Abstract
Deluded people differ from nondeluded controls on attributional style questionnaires and probabilistic-reasoning and theory-of-mind (ToM) tasks. No study to date has examined the relations between these 3 reasoning anomalies in the same individuals so as to evaluate their functional independence and potentially inform theories of delusion formation. We did so in 35 schizophrenic patients with a history of delusions, 30 of whom were currently deluded, and 34 healthy controls. Compared with healthy controls, patients showed (a) a jumping-to-conclusions bias and a bias to overadjust when confronted with a change of evidence on probabilistic-reasoning tasks, (b) an excessive externalizing attributional bias, and (c) performance deficits on 3 ToM tasks. Probabilistic-reasoning and ToM measures correlated, while attributional-bias scores were independent of other task measures. A general proneness to delusional ideation correlated with probabilistic-reasoning and ToM measures, while externalizing bias was unrelated to the study measures of delusional ideation. Personalizing bias associated specifically with paranoia across the clinical and nonclinical participants. Findings are consistent with a common underlying mechanism in schizophrenia which contributes to the anomalies on probabilistic-reasoning and ToM tasks associated with delusions. We speculate that this mechanism is impairment of the normal capacity to inhibit “perceived reality” (the evidence of our senses), a capacity that evolved as part of the “social brain” to facilitate intersubjective communication within a shared reality.
Keywords: psychosis, reality distortion, social cognition, belief
Introduction
Delusions resist definition according to a set of necessary and sufficient criteria.1–3 Yet these symptoms are a clinical reality, readily recognizable in most instances, and of particular importance in psychotic conditions like schizophrenia. The characteristic features that help define the presence of delusions are incorrigibility and incomprehensibility; delusions are espoused with a degree of conviction that is unwarranted by the evidence to hand and resist revision even when the delusional content is fantastic. A traditional psychiatric approach to incorrigible, incomprehensible delusions is to conceive of these as nonbelief-like statements or kinds of judgments that spring into existence without a meaningful context4; primary delusions, in particular, are described historically as psychologically un-understandable. An alternative approach is to conceive of delusions as resulting from breakdowns in the normal cognitive (ie, mental-processing) system for belief generation and evaluation; this is the cognitive neuropsychological approach.5 On this latter approach, delusions count as beliefs, albeit abnormal beliefs, and can be understood “psychologically,” albeit sometimes only in terms of cognitive-psychological processes that are not directly accessible to consciousness.
The “2-factor theory” developed from the application of cognitive neuropsychology to the study of delusions5–8 proposes that 2 distinct types of cognitive disturbance contribute, in combination, to the generation and adoption of a delusion. The first disturbance explains why an implausible thought with a particular content comes to mind in the first place, thus accounting for the delusional theme. The second disturbance explains why the implausible thought is accepted uncritically as true.
Recent advances in the cognitive sciences have highlighted a number of cognitive disturbances that contribute to the generation of specific delusional themes. Capgras delusion, eg, the delusional belief that a loved one has been replaced by an identical looking impostor, arises when intact visual processing of a familiar face combines with a loss of the normal autonomic (measured using skin conductance responses) sense of familiarity that ought to be triggered by the sight of that face .9,11 This disconnection generates the implausible thought that the person in front of one, despite claiming to be a loved one and recognized as such by other family members, is a stranger whose face merely resembles that of the loved one. Frith, Blakemore, and colleagues11–13 have provided evidence that alien control thoughts come to mind when the internal monitoring of intended actions is disrupted such that actions are experienced with no accompanying sense of agency. A further example is implausible referential ideas, which, several authors14–16 have suggested, arise when heightened salience is assigned inappropriately to mundane events in the environment.
Such disruptions contribute to the understanding of delusions but fall short of a full account because they fail to explain the uncritical adoption of the implausible thoughts (generated by the first cognitive disturbance) as true. People with depersonalization disorder, eg, might think it is as if others are controlling their actions but do not believe this to be true. Likewise, people who experience déjà vu might feel as if they have been in a particular situation before but know this cannot be so. Recent reviews17,53 have highlighted 3 reasoning anomalies that might contribute to the uncritical adoption of implausible thoughts in deluded people: (a) a jumping-to-conclusions (JTC) bias on probabilistic-reasoning tasks, (b) extreme causal attributional biases, and (c) difficulties with appreciating other people's perspectives on theory-of-mind (ToM) tasks.
Garety and colleagues19–22 pioneered the work on JTC and delusions. In a typical paradigm, participants are shown 2 jars of colored beads (in reverse ratios) and are asked to nominate from which jar a purportedly random series of beads is drawn. The common finding is that deluded people decide after fewer draws than nondeluded people; up to half of deluded participants might decide after 1–2 draws. Bentall and colleagues23 pioneered the work on attributional biases and delusions. Kaney and Bentall24 first used an attributional style questionnaire to show that persecutory-deluded people excessively externalize blame for negative events. Kinderman and Bentall25 later showed that these individuals also excessively blame other people rather than circumstances. Frith26 pioneered work on ToM and delusions; he proposed that a difficulty with making appropriate inferences of others’ thoughts and intentions (ie, a poor ToM) might explain persecutory and referential delusions. Numerous studies have since demonstrated pervasive ToM deficits in schizophrenia, although results are equivocal concerning a specific link with persecutory/referential delusions (see Brune28 and Langdon28,29 for reviews).
While theorizing about JTC has focused on the contribution of a liberal acceptance (or data-gathering) bias to delusion-proneness (irrespective of delusional theme), theorizing about the attributional-bias and ToM findings has focused, instead, on explaining specific delusional themes. The attributional-bias findings, eg, have been interpreted to support the defensive attribution model30; people with specifically persecutory delusions subconsciously defend against activating latent-negative self-beliefs by externalizing blame. Because JTC purportedly associates with a general vulnerability to delusions, while attributional biases and ToM purportedly associate with specific delusional themes, it has been implicitly assumed that the 3 reasoning anomalies are quite distinct, indeed functionally independent. This assumption has not, however, been empirically evaluated.
