-adjusted MMSE scores above the 25th percentile. Second, patients were not demented as defined in DSM-IV; cognitive impairment was not severe enough to impede routine activities of daily living. Third, patients demonstrated abnormal performance at least 1.5 SD below age-referenced means on the Hopkins Verbal Learning Test 3-trial immediate recall or percent delayed recall, and scores within 1 SD of norms on other tests. Patients were diagnosed with AD based on meeting DSM-IV criteria for dementia and NINCDS-ADRDA criteria for probable diagnosis. The modified Hachinski Ischemic Scale scores included patients indicating a low or indeterminate likelihood of vascular injury (<7). Similar to the healthy controls, exclusion criteria consisted of additional chronic neurological conditions or if the neurological examination at the initial evaluation suggested such conditions. Finally, patients were excluded if they met criteria for major depression or any other major psychiatric disorder or alcohol/substance abuse within 2 years prior to evaluation.
All Participants also underwent standard neuropsychological (NP) and IQ testing to assess cognitive abilities within individuals and between diagnostic groups. A subset of these tests deemed to have a somatomotor component to them was correlated with the MEG results (WAIS-R Block Design, WAIS-R Digit Symbol, Rey Complex Figure Copy, Trails A time, Trails B time).
Data acquisition
MEG data were acquired using a whole-head 275-channel CTF biomagnetometer located within a 2-layer magnetically shielded room (Vacuumschmelze, GmbH & Co. KG, Nanau, Germany). Anatomical MRIs were acquired on a 1.5-T Siemens Sonata MR scanner at the Mind Research Network. Standard T1-weighted MPRAGE (1.5 mm slices) and T2-weighted Turbo Spin Echo (1.8 mm slices) sequences were acquired. A board certified neuroradiologist read the MRIs and those with abnormalities were reported to the participant’s physician. Patients with gross MRI abnormalities were excluded from the study. For MEG data collection, three coils were attached to the participant’s head; position of the coils and head shape was obtained with a Polhemus head position device. Prior to data collection, the coils were activated to determine the participant’s head position within the MEG helmet. This allowed for co-registration of the MEG data with the anatomical MRIs using automatic transformations available in MRIVIEW (Ranken and George 1993).
The left and right median nerves were stimulated randomly (1 s inter-stimulus interval) using two surface electrodes placed on each inner wrist. A 0.3-ms current pulse was applied using a Grass Constant Current Stimulator. The electrodes and voltage were adjusted until a thumb twitch was obtained in each hand. If the maximum voltage was reached without a thumb twitch, a just-detectable threshold value was determined and the voltage was set at twice the threshold value. Participants were instructed to relax but not to fall asleep. MEG data were digitized at 2.4 kHz with anti-aliasing filters applied. Continuous recordings were made and saved for off-line processing. The data were preprocessed using CTF software and digital filters were applied (1.5–100 Hz band-pass and 58–62 Hz notch filter to eliminate power line noise). The data were then epoched (−0.1 to 0.4 s) relative to the markers for left and right stimulation conditions. Epochs with eye blinks and large amplitude noise were eliminated prior to averaging. MEGAN (an MEG ANalysis tool developed by E. Best at Los Alamos National Laboratory) was used to remove baseline noise (estimated from the −100 to −5 ms pre-stimulus), DC shifts identified in the baseline, and channels with flux jumps or large amplitude non-physiological responses.
Dipole modeling
As mentioned above, MRIVIEW (Ranken and George, 1993; Ranken et al., 2002) was used to co-register the MRI and the MEG data. Then, the cortical surface was identified and labeled using the automated segmentation tool in order to estimate the multiple best fitting spheres for the modeling procedure (Huang et al. 1998). A sparse sampling of the brain volume provided the set of random starting locations for the source localization algorithm described below.