Moreover, the attributional-bias and ToM anomalies might, just like the JTC bias, promote a general vulnerability to accept implausible thoughts as true (irrespective of delusional theme). Externalizing bias (EB) might, eg, be associated with a general failure to self-correct an initial implausible thought (“God is speaking to me”) with an internalizing attribution (“something wrong with my mind is causing me to hear voices”).31,32 Likewise, ToM impairment might reflect an egocentric difficulty with taking on, in imagination, other points of view,28,29 thus compromising general reality-testing. If all 3 reasoning anomalies promote a general vulnerability to delusions, as suggested, it seems plausible to hypothesize that they might also be related, and, if related, dependent upon a common underlying mechanism. In order to explore these possibilities and potentially inform theories of delusions, we examined probabilistic reasoning, attributional biases, and ToM in the same samples of people with schizophrenia and healthy controls and employed correlation techniques to examine the relationships of task measures with state and trait ratings of delusional thinking and paranoid ideation.
Methods
Participants
Thirty-five clinical participants took part. Of them, 30 had a Diagnostic and Statistical Manual of Mental Disorder, Fourth Edition (DSM-IV), diagnosis of schizophrenia and 5 were diagnosed with schizoaffective disorder. Patients were recruited from the New South Wales Area Health Services of the Sydney South West and the Hunter Areas and volunteer registers established by the Macquarie Centre for Cognitive Science and the Schizophrenia Research Institute of Australia. Diagnosis was confirmed using the Diagnostic Interview for Psychosis (DIP).33 The DIP revealed that all patients had a history of marked delusions, although not all patients were acutely delusional at the time of testing. Thirty were currently deluded and 15 had current delusions of moderate-to-severe levels (ie, all 15 were convinced of their delusions with variable impact on the patients’ daily preoccupations and activities). Persecution was the most common theme, being present in 23 of the patients, with loss of boundary delusions being the next most common, present in 17 of the patients. The persecutory delusions were moderate to severe in 10 of these 23 patients. Thirteen of the persecutory-deluded patients also reported grandiose delusions, while 12 also reported delusions of reference. Among those patients with current nonpersecutory delusions, 5 patients reported loss of boundary delusions and 2 had delusions of guilt. Hallucinations were present in 19 of the patients. Table 1 summarizes the ranges and the mean severity levels of the clinical symptoms.
Table 1.
Demographics and Levels of Depression, Delusion-Proneness, and Paranoia for Both Groups, as well as Clinical Demographics and Symptom Ratings for Patients
| Patients | Healthy Controls | Significance Test | |
| N | 35 | 34 | |
| Males:females | 23:12 | 26:8 | χ21 = 0.97 |
| Age (years) | 35.9 ± 10.4 (18–59) | 32.0 ± 12.9 (20–58) | t67 = 1.37 |
| Depression | 7.9 ± 6.6 (0–28) | 4.1 ± 4.0 (0–14) | t67 = 2.86** |
| Delusion-proneness | 9.9 ± 4.3 (2–20) | 6.6 ± 2.6 (3–12) | t67 = 3.82**** |
| Paranoia | 56.9 ± 15.7 (29–89) | 43.0 ± 11.3 (24–66) | t58 = 4.20**** |
| Clinical demographics | |||
| Age of illness onset (years) | 22.8 ± 6.8 (15–42) | ||
| Duration of illness (years) | 12.6 ± 9.1 (1.5–41) | ||
| Clinical symptomsa | |||
| Delusions | 2.4 ± 1.6 (0–5) | ||
| Persecutory delusions | 1.7 ± 1.6 (0–5) | ||
| Hallucinations | 1.8 ± 1.9 (0–5) | ||
| Bizarre behavior | 1.0 ± 1.1 (0–3) | ||
| Positive thought disorder | 1.5 ± 1.2 (0–4) | ||
| Negative symptoms | 1.6 ± 0.9 (0–4) | ||
Note: Data expressed as means ± SD (range in parentheses).
0 = not present; 1 = questionable; 2 = mild; 3 = moderate; 4 = marked; 5 = severe.
**P < .01; ****P < .0005.
Thirty-four healthy controls were recruited from the general community and from among mature-age University students to match the patient group on years of age, gender ratio, and IQ estimated using the National Adult Reading Test (NART: see Table 1). Healthy controls were screened using the affective, psychotic, and substance abuse screening modules from the Structured Clinical Interview for DSM-IV Axis 1 Disorders (SCID-I).34 Exclusion criteria for both groups included past history of central nervous system disease or head injury, current substance abuse, and less than 8 years of formal education. All participants were English-speaking and gave written informed consent.
Materials and Procedure
Baseline Measures.
IQ was assessed using the NART, while memory span was assessed using the Wechsler Adult Intelligence Scale-Revised Digits Forward and Backward subtests. The Wechsler Memory Scale-Revised Logical Memories subtests (LMI, LMII) provided a measure of prose recall. The memory measures were included because previous work suggests reduced memory span contributes to a JTC bias35 and because the verbal eliciting stimuli used in traditional ToM tasks place heavy demands on prose recall.36
Probabilistic Reasoning.
There were 2 versions of the “beads task.”19 In the first “decide” version, participants were shown 2 jars of red/green beads in complementary ratios (85:15) and asked to decide from which jar a purportedly random sequence of beads was drawn (McKay, Langdon, Coltheart37,38 for details). The number of draws taken to reach a decision was recorded as was decision confidence rated using a 6-point Likert scale (from 50-50 unsure to 100% certain).