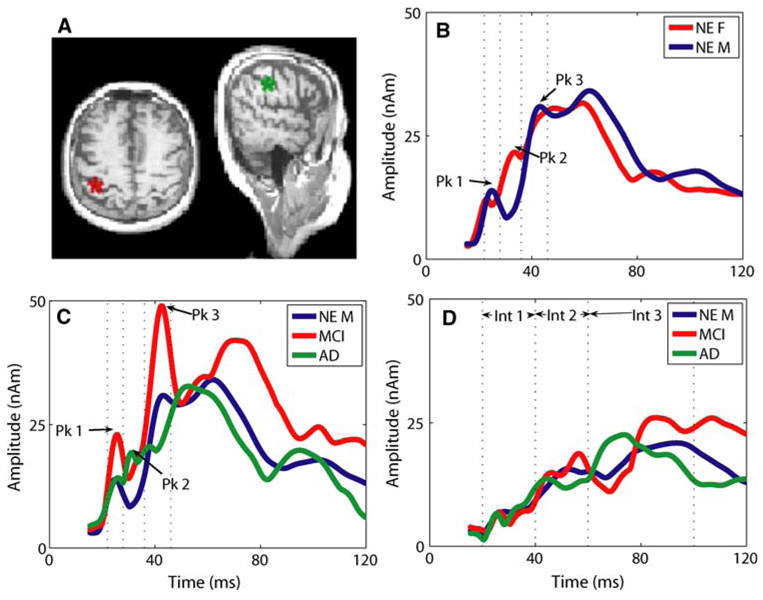
As described previously (Ranken et al. 2002; Stephen et al. 2003a, b) and similar to Huang et al. (1998) and Aine et al. (2000, 2003, 2005), a calibrated-start spatio-temporal multi-dipole modeling technique was used to determine the sources and source timecourses of the MEG activity. Once a best model was obtained for each participant, the individual source timecourses were characterized. Three intervals were identified for SI and SII with the intervals corresponding to the initial peaks seen in the individual timecourses across participants: SI: (1) 22–28 ms; (2) 28–32 ms; (3) 39–46 ms (see Fig. 2); SII: (1) 20–40 ms; (2) 40–60 ms; (3) 60–100 ms. Within each interval, the maximum amplitude was determined for each individual using an automated Matlab routine. The peak amplitudes and latencies were then compiled across participants. The data were tested for outliers to eliminate the possibility that the results could be skewed by individual results.
Fig. 2.
Source locations and timecourses. a The left source (red) is an example of cSI source location in one of the NE participants. The right source (green) denotes cSII cortex activation in a NE participant. All MEG source analysis results were projected onto the individual’s MRI to confirm source location prior to averaging the source timecourses across participants. b The averaged cSI timecourses in NE participants. For each individual the peak amplitude was obtained from the individual timecourses within the three intervals (see dotted lines). These peak amplitudes were used for statistical comparisons across gender and diagnostic category. The peak amplitude across participants within interval 2 was significantly different (see text). c The averaged cSI timecourses for all male participants including NE (blue), MCI (red) and AD (green). d Averaged cSII timecourses for all male participants comparing NE (blue), MCI (red), and AD (green). No clear difference was identified in the intervals tested. The variance increased across time making any apparent differences later in time, not significant
Statistical analyses
We began with a fully saturated model with all interactions and eliminated all insignificant higher-order interactions using PROC GLM in SAS. We conducted analysis of variance (ANOVA) on maximum amplitudes and latencies identified in the intervals described above. Interval and diagnosis were factors. Side of stimulation and gender were eliminated from the model due to lack of significance (side) and small n (gender). The significance from the level three sums of squares is reported, which accounts for multiple comparisons within a GLM model. Post hoc comparisons were performed using the Tukey Least Significant Difference t test to isolate interaction effects.
Results
We obtained good somatosensory responses from 25 participants from both left and right median nerve stimulation. Demographic information for the different participant categories is shown in Table 1. There were no significant differences between the NE males and females in general demographics (age, years of education) and their test scores differed on only one of the NP measures (Rey complex figure—copy raw—t test F < M t = −2.2, P < 0.05). Because MCI and AD patients were recruited through the Albuquerque VA Medical Center, we had a gender imbalance in the study with only one female AD patient. For this reason and due to the reported gender differences described below, analyses based on diagnosis focused on the male individuals. With a one-way ANOVA, the males were compared across diagnostic category (NE, MCI, and AD). Again, there were no significant differences in general demographics (age, years of education) across diagnosis. As expected, there was a significant difference in MMSE score (NE > MCI > AD, F = 27.4, df(2,15), P < 0.0001). Additional significant differences were noted in three of the NP measures (RCF—copy raw, F = 5.7, df(2,15), P = 0.014 post hoc showed NE > AD; Trails A time, F = 7.5, df(2,15), P = 0.0055, post hoc showed AD > NE and AD > MCI; Trails B time, F = 4.3, df(2,15), P = 0.034, post hoc showed AD > NE).
Table 1.
Mean (standard deviations) demographics by gender and diagnostic category
| Group | Years of education |
Age (years) |
MMSE | IQ | Block design |
Digit symbol |
RCF—copy raw |
Trails A time (s) |
Trails B time (s) |
|---|---|---|---|---|---|---|---|---|---|
| NE females (N = 6) | 15 (4) | 71 (5) | 28.5 (1.9) | 116 (18) | 9 (5) | 7 (3) | 31 (4)* | 34 (11) | 77 (29) |
| NE males (N = 10) | 16 (4) | 72 (5) | 29.2 (1.3)† | 126 (16) | 11 (4) | 7 (2) | 35 (2)*† | 40 (10)† | 87 (39)† |
| MCI males (N = 4) | 14 (3) | 80 (7) | 26 (2)† | 108 (31) | 7 (2) | 5 (2) | 31 (5) | 65 (15)† | 155 (68) |
| AD males (N = 4) | 14 (2) | 75 (6) | 22 (2.2)† | 101 (14) | 10 (5) | 4 (3) | 22 (13)† | 160 (115)† | 241 (201)† |
P<0.05 in a t-test comparison between NE Females and NE males
P<0.05 in a one-way ANOVA with diagnosis as comparison in all male participants
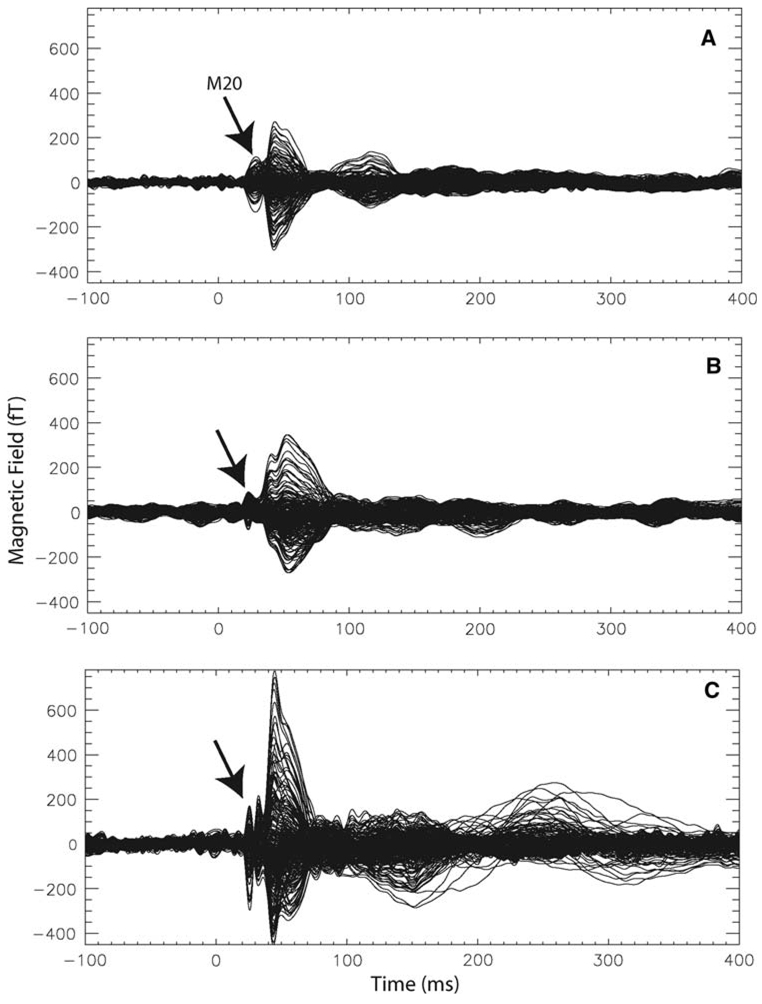
Example MEG waveforms are shown in Fig. 1. The individual waveforms are representative of the pattern identified in the source timecourses with a decreased response in interval 2, immediately following the initial peak in the NE males compared to NE females as well as larger amplitude overall in the MCI individual.
Fig. 1.