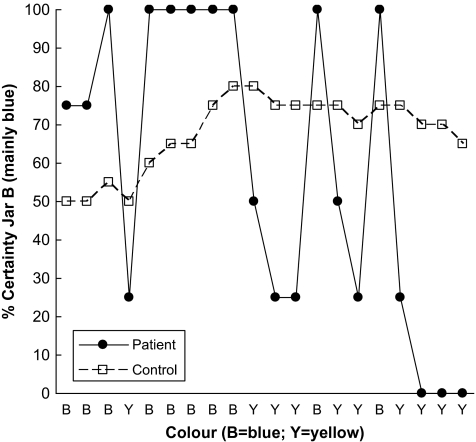
In the second “estimate” version, participants were again presented with 2 jars of beads, now blue/yellow, in similar ratios. For each consecutive draw of a total of 20 draws, they now indicated how certain they were the beads came from one or the other jar. This they did by marking a cross anywhere on a 10-cm rating scale ranging from “100% sure jar A” to “100% sure jar B.” The sequence of draws was in fact predetermined (as it was for the decide version); the first 10 draws were consistent with the beads being drawn from jar B, while the next 10 draws were consistent with the beads being drawn from jar A. All ratings were converted to scores ranging from 0 to 100 (indicating certainty in jar B) and ratings at trials 1, 10, and 20 were analyzed. We also calculated “shift in certainty”: the average change in certainty whenever the bead changed color. This was to capture the effect that many patients “overadjusted,” an effect observed previously, particularly in deluded schizophrenic patients21,40 (see figure 1 for an illustration).
Fig. 1.
Certainty Ratings From Trials 1 to 20 on the “Estimate” Probabilistic-Reasoning Task for One of the Most Extreme Patients and a Cautions Healthy Control.
Three sequences of draws were taken from Garety et al21 and allocated to the decide version, the first 10 trials of the estimate version and the next 10 trials in a counterbalanced manner so as to create 3 versions of the task. Because the draws-to-decision variable has most reliably demonstrated extreme biases in previous studies,39,40 this version always came first.
Attributional Biases.
The Internal, Personal and Situational Attributions Questionnaire (IPSAQ41) assessed attributional biases. The IPSAQ is a self-report instrument comprising 16 positive and 16 negative events presented to participants in a fixed pseudorandom order. Participants imagine each event, generate the single, most likely reason for that event, and then indicate its primary cause by selecting whether that reason is due primarily to something about themselves (self attribution), something about other people (external-personal attribution), or something about the situation (external-situational attribution). EB was calculated as percent positive self attributions minus percent negative self-attributions. Personalizing bias (PB) was calculated as percent externally attributed negative events (external-personal or external-situational) that were attributed to other people.
Theory-of-Mind.
There were 3 ToM tasks. The first comprised 8 ToM stories testing comprehension of story characters’ mental states and 8 control stories which controlled for general comprehension abilities, with the length of stories and the sentence complexities matched across ToM and control conditions.42 Participants read stories, presented in a fixed pseudorandom order, silently at their own pace, and then turned the page to reveal a question (eg, “Why did the burglar do that?”). Responses were recorded and scored 0–2 as recommended. Scores were averaged across the 8 stories/set. The second task was a joke-appreciation task based on Happé et al42 and comprised 11 ToM cartoons that required appreciation of cartoon characters’ mental states in order to “get the joke” and 11 control cartoons which depicted slapstick or behavioral/situational humor and could be understood without inferring mental states. The 22 cartoons were intermixed and presented in a fixed pseudorandom order with participants instructed to “explain the joke.” Responses were recorded and scored 0–3 as recommended. Scores were averaged across the 11 cartoons/set. For both the story-comprehension and the joke-appreciation tasks, the number of words generated by participants was also recorded and averaged across each type of story/joke (having excluded “don't know” responses). The third task was a picture-sequencing task43 comprising 4 sets of 4-card pictures stories. False-belief stories test ToM by assessing the capacity to go beyond objective facts so as to infer a character's mistaken belief, while social-script, mechanical, and capture stories control for logical social-script reasoning, cause-and-effect reasoning, and inhibitory control, respectively. Participants rearrange picture cards to show a logical sequence of events. The order of cards was recorded and scored as recommended. Scores were averaged across each type of story (range 0–6).
Questionnaires.
The Peters et al44 Delusions Inventory (PDI) indexed a general proneness to delusional ideation. The PDI demonstrates good internal consistency and concurrent validity and has been used with both clinical and nonclinical groups.45 Participants responded “yes/no” to 21 questions and the number of “yes” responses was used to index delusion-proneness (range 0–21). Participants also completed the Paranoia Scale (PS46) to index a more specific proneness to paranoid ideation. The PS is a 20-item self-report inventory using a 5-point Likert scale with good internal consistency, test-retest reliability, and construct validity.46 Total scores range from 20 to 100. The PS was developed for use with nonclinical adults but has since been used to assess paranoid ideation (not necessarily of delusional intensity) in schizophrenia.47,48
Interviews.
Patients were interviewed using the DIP to confirm diagnosis and the Scales for Assessing Positive and Negative Symptoms of Schizophrenia49,50 to rate symptom severity. Because an excessive internalizing, as opposed to an excessive externalizing, bias is associated specifically with depression, we also assessed levels of depression in all the participants using the Hamilton51 Depression Scale (HDS: range 0–52). The HDS is reported to have good reliability and validity.52 Healthy controls were screened for the presence of psychotic and affective disorders, as well as substance abuse, using the SCID screening modules.
Results
Patients were more depressed than controls and self-reported more paranoia and higher levels of delusion-proneness (see table 1).
Table 2 summarizes IQ, memory, and probabilistic-reasoning results. Group differences in IQ were nonsignificant; however, patients showed significant memory impairments.
Table 2.
Basic Abilities (IQ and Memory) and Probabilistic-Reasoning Measures for Patients and Controls
| Digit Span |
Logical Memories |
Probabilistic Reasoning |
|||||||||
| IQ | DF | DB | LMI | LMII | Draws to Decisiona | Confidence | Draw 1 Certainty | Draw 10 Certainty | Draw 20 Certainty | Shift in Certaintya | |
| Patients | 100.0 ± 13.3 | 7.3 ± 1.9 | 5.7 ± 2.2 | 17.0 ± 7.1 | 12.0 ± 7.9 | 2.0 | 83.7 ± 13.3 | 69.0 ± 20.4 | 71.2 ± 31.7 | 43.2 ± 33.4 | 28.6 |
| Controls | 105.0 ± 8.2 | 9.3 ± 2.5 | 8.4 ± 2.7 | 27.7 ± 6.3 | 22.7 ± 6.7 | 4.0 | 84.8 ± 12.6 | 62.4 ± 18.9 | 86.8 ± 18.9 | 56.3 ± 29.8 | 4.2 |
| t67 = 1.86 | t67 = 3.74*** | t67 = 4.57*** | t67 = 6.43*** | t67 = 6.15*** | M-W Z = 2.24* | t67 = 0.72 | Wilk's F3,65 = 2.46 | M-W Z = 4.36*** | |||
Note: Data expressed as means ± SD for parametric results and median for nonparametric results. M-W, Mann-Whitney; DF, Digits Forward; DB, Digits Backward.
Median values reported.
*P < .05; ***P < .0005.
In the following analyses, nonparametric statistics are reported whenever distributions were skewed.
Probabilistic-Reasoning Measures
Neither the memory measures nor the IQ were significant predictors of any probabilistic-reasoning measure.
Decide Version.
While 4 healthy controls (11.8%) reached a decision after only one draw, the proportion of patients doing so was significantly greater (34.3%), χ21 = 4.91, P = .03. Because the taking of 2 or fewer draws has also been used as a criterion for extreme JTC53 we likewise compared the proportions of patients and controls who decided within 2 draws, and the results were generally similar, χ21 = 3.47, P = .05. A Mann-Whitney test also revealed a significant group difference in draws to decision, Mann-Whitney Z = 2.24, P = .03, with patients, as a group, deciding after fewer draws. Of note, it was the healthy controls with higher PDI scores (indexing a greater propensity to delusional ideation) who decided after fewer draws, rho = −.51, P = .002.
The group difference in decision confidence was nonsignificant, P = .72.
Estimate Task Results.
Certainty ratings at trials 1, 10, and 20 were analyzed using a global test, revealing nonsignificant group differences, Wilk's F3,65 = 2.46, P = .07.
However, while patients’ and controls’ certainty levels at trials 1, 10, and 20 were generally similar, the 2 groups varied significantly in their pattern of responding from trial to trial. In particular, a Mann-Whitney test revealed a highly significant group difference in the shift in certainty, Z = 4.36, P < .0005. The median shift in certainty for patients was 28.6% compared with only 4.2% for controls.
Attributional Biases
Table 3 summarizes results. (One patient failed to generate likely causes for a majority of events and was excluded from these analyses.) While neither the memory measures nor the IQ were significant predictors of EB, there was a significant negative correlation with depression in the patients, r = −.37, P = .03; patients who were more depressed were more inclined to internalize, rather than externalize, the blame for negative events. While an initial t test revealed a significant group difference in EB, t67 = 2.05, P = .04, this difference was far more marked when an analysis of covariance (ANCOVA) was used to adjust for the levels of depression, F1,65 = 7.10, P = .01. Overall, patients externalized blame more than controls.
Table 3.
Estimated Marginal Means (±SD) for Attributional-Bias and ToM Scores for Patients and Controls
| ToM |
||||||||||
| Attributional Biases |
Story-Comprehension Taska |
Joke-Appreciation Taska |
Picture-Sequencing Taskb |
|||||||
| EBc | PBd | ToM | Physical | ToM | Physical | False-Belief (ToM) | Social-Script | Mechanical | Capture | |
| Patients | 20.6 ± 22.3 | 70.0 ± 30.0 | 0.77 ± 0.27 | 0.85 ± 0.39 | 1.42 ± 0.34 | 1.95 ± 0.49 | 3.83 ± 1.59 | 5.53 ± 0.65 | 5.15 ± 0.93 | 3.40 ± 1.02 |
| Controls | 4.4 ± 25.9 | 57.0 ± 26.0 | 1.10 ± 0.27 | 0.97 ± 0.31 | 1.66 ± 0.55 | 2.00 ± 0.30 | 5.52 ± 0.49 | 5.83 ± 0.28 | 5.50 ± 0.75 | 4.45 ± 0.87 |
| Statistics | F1,65 = 7.10** | F1,65 = 2.74 | Interaction: F1,66 = 3.79# | Interaction: F1,66 = 16.97*** | Interaction: F3,192 = 10.94*** | |||||
#P = .056; **P < .1; ***P < .0005.
Adjusted for prose recall (LM) and word count.
Adjusted for both memory measures (LM and Digits) and IQ.
Adjusted for depression levels.
Adjusted for IQ and memory (Digits).
With regards PB, patients with better cognitive function, whether indexed by IQ, r = .43, P = .01, or Digits, r = .41, P = .02, were more inclined to blame other people rather than circumstances for negative events. An initial t test revealed a nonsignificant group difference in PB, t67 = .76, P = .45, with results remaining nonsignificant when an ANCOVA was used to adjust for IQ and memory, F1,65 = 2.74, P = .10.
Theory of Mind
ToM results are summarized in table 3. The designs for the story-comprehension and the joke-appreciation tasks were similar: (2 × 2) mixed models with 2 levels on the between-factor group (patients vs controls) and 2 levels on the repeated factor story/cartoon type (ToM vs control). Neither IQ nor Digits, P > .10, were significant predictors of story-comprehension scores, once LM, indexing prose recall, and word count had been taken into account. The latter 2 variables were significant independent predictors, P < .03; the more words the participants generated and the better their verbal comprehension/memory, the higher their story-comprehension scores. LM and word count were therefore included as covariates in an ANCOVA, the results of which revealed a significant main effect of group, F1,65 = 10.72, P = .002, and a near-significant 2-way interaction of group × story type, F1,66 = 3.79, P = .056. Simple contrasts investigating the 2-way interaction, adjusting for covariates, revealed that the patients’ ToM scores were significantly lower than those of the controls, P < .0005, while the scores for the control stories did not differ between groups, P = .22.
While the pattern of results was generally as expected, we were concerned that we may have been too conservative in using word count as a covariate. This is because the direction of causation might have been such that a basic difficulty with comprehending stories caused both a poor story-comprehension score and the generation of few words. In other words, by covarying word count, we might have been reducing power by adjusting for another index (perhaps, a less sensitive index) of the primary construct of interest—ie, story-comprehension capacity. We therefore reanalyzed results excluding word count as a covariate and relied solely upon the independent LM measure of verbal comprehension/memory as the covariate. This reanalysis revealed a significant 2-way interaction of group × story type, F1,66 = 5.96, P = .02.