Example waveforms. The waveform responses to left median nerve stimulation are shown for three individuals. All channels from the 275-channel array system are plotted on one axis to reveal the timing and amplitudes associated with the responses. A clear M20 is visible in all three participants as is expected from median nerve stimulation. a A sample waveform for a NE female participant. b A sample waveform from a NE male participant. c A sample waveform from a male MCI participant. Notice the larger amplitude response. These waveforms are consistent with the results seen across participant groups in the SI timecourses shown in Fig. 2
From the source modeling, we obtained source activations in primary somatosensory cortex (SI), contralateral and ipsilateral secondary somatosensory cortex (cSII and iSII) as well as posterior parietal and cingulate cortex. Since contralateral SI and SII were the most reliably identified sources, they are the focus of this report. Example source locations for cSI and cSII are shown in Fig. 2a. Timecourses for cSI and cSII were averaged across participants within each diagnostic group for visualization purposes. As described in the methods, the statistical analyses were performed on the individual participant peak amplitude and latency results. Averaged SI timecourses are shown in Fig. 2b for males versus females. Consistent with our previously reported results (Stephen et al. 2006), we identified a lower amplitude for peak 2 in NE male compared to NE female participants [F = 5.0, df(1,29), P = 0.03]. No significant gender differences for latency within intervals were found. By comparing the male participants by diagnosis (Fig. 2c), we found a significant difference in amplitude across the three intervals, which included peaks 1–3 [F = 3.7, df(2,94), P < 0.03]. Post hoc tests showed that across time the amplitudes were significantly greater for the MCI male participants compared to both NE and AD male participants. There was no significant interaction effect, despite the apparent change in pattern across intervals 1–3. In addition, no significant difference in latency within intervals was found across diagnostic categories. Analysis of the cSII timecourses revealed no significant differences in amplitude or latency across diagnostic categories (Fig. 2d).
The individual NP measures were introduced to the model as covariates to determine their affect on the cSI amplitude by diagnosis result. This limited the analysis to individuals with NP measures (partial NPs in 24/25 participants, complete NPs 21/25). The larger amplitude cSI response in MCI individuals remained significant within the smaller sample (F = 5.5, df(2,77), P < 0.005). Two covariates showed significant effects: Block Design and Trails B time (see Table 2 – bolded values). Since only two covariates had an effect, this suggests that diagnosis alone is a better predictor of cSI amplitude than any individual NP measure. In addition, the difference between the cSI amplitudes for MCI and AD was increased when time of Trails B was included as a covariate (implying that amplitude increased with increasing time). In addition, the difference between MCI and NE cSI amplitudes decreased when Block Design was included as a covariate (implying that amplitude decreased as block design score increased). These results are consistent with an overall pattern that larger amplitude responses corresponded with poorer performance on NP measures.
Table 2.
Neuropsychological measures as covariates to diagnostic category and interval in the general linear model
| Covariates | Significance | Amplitude difference (significance) | ||||
|---|---|---|---|---|---|---|
| Dx | Interval | Covariate | NE vs. AD | MCI vs. AD | MCI vs. NE | |
| None | 0.005 | <0.001 | N/A | −3.4 (0.4) | 10.5 (0.036) | 13.9 (0.01) |
| Block design | 0.06 | <0.001 | 0.002 | 4.7 (0.3) | 11.7 (0.02) | 7.0 (0.13) |
| Digit symbol | 0.06 | <0.001 | 0.37 | 1.8 (0.7) | 11.6 (0.03) | 9.8 (0.07) |
| RCF—copy raw | 0.003 | <0.001 | 0.09 | 2.0 (0.7) | 15.4 (0.008) | 13 (0.01) |
| Trails A time | 0.02 | <0.001 | 0.9 | −3.4 (0.4) | 10.8 (0.05) | 14.2 (0.01) |
| Trails B time | 0.005 | <0.001 | 0.02 | 7.5 (0.19) | 17.5 (0.002) | 10.2 (0.02) |
| Full scale IQ | 0.03 | <0.001 | 0.58 | −0.5 (0.9) | 11.7 (0.04) | 12.2 (sign) |
| Performance IQ | 0.02 | <0.001 | 0.22 | 2.1 (0.7) | 13.3 (0.02) | 11.3 (sign) |
| Verbal IQ | 0.02 | <0.001 | 0.61 | −0.6 (0.9) | 11.9 (0.05) | 12.5 (sign) |
Discussion
The primary result of this study was that the amplitude of cSI across the first three peaks was significantly larger for male individuals diagnosed as MCI compared to NE and AD male participants. In addition, larger amplitudes in cSI across participants corresponded with poorer performance on two of the NP measures tested. These results show involvement of the SI cortex in patients with MCI, which is considered a precursor to AD in some individuals, and thus provides a potential early marker for cortical involvement in MCI.
The current results replicate our previous results in the NE (Stephen et al. 2006) which used a Neuromag 122-channel planar gradiometer MEG system compared to the CTF axial gradiometer system used in the current study. The replication across MEG systems is consistent with our preliminary study by Weisend et al. (2007) showing essentially identical results within participants across three commercially available MEG systems. Since the current study recruited a different group of NE individuals from the previous normal aging study (three individuals were tested using both MEG systems), this provides additional evidence supporting a gender difference in SI timecourses, suggesting that gender should be accounted for when evaluating sensory responses in the elderly.
The staging of AD presented by Braak and Braak (1991), based on neuropathology identified in 83 brains, showed involvement of sensorimotor cortex only at the latest stages of the disease based on postmortem anatomical criteria. Sensory areas were not shown to be involved until Stage VI. However, initial involvement of subcortical structures was identified as early as stage IV, at a time when cortical structures were only mildly affected. While they suggested that the limbic stages (I–IV) should correspond to the pre-clinical stages of AD or potentially to MCI stages, and they specifically stated that they did not intend to link their stages of AD progression with the progression of clinical stages. Despite continued work in both arenas, the correspondence between neuropathological staging and clinical staging in AD is not yet well established. Based on the results identified in the current study, we consider the link between the clinical diagnosis at the MCI stage to correspond potentially with stages IV–VI of Braaks’ neuropathological staging. We expect that the amplitude differences identified in cSI would primarily be associated with cortical changes in SI, which would suggest Stage VI involvement; however, involvement of subcortical structures could also result in changes of cSI amplitudes and/or latencies, which would suggest a stage IV correspondence. If the cSI amplitude differences were related to subcortical changes, then it would help explain why cSII measures did not show group differences.
Recent studies have begun to suggest that sensorimotor dysfunction occurs earlier than previously thought in the progression of AD. Suva et al. (1999) found senile plaques in sensorimotor areas as soon as they were identified in other cortical association areas, with only hippocampus showing isolated plaques initially. Consistent with this finding, Ortiz Alonso et al. (2000) identified sensorimotor deficits in patients with AD (MMSE < 24). Ugawa et al. (1987) also identified patients with mild AD who had myoclonus, again suggesting abnormal motor processing at a much earlier stage than previously thought. At the same time, Abbruzzese et al. (1984) reported that patients with AD had preserved SEP responses to median nerve stimulation relative to NE. This is consistent with our results of no difference between NE and AD. However, the results of the abnormal responses in MCI suggest a different process may be at play in the early stages of the disease. Similar results were found by Golob et al. (2007) in an auditory P300 paradigm with increased amplitude of the P50 peak in patients with MCI. The MCI patients who later converted to probable AD had larger amplitude P50s than those with a stable MCI diagnosis. Our recent study (Aine et al. 2010) also noted larger amplitude responses in the anterior temporal region during an auditory verbal memory task in the MCI/AD group compared to NE. Motor results from Di Lazzaro et al. (2004) suggest that hyperexcitability in motor cortex of patients with AD is related to abnormal excitatory responses rather than decreased inhibition, commonly reported in NE, which may be one of the mechanisms that leads to abnormal responses in MCI individuals. Papaliagkas et al. (2008) performed a large auditory ERP study of MCI patients and identified greater amplitude P200 responses and no latency changes in the early components compared to normal participants. This suggests a consistent amplitude effect across basic sensory areas underlining the likelihood that much of cortex is involved by the time behavioral measures detect MCI. Given the reduced variability in brain responses across individuals in sensory tasks compared to cognitive tasks (Ojemann et al. 1989 and Aine et al. 2010), the reported early changes in cSI may provide a sensitive marker for cognitive decline.
There were no significant differences in timing across diagnostic groups. This is consistent with Teipel et al. (2007) where no differences were found in somatosensory white matter tracts; it is generally assumed that white matter tract integrity correlates with timing. This result is also consistent with the overall framework presented by Bartzokis (2004) suggesting a staged involvement of white matter tracts in AD. In the Bartzokis model, somatosensory white matter tracts would be one of the last sites to be affected since primary/secondary somatosensory cortices are one of the first to myelinate and the last to reveal myelin breakdown across the lifespan. These studies are consistent with the notion that amplitude/latency changes in association areas and consequent cognitive deficits should be witnessed first, followed by amplitude/latency changes in primary/secondary sensory areas which is also concordant with the Braak and Braak staging of AD (Braak and Braak 1991).