But we think that the better test of the specificity of any ToM difficulty in the patients on this task is to compare the patients’ and the healthy controls’ ToM scores, while adjusting for their performances on the control stories. This is because the control stories were designed specifically to control for baseline comprehension of written stories of matched length and sentence complexity. This ANCOVA revealed a highly significant group difference, F1,66 = 25.20, P < .0005.
In order to be consistent, we then carried out the same series of analyses for the joke-appreciation task. LM and word count, P < .01, and neither IQ nor Digits, P > .30, were once again significant predictors of joke-appreciation scores; the more words the participants generated and the better their verbal comprehension/memory, the higher their joke-appreciation scores. An ANCOVA adjusting for these 2 measures then revealed a significant main effect of joke type, F1,66 = 115.35, P < .0005, and a highly significant 2-way interaction of group × joke type, F1,66 = 16.97, P < .0005. Simple contrasts, adjusting for the 2 covariates, indicated that there were group differences on the ToM jokes, P = .047, but not on the control jokes, P = .66. Results were similar when word count was removed as a covariate; the 2-way interaction was similarly significant, F1,66 = 6.46, P < .01. As argued above, however, we think that the more telling test of the specificity of the patients’ ToM difficulty on this task is to compare the patients’ and the healthy controls’ scores on the ToM jokes, adjusting for the participants’ performances on the control jokes. This analysis revealed a highly significant group difference, F1,66 = 17.77, P < .0005.
The design for the picture-sequencing task was a (2 × 4) mixed model with 2 levels on the between-factor group (patients vs controls) and 4 levels on the repeated factor sequence type (social-script vs mechanical vs capture vs false-belief). Both memory measures were significant predictors of false-belief scores across groups, P < .005, while IQ predicted performances on some control sequences in the controls (but not the patients), P < .03. In order to be conservative, we therefore included both memory measures and IQ as covariates in an initial ANCOVA which revealed the only significant result to be a 2-way interaction of group × sequence type, F2.62,165.30 = 6.66, P = .001 (Greenhouse-Geisser corrections are reported because the assumption of sphericity was violated). Simple adjusted contrasts revealed that the patients performed more poorly than the controls on both the false-belief, P < .0005, and the capture sequences, P < .002, with no significant group differences on the social-script and mechanical sequences, P > .08. A post hoc ANCOVA, adjusting for the capture scores, as well as the memory and IQ scores, then revealed that the patients still made significantly more errors than the controls when sequencing the false-belief stories (testing ToM), F1,62 = 8.00, P = .006. Similarly, when the false-belief scores of the patients and the controls were compared, adjusting for the participants’ performances on the control sequences, the patients continued to show a selective deficit on the false-belief sequences (which test ToM understanding), F1,64 = 14.96, P < .0005.
Findings thus far are consistent with the co-occurrence, in a general sample of schizophrenia patients who have a history of delusions of (a) a JTC bias and a bias to overadjust in the face of changing evidence on probabilistic-reasoning tasks, (b) a more extreme externalizing (but not personalizing) attributional bias, and (c) ToM difficulties across 3 tasks. Next, we examine whether there is correlation as well as co-occurrence.
Interrelationships Between Task Measures and Relations Between Task Measures and Ratings of Delusional Ideation
First, a composite ToM score was calculated by standardizing and summing z scores for the story-comprehension, joke-appreciation, and false-belief ToM scores. (Results were similar if the ToM story-comprehension score was excluded from this composite measure.) This composite score was then correlated with draws to decision, shift in certainty, EB, and PB. Alpha was set at .01. All results involving EB and PB were nonsignificant. (We also used partial correlations to adjust for levels of depression in the case of EB and to adjust for cognitive function indexed by IQ and Digits scores in the case of PB and results were similar.) There was, however, a significant negative correlation between draws to decision and shift in certainty which was present across groups, rho = −.53, P < .0005, within patients, rho = −.53, P = .001, and within controls, rho = −.47, P = .005. Draws to decision also correlated with the ToM score across groups, rho = .44, P < .0005, and within patients, rho = .57, P < .0005, although not within controls, rho = .14, P = .42. Examination of scatterplots revealed that the latter null result reflected a tendency to ceiling effects on the composite ToM score in the controls. The correlation between ToM and shift in certainty was also significant across groups, rho = −.50, P < .0005, although not within either the patients or the controls, P > .10.
Correlations between task measures and trait and state levels of delusional ideation were examined next. Somewhat surprisingly, all results involving EB were nonsignificant. There was, however, a tendency for PB to be associated with paranoia across groups, r = .27, P = .02, although not within either the patients or the controls, P > .04. Draws to decision correlated negatively with levels of delusion-proneness across groups, rho = −.44, P < .0005, and within controls, rho = −.51, P = .002, although not within patients, rho = −.28, P = .10. Once again, examination of scatterplots revealed that the latter null result reflected a tendency to ceiling effects for the delusion-proneness measure in the patients. There was also a negative correlation between draws to decision and the clinical ratings of delusions, although this was nonsignificant, rho = −.27, P = .12. Moreover, there was a significant negative correlation between ToM and clinical ratings of delusions, rho = −.44, P = .008, while the correlation with levels of delusion-proneness was also significant across groups, rho = −.38, P = .001, although not within either the patients or the controls, P > .10.