In addition to the Bartzokis model, which focused on white matter, Delbeuck et al. (2007) hypothesized that AD is in part related to a disconnection syndrome where lack of communication between critical cortical areas leads to cognitive impairment. Disconnection has been evidenced by reduced coherence using ERPs (Leuchter et al. 1992 and others) and reduced multisensory integration using behavioral tests (Golob et al. 2001). While Delbeuck and colleagues identified equivalent responses in unisensory and congruent auditory/visual tasks across patients with AD and normal controls, patients with AD did not fuse the incongruent auditory/visual presentation of syllables as frequently as normal controls, suggesting a higher-order deficit. The similarity of our latency results across diagnostic categories for secondary somatosensory cortex, along with evidence of early amplitude changes in primary somatosensory cortex, may appear to refute the disconnection hypothesis discussed by Delbeuck and others (e.g. Lakmache et al. 1998, Tomimoto et al. 2004, Wiltshire et al. 2005). However, given the staged development and regression of white matter tracts proposed by Bartzokis, we feel that our results do not confirm or refute the general disconnection hypothesis, but may reflect a change in cSI that occurs at an earlier stage than an association area disconnection, as proposed by Delbeuck et al. (2007). Alternatively, the disconnection results identified by Delbueck and others may be related to a more subtle change since the congruent multisensory condition was not different between groups, only the incongruent condition differed. Basic somatosensory processing as seen in cortical cSII area may also be a robust response that does not differentiate between groups until later stages in the progression of the disease.
In conclusion, the larger amplitude cSI response in MCI patients relative to NE and AD suggests a possible early marker for abnormal brain function leading to AD. Increased amplitude measures seen across groups also corresponded with poorer performance on some NP tests with a sensorimotor component. Follow-up longitudinal measures are needed to confirm that MCI patients revealing larger amplitude cSI responses are the same individuals who convert from MCI to AD. Finally, the current study does not provide information about the specificity of these measures for distinguishing various types of dementia. Follow-up studies focusing on the specificity of the measures (e.g., do patients with probable AD reveal larger amplitude responses than patients diagnosed with vascular dementia?) will provide important information about the feasibility of using this potential marker as a tool for differential diagnosis.
Acknowledgments
This work was supported by NIH R01AG020302, DE-FG02-07ER64415, UNM HSC GCRC—NIH 5M01-RR00997. We thank the Mind Research Network for their excellent IT support as well as UNM Radiology and the VA Research Office for their support. We also thank Denise Padilla for coordinating the project, Jennifer Bryant for her help acquiring the data, and S. Laura Lundy for help with neuropsychological testing.
Contributor Information
Julia M. Stephen, Email: jstephen@mrn.org, The Mind Research Network, 1101 Yale Boulevard NE, Albuquerque, NM 87106, USA.
Rebecca Montaño, Department of Radiology, University of New Mexico Health Sciences Center, Albuquerque, NM, USA.
Christopher H. Donahue, Department of Radiology, University of New Mexico Health Sciences Center, Albuquerque, NM, USA
John C. Adair, Department of Neurology, University of New Mexico Health Sciences Center, Albuquerque, NM, USA Neurology Service, New Mexico VA Healthcare System, Albuquerque, NM, USA.
Janice Knoefel, Department of Internal Medicine, University of New Mexico Health Sciences Center, Albuquerque, NM, USA; Internal Medicine Service, New Mexico VA Healthcare System, Albuquerque, NM, USA.
Clifford Qualls, General Clinical Research Center, University of New Mexico Health Sciences Center, Albuquerque, NM, USA.
Blaine Hart, Department of Radiology, University of New Mexico Health Sciences Center, Albuquerque, NM, USA.
Doug Ranken, Biophysics Group (P-21), Los Alamos National Laboratory, Los Alamos, NM, USA.
Cheryl J. Aine, Department of Radiology, University of New Mexico Health Sciences Center, Albuquerque, NM, USA
References
- Abbruzzese G, Reni L, Cocito L, Ratto S, Abbruzzese M, Favale E. Short-latency somatosensory evoked potentials in degenerative and vascular dementia. J Neurol Neurosurg Psychiatry. 1984;47:1034–1037. doi: 10.1136/jnnp.47.9.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aine C, Huang M, Stephen J, Christner R. Multistart algorithms for MEG empirical data analysis reliably characterize locations and time courses of multiple sources. Neuroimage. 2000;12:159–172. doi: 10.1006/nimg.2000.0616. [DOI] [PubMed] [Google Scholar]
- Aine C, Stephen J, Christner R, Hudson D, Best E. Task relevance enhances early transient and late slow-wave activity of distributed cortical sources. J Comp Neurosci. 2003;15:203–221. doi: 10.1023/a:1025864825200. [DOI] [PubMed] [Google Scholar]
- Aine C, Adair J, Knoefel J, Hudson D, Qualls C, Kovacevic S, Woodruff C, Cobb W, Padilla D, Lee R, Stephen J. Temporal dynamics of age-related differences in auditory incidental verbal learning. Cog Brain Res. 2005;24:1–18. doi: 10.1016/j.cogbrainres.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Aine CJ, Bryant JE, Knoefel JE, Adair JC, Hart B, Donaue CH, Montano R, Hayek R, Qualls C, Ranken D, Stephen JM. Different stategies for auditory word recognition in healthy versus normal aging. Neuroimage. 2010;49:3319–3330. doi: 10.1016/j.neuroimage.2009.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G. Age-related myelin breakdown: a developmental model of cognitive decline and Alzheimer’s disease. Neurobiol Aging. 2004;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. author reply 49–62. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brumback RA, Leech RW. Alzheimer’s disease: pathophysiology and the hope for therapy. J Okla State Med Assoc. 1994;87:103–111. [PubMed] [Google Scholar]
- Delbeuck X, Collette F, Van der Linden M. Is Alzheimer’s disease a disconnection syndrome? Evidence from a cross-modal audio-visual illusory experiment. Neuropsychologia. 2007;45:3315–3323. doi: 10.1016/j.neuropsychologia.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Marra C, Daniele A, Ghirlanda S, Gainotti G, Tonali PA. Motor cortex hyperexcitability to transcranial magnetic stimulation in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004;75:555–559. doi: 10.1136/jnnp.2003.018127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geula C, Mesulam MM. Systematic regional variations in the loss of cortical cholinergic fibers in Alzheimer’s disease. Cereb Cortex. 1996;6:165–177. doi: 10.1093/cercor/6.2.165. [DOI] [PubMed] [Google Scholar]
- Golob EJ, Miranda GG, Johnson JK, Starr A. Sensory cortical interactions in aging, mild cognitive impairment, and Alzheimer’s disease. Neurobiol Aging. 2001;22:755–763. doi: 10.1016/s0197-4580(01)00244-5. [DOI] [PubMed] [Google Scholar]
- Golob EJ, Irimajiri R, Starr A. Auditory cortical activity in amnestic mild cognitive impairment: relationship to subtype and conversion to dementia. Brain. 2007;130:740–752. doi: 10.1093/brain/awl375. [DOI] [PubMed] [Google Scholar]
- Huang M, Aine CJ, Supek S, Best E, Ranken D, Flynn ER. Multi-start downhill simplex method for spatio-temporal source localization in magnetoencephalography. Electroencephalogr Clin Neurophysiol. 1998;108:32–44. doi: 10.1016/s0168-5597(97)00091-9. [DOI] [PubMed] [Google Scholar]
- Lakmache Y, Lassonde M, Gautheir S, Frigon JY, Lepore F. Interhemispheric disconnection syndrome in Alzheimer’s disease. Proc Natl Acad Sci USA. 1998;95:9042–9046. doi: 10.1073/pnas.95.15.9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuchter AF, Newton TF, Cook IA, Walter DO. Changes in brain functional connectivity in Alzheimer-type and multiinfarct dementia. Brain. 1992;115:1543–1561. doi: 10.1093/brain/115.5.1543. [DOI] [PubMed] [Google Scholar]