Discussion
When compared with controls, a general sample of 35 schizophrenic patients with a history of delusions and 30 reporting current delusions showed (a) a JTC bias and a bias to overadjust certainty in line with current data on probabilistic-reasoning tasks, (b) an extreme externalizing attributional bias, and (c) performance deficits on 3 ToM tasks. While levels of PB did not differ between patients and controls, PB was found to be associated with levels of paranoid ideation across clinical and nonclinical participants. Our findings are thus generally consistent with the results of previous studies which have separately examined each of probabilistic reasoning, attributional style, and ToM. Our findings add to this literature by indicating that the attributional biases are unrelated to the other reasoning anomalies, while the probabilistic-reasoning and ToM anomalies appear related. ToM scores also correlated with ratings of delusional ideation, although it was the draws-to-decision measure that associated most robustly with a general vulnerability to delusions. PB, but not EB, was found to be associated more specifically with levels of paranoia across the clinical and nonclinical participants. While there was no evidence in our clinical and nonclinical samples of an association between EB and a proneness to paranoid ideation, it may have been that our measure of paranoid ideation (the PS) was not specific enough. Freeman,54 eg, have argued that paranoia is a much broader construct, with connotations going well beyond persecution (including, eg, jealousy, ideas of reference, and even grandiosity). Martin and Penn,55 like ourselves, also failed to find evidence of a relationship between EB and paranoid ideation, and these authors suggested that such a relationship may only exist with more specific persecutory paranoid ideation.
Overall, our results suggest that a common underlying mechanism in people with schizophrenia contributes to the probabilistic-reasoning anomalies and the ToM deficits (but not the attributional biases) that have been linked to delusional ideation. Results also suggest that this commonality is not attributable to poor IQ or impaired memory. Theorizing about the role of probabilistic and ToM anomalies in delusion formation thus need to consider mechanisms which are more general than task-specific processes such as setting decision thresholds on probabilistic-reasoning tasks or representing other people's thoughts as decoupled from reality (purportedly underpinned by medial prefrontal circuitry) on ToM tasks.
As to what this mechanism might be, we draw upon the following:
From the delusion literature, that deluded people have a difficulty with overriding the automatic salience of first-person evidence in order to give equal weight to one's own experience, the views of other people, and third-person general knowledge5;
From the ToM literature, that performance deficits on ToM tasks in schizophrenia reflect an egocentric difficulty with representing multiple concurrent, yet potentially discordant, viewpoints of the same here-and-now reality29,30,56; and
From Hemsley's57–59 work, that schizophrenia is characterized by a weakening of the influence of stored memories of past regularities on current perception.
While acknowledging that our ideas are preliminary because they rely on correlational data rather than any direct test of our theory, we speculate that the common underlying mechanism in schizophrenia is a difficulty with inhibiting thoughts that present relatively directly to consciousness as the “perceived reality.” This would explain the tendencies of people with schizophrenia to be swayed more by current data when making decisions on probabilistic-reasoning tasks and the difficulties with going beyond objective facts, as presented on ToM tasks, so as to infer other people's beliefs. This inhibitory impairment also dissociates from the excessive attributional biases also seen in schizophrenia. While the latter may reflect self-defense mechanisms triggered by perceived threat, the former may reflect brain dysfunction, consistent with recent evidence that a failure to inhibit the “self-perspective” (information presented directly to participants on classic false-belief ToM tasks) causes poor ToM task performances in patients with right-frontal brain injury,60,61 an area of the brain associated with normal belief revision.7
In conclusion, we suggest that the probabilistic-reasoning anomalies and the ToM deficits that have been associated with delusions are, in part, functionally related in schizophrenia and depend upon a common impairment of the capacity to inhibit perceived reality. Moreover, we note that this inhibitory impairment cannot be reduced to a generalized frontal dysfunction because ToM deficits are independent of co-occurring executive deficits in schizophrenia.62,63 We suggest, instead, that specific inhibitory processes for distancing ourselves from perceived reality (“the evidence of our senses”) likely evolved as a part of the “social brain” so as to facilitate the imagining of other subjective viewpoints, thus promoting intersubjective communication within a shared reality.
Funding
Australian Research Council Research Fellowship to R.L.
Acknowledgments
Thanks to the Schizophrenia Research Institute, Australia, for assistance with clinical participant recruitment, and to Jennifer McLaren and Tonia Corner of the Macquarie Centre for Cognitive Science for research assistance support.
References
- 1.David AS. On the impossibility of defending delusions. Philos Psychiatr Psychol. 1999;6:17–20. [Google Scholar]
- 2.Oltmanns TF. Approaches to the definition and study of delusions. In: Oltmanns TF, Maher BA, editors. Delusional Beliefs. Oxford, England: John Wiley & Sons; 1988. pp. 3–11. [Google Scholar]
- 3.Spitzer M. On defining delusions. Compr Psychiatry. 1990;31:377–397. doi: 10.1016/0010-440x(90)90023-l. [DOI] [PubMed] [Google Scholar]
- 4.Spitzer M. The phenomenology of delusions. Psychiatr Ann. 1992;22:252–259. [Google Scholar]
- 5.Langdon R, Coltheart M. The cognitive neuropsychology of delusions. Mind Lang. 2000;15:184–218. [Google Scholar]
- 6.Davies M, Coltheart M, Langdon R, Breen N. Monothematic delusions: towards a two factor account. Philos Psychiatr Psychol. 2002;8:133–158. [Google Scholar]
- 7.Coltheart M, Langdon R, McKay R. Schizophrenia and monothematic delusions. Schizophr Bull. 2007;33:642–647. doi: 10.1093/schbul/sbm017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langdon R, McKay R, Coltheart M. The cognitive neuropsychological understanding of persecutory delusions. In: Freeman D, Bentall R, Garety P, editors. Persecutory Delusions: Assessment, Theory and Treatment. Oxford, England: Oxford University Press; In press. [Google Scholar]
- 9.Ellis HD, Young AW, Quayle AH, DePauw KW. Reduced autonomic responses to faces in Capgras delusion. Proc Biol Sci. 1997;264:1085–1092. doi: 10.1098/rspb.1997.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellis HD, Young AW. Accounting for delusional misidentifications. Br J Psychiatry. 1990;157:239–248. doi: 10.1192/bjp.157.2.239. [DOI] [PubMed] [Google Scholar]
- 11.Blakemore SJ. Deluding the motor system. Conscious Cogn. 2003;12:647–655. doi: 10.1016/j.concog.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Blakemore SJ, Wolpert DM, Frith CD. Abnormalities in the awareness of action. Trends Cogn Sci. 2002;6:237–242. doi: 10.1016/s1364-6613(02)01907-1. [DOI] [PubMed] [Google Scholar]
- 13.Frith C. The self in action: lessons from delusions of control. Conscious Cogn. 2005;14:752–770. doi: 10.1016/j.concog.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Anscombe R. The disorder of consciousness in schizophrenia. Schizophr Bull. 1987;13:241–260. doi: 10.1093/schbul/13.2.241. [DOI] [PubMed] [Google Scholar]
- 15.Gray JA. The contents of consciousness: a neuropsychological conjecture. Behav Brain Sci. 1995;18:659–722. [Google Scholar]
- 16.Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 17.Bell V, Halligan PW, Ellis HD. Explaining delusions: a cognitive perspective. Trends Cogn Sci. 2006;10:219–226. doi: 10.1016/j.tics.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Garety PA, Freeman D. Cognitive approaches to delusions: a critical review of theories and evidence. Br J Clin Psychol. 1999;38:113–154. doi: 10.1348/014466599162700. [DOI] [PubMed] [Google Scholar]
- 19.Huq SF, Garety PA, Hemsley DR. Probabilistic judgements in deluded and non-deluded subjects. Q J Exp Psychol. 1988;40A:801–812. doi: 10.1080/14640748808402300. [DOI] [PubMed] [Google Scholar]
- 20.Garety P. Reasoning and delusions. Br J Psychiatry. 1991;159:14–18. [PubMed] [Google Scholar]
- 21.Garety PA, Hemsley DR, Wessely S. Reasoning in deluded schizophrenic and paranoid patients: biases in performance on a probabilistic inference task. J Nerv Ment Dis. 1991;179:194–201. doi: 10.1097/00005053-199104000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Garety PA, Freeman D, Jolley S, et al. Reasoning, emotions, and delusional conviction in psychosis. J Abnorm Psychol. 2005;114:373–384. doi: 10.1037/0021-843X.114.3.373. [DOI] [PubMed] [Google Scholar]
- 23.Bentall RP, Corcoran R, Howard R, Blackwood N, Kinderman P. Persecutory delusions: a review and theoretical integration. Clin Psychol Rev. 2001;21:1143–1192. doi: 10.1016/s0272-7358(01)00106-4. [DOI] [PubMed] [Google Scholar]
- 24.Kaney S, Bentall RP. Persecutory delusions and attributional style. Br J Med Psychol. 1989;62:191–198. doi: 10.1111/j.2044-8341.1989.tb02826.x. [DOI] [PubMed] [Google Scholar]
- 25.Kinderman P, Bentall RP. Causal attributions in paranoia and depression: internal, personal, and situational attributions for negative events. J Abnorm Psychol. 1997;106:341–345. doi: 10.1037//0021-843x.106.2.341. [DOI] [PubMed] [Google Scholar]
- 26.Frith CD. The Cognitive Neuropsychology of Schizophrenia. Hillsdale, NJ: Lawrence Erlbaum Associates; 1992. [Google Scholar]
- 27.Brune M. “Theory of Mind” in schizophrenia: a review of the literature. Schizophr Bull. 2005;31:21–42. doi: 10.1093/schbul/sbi002. [DOI] [PubMed] [Google Scholar]
- 28.Langdon R. Theory of mind and social dysfunction: psychotic solipsism versus autistic asociality. In: Repacholi B, Slaughter V, editors. Individual Differences in Theory of Mind: Implications for Typical and Atypical Development. New York, NY: Psychology Press; 2003. pp. 241–270. Macquarie Monographs in Cognitive Science. [Google Scholar]
- 29.Langdon R. Theory of mind in schizophrenia. In: Malle B, Hodges S, editors. Other Minds; How Humans Bridge the Divide between Self and Others. New York, NY: Guilford Press; 2005. pp. 333–342. [Google Scholar]
- 30.Bentall RP, Kinderman P, Kaney S. The self, attributional processes and abnormal beliefs: towards a model of persecutory delusions. Behav Res Ther. 1994;32:331–341. doi: 10.1016/0005-7967(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 31.Garety PA, Kuipers E, Fowler D, Freeman D, Bebbington PE. A cognitive model of the positive symptoms of psychosis. Psychol Med. 2001;31:189–195. doi: 10.1017/s0033291701003312. [DOI] [PubMed] [Google Scholar]
- 32.Langdon R, Corner T, McLaren J, Ward PB, Coltheart M. Externalizing and personalizing biases in persecutory delusions: the relationships with poor insight and theory of mind. Behav Res Ther. 2006;44:699–713. doi: 10.1016/j.brat.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Castle DJ, Jablensky A, McGrath JJ. The diagnostic interview for psychoses (DIP): development, reliability and applications. Psychol Med. 2006;36:69–80. doi: 10.1017/S0033291705005969. [DOI] [PubMed] [Google Scholar]
- 34.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P, Version 2.0) New York, NY: New York State Psychiatric Institute, Biometrics Research Department; 1996. [Google Scholar]