- Ojemann G, Ojemann J, Lettich E, Berger M. Cortical language localization in left, dominant hemisphere. An electrical stimulation mapping investigation in 117 patients. J Neurosurg. 1989;71:316–326. doi: 10.3171/jns.1989.71.3.0316. [DOI] [PubMed] [Google Scholar]
- Ortiz Alonso T, Lopez-Ibor MI, Martinez Castillo E, Fernandez Lucas A, Maestu Unturbe F, Lopez-Ibor JJ. Deficit in sensory motor processing in depression and Alzheimer’s disease: a study with EMG and event related potentials. Electromyogr Clin Neurophysiol. 2000;40:357–363. [PubMed] [Google Scholar]
- Papaliagkas V, Kimiskidis V, Tsolaki M, Anogianakis G. Usefulness of event-related potentials in the assessment of mild cognitive impairment. BMC Neurosci. 2008;9:107. doi: 10.1186/1471-2202-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- Ranken D, George J. MRIVIEW: an interactive computational tool for investigation of brain structure and function. Proceedings of IEEE visualization ‘93; Los Alamitos: IEEE Computer Society Press; 1993. pp. 321–324. [Google Scholar]
- Ranken D, Best E, Stephen J, Schmidt D, George J, Wood C, Huang M. MEG/EEG forward and inverse modeling using MRIVIEW. In: Nowak H, Haueisen J, Gießler F, Huonker R, editors. Proceedings of the 13th international conference on biomagnetism; Jena. 2002. pp. 785–787. [Google Scholar]
- Stephen JM, Aine CJ, Ranken D, Hudson D, Shih JJ. Multidipole analysis of simulated epileptic spikes with real background activity. J Clin Neurophysiol. 2003a;20:1–16. doi: 10.1097/00004691-200302000-00001. [DOI] [PubMed] [Google Scholar]
- Stephen JM, Davis LE, Aine CJ, Ranken D, Herman M, Hudson D, Huang M, Poole J. Investigation of the normal proximal somatomotor system using magnetoencephalography. Clin Neurophysiol. 2003b;114:1781–1792. doi: 10.1016/s1388-2457(03)00150-0. [DOI] [PubMed] [Google Scholar]
- Stephen JM, Ranken D, Best E, Adair J, Knoefel J, Kovacevic S, Padilla D, Hart B, Aine CJ. Aging changes and gender differences in response to median nerve stimulation measured with MEG. Clin Neurophysiol. 2006;117:131–143. doi: 10.1016/j.clinph.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Suva D, Favre I, Kraftsik R, Esteban M, Lobrinus A, Miklossy J. Primary motor cortex involvement in Alzheimer disease. J Neuropathol Exp Neurol. 1999;58:1125–1134. doi: 10.1097/00005072-199911000-00002. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Stahl R, Dietrich O, Schoenberg SO, Perneczky R, Bokde AL, Reiser MF, Moller HJ, Hampel H. Multivariate network analysis of fiber tract integrity in Alzheimer’s disease. Neuroimage. 2007;34:985–995. doi: 10.1016/j.neuroimage.2006.07.047. [DOI] [PubMed] [Google Scholar]
- Tomimoto H, Lin JX, Matsuo A, Ihara M, Ohtani R, Shibata M, Miki Y, Shibasaki H. Different mechanisms of corpus callosum atrophy in Alzheimer’s disease and vascular dementia. J Neurol. 2004;251(4):398–406. doi: 10.1007/s00415-004-0330-6. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Kohara N, Hirasawa H, Kuzuhara S, Iwata M, Mannen T. Myoclonus in Alzheimer’s disease. J Neurol. 1987;235:90–94. doi: 10.1007/BF00718016. [DOI] [PubMed] [Google Scholar]
- Uylings HB, de Brabander J. Neuronal changes in normal human aging and Alzheimer’s disease. Brain Cogn. 2002;49:268–276. doi: 10.1006/brcg.2001.1500. [DOI] [PubMed] [Google Scholar]
- Weisend M, Hanlon F, Montano R, Donahue C, Ahlfors S, Leuthold A, Mosher J, Georgeopoulos A, Hamalainen M, Aine C. Paving the way for cross-site pooling of MEG data. In: Cheyne D, Ross B, Stroink G, Weinberg H, editors. International congress series: New Frontiers in biomagnetism: proceedings of 15th international conference biomagnetism; Vancouver. 2007. [Google Scholar]
- Wiltshire K, Foster S, Kaye JA, Small BJ, Camicioli R. Corpus callosum in neurodegenerative diseases: findings in Parkinson’s disease. Dement Geriatr Cogn Disord. 2005;20(6):345–351. doi: 10.1159/000088526. [DOI] [PubMed] [Google Scholar]