- 35.Menon M, Pomarol-Clotet E, McKenna PJ, McCarthy RA. Probabilistic reasoning bias in schizophrenia—the role of memory. Schizophr Res. 2003;60(suppl. 1):176. [Google Scholar]
- 36.Corcoran R, Frith CD. Autobiographical memory and theory of mind: evidence of a relationship in schizophrenia. Psychol Med. 2003;33:897–905. doi: 10.1017/s0033291703007529. [DOI] [PubMed] [Google Scholar]
- 37.McKay R, Langdon R, Coltheart M. Jumping to delusions? Paranoia, probabilistic reasoning and need for closure. Cognit Neuropsychiatry. 2007;12:362–376. doi: 10.1080/13546800701203769. [DOI] [PubMed] [Google Scholar]
- 38.McKay R, Langdon R, Coltheart M. Need for closure, jumping to conclusions and decisiveness in delusion-prone individuals. J Nerv Ment Dis. 2006;194:422–426. doi: 10.1097/01.nmd.0000221353.44132.25. [DOI] [PubMed] [Google Scholar]
- 39.Dudley REJ, Over DE. People with delusions jump to conclusions: a theoretical account of research findings on the reasoning of people with delusions. Clin Psychol Psychother. 2003;10:263–274. [Google Scholar]
- 40.Moritz S, Woodward TS. Jumping to conclusions in delusional and non-delusional schizophrenic patients. Br J Clin Psychol. 2005;44:193–207. doi: 10.1348/014466505X35678. [DOI] [PubMed] [Google Scholar]
- 41.Kinderman P, Bentall RP. The development of a novel measure of causal attributions: the Internal Personal and Situational Attributions Questionnaire. Pers Individ Dif. 1996;20:261–264. [Google Scholar]
- 42.Happe F, Brownell H, Winner E. Acquired “theory of mind” impairments following stroke. Cognition. 1999;70:211–240. doi: 10.1016/s0010-0277(99)00005-0. [DOI] [PubMed] [Google Scholar]
- 43.Langdon R, Coltheart M. Mentalising, schizotypy, and schizophrenia. Cognition. 1999;71:43–71. doi: 10.1016/s0010-0277(99)00018-9. [DOI] [PubMed] [Google Scholar]
- 44.Peters E, Joseph S, Day S, Garety P. Measuring delusional ideation: the 21-item Peters et al. Delusions Inventory (PDI) Schizophr Bull. 2004;30:1005–1022. doi: 10.1093/oxfordjournals.schbul.a007116. [DOI] [PubMed] [Google Scholar]
- 45.Peters ER, Joseph SA, Garety PA. Measurement of delusional ideation in the normal population: introducing the PDI (Peters et al. Delusions Inventory) Schizophr Bull. 1999;25:553–576. doi: 10.1093/oxfordjournals.schbul.a033401. [DOI] [PubMed] [Google Scholar]
- 46.Fenigstein A, Vanable PA. Paranoia and self-consciousness. J Pers Soc Psychol. 1992;62:129–138. doi: 10.1037//0022-3514.62.1.129. [DOI] [PubMed] [Google Scholar]
- 47.Craig JS, Hatton C, Craig FB, Bentall RP. Persecutory beliefs, attributions and theory of mind: comparison of patients with paranoid delusions, Asperger's syndrome and healthy controls. Schizophr Res. 2004;69:29–33. doi: 10.1016/S0920-9964(03)00154-3. [DOI] [PubMed] [Google Scholar]
- 48.Smári J, Stefánsson S, Thorgilsson H. Paranoia, self-consciousness, and social cognition in schizophrenics. Cognit Ther Res. 1994;18:387–399. [Google Scholar]
- 49.Andreasen NC. Negative symptoms in schizophrenia: definition and reliability. Arch Gen Psychiatry. 1982;39:784–788. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- 50.Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, IA: The University of Iowa; 1984. [Google Scholar]
- 51.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bech P, Bolwig TG, Kramp P, Rafaelsen OJ. The Bech-Rafaelsen Mania Scale and the Hamilton Depression Scale: evaluation of homogeneity and inter-observer reliability. Acta Psychiatr Scand. 1979;59:420–430. doi: 10.1111/j.1600-0447.1979.tb04484.x. [DOI] [PubMed] [Google Scholar]
- 53.Freeman D. Suspicious minds: the psychology of persecutory delusions. Clin Psychol Rev. 2007;27:425–457. doi: 10.1016/j.cpr.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 54.Freeman D, Garety PA. Comments on the content of persecutory delusions: does the definition need clarification? Br J Clin Psychol. 2000;39:407–414. doi: 10.1348/014466500163400. [DOI] [PubMed] [Google Scholar]
- 55.Martin JA, Penn DL. Social cognition and subclinical paranoid ideation. Br J Clin Psychol. 2001;40:261–265. doi: 10.1348/014466501163670. [DOI] [PubMed] [Google Scholar]
- 56.Langdon R, Coltheart M, Ward P, Catts S. Visual and cognitive perspective-taking impairments in schizophrenia: a failure of allocentric simulation? Cognit Neuropsychiatry. 2001;6:241–269. [Google Scholar]
- 57.Hemsley DR. An experimental psychological model for schizophrenia. In: Hafner H, Fattaz WF, Janzavik W, editors. Search for the Causes of Schizophrenia. Stuttgart, Germany: Springer-Verlag; 1987. pp. 179–188. [Google Scholar]
- 58.Hemsley DR. The schizophrenic experience: taken out of context? [First published on February 16, 2005] Schizophr Bull. 2005;31:43–53. doi: 10.1093/schbul/sbi003. doi:10.1093/schbul/sbi003. [DOI] [PubMed] [Google Scholar]
- 59.Hemsley DR. The development of a cognitive model of schizophrenia: placing it in context. Neurosci Biobehav Rev. 2005;29:977–988. doi: 10.1016/j.neubiorev.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 60.Samson D, Apperly IA, Kathirgamanathan U, Humphreys GW. Seeing it my way: a case of a selective deficit in inhibiting self-perspective. [First published on March 17, 2005] Brain. 2005;128:1102–1111. doi: 10.1093/brain/awh464. doi:10.1093/brain/awh464. [DOI] [PubMed] [Google Scholar]
- 61.Samson D, Connolly C, Humphreys GW. When “happy” means “sad”: neuropsychological evidence for the right prefrontal cortex contribution to executive semantic processing. [First published on September 27, 2006] Neuropsychologia. 2007;45:896–904. doi: 10.1016/j.neuropsychologia.2006.08.023. doi:10.1016/j.neuropsychologia.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 62.Langdon R, Coltheart M, Ward P, Catts S. Mentalising, executive planning and disengagement in schizophrenia. Cognit Neuropsychiatry. 2001;6:81–108. [Google Scholar]
- 63.Harrington L, Siegert R, McClure J. Theory of mind in schizophrenia: a critical review. Cognit Neuropsychiatry. 2005;10:249–286. doi: 10.1080/13546800444000056. [DOI] [PubMed] [Google Scholar]