Abstract
The identification of schizophrenia's negative symptoms dates back to the earliest descriptions of Kraepelin and Bleuler, who each highlighted the central role of avolition in the phenomenology and course of this illness. Since, there have been numerous advances in our understanding of schizophrenia, and the present review tracks the changes that have taken place in our understanding of negative symptoms, their description and measurement. That these symptoms represent a distinct domain of the illness is discussed in the context of their ties to other symptoms and functional outcome. The underlying structure of the negative symptom construct is explored, including several lines of investigation that point towards diminished expression and amotivation as key underlying subdomains. We also discuss findings of intact emotional experience and consummatory pleasure in individuals with schizophrenia, calling into question the presence of anhedonia in this illness. We conclude with a reconceptualization of the negative symptoms, suggesting amotivation (ie, avolition) represents the critical component, particularly in regard to functional outcome. Further exploration and clarification of this core deficit will ultimately enhance our neurobiological understanding of schizophrenia, as well as strategies that may improve outcome.
Keywords: motivation, avolition, apathy, anhedonia, functional outcome, cognitive symptoms
Dementia praecox … “a weakening of those emotional activities which permanently form the mainsprings of volition …” Kraepelin (1919).1
With his conceptualization of “dementia praecox,” Kraepelin is best remembered for his position that schizophrenia represents an illness of early and progressive deterioration. Perhaps not as well known is the central role he attributed to avolition in dictating the changes characterizing this decline. Although somewhat less pessimistic regarding long-term outcome, Bleuler was no less sensitive to this issue, noting that “indifference seems to be the external sign of their state …. The will … disturbed in a number of ways, but above all by the breakdown of the emotions … The patients appear lazy and negligent because they no longer have the urge to do anything either of their own initiative or at the bidding of another.”2
The introduction of modern psychopharmacology in the 1950s revolutionized the treatment of major mental disorders, including schizophrenia. The efficacy of chlorpromazine in alleviating the positive symptoms of this illness (ie, delusions, hallucinations) confirmed the biological underpinnings of schizophrenia, and the so-called “neuroleptics” quickly established themselves as the cornerstone of treatment. The benefit they bestowed in terms of positive symptoms drove the field's focus in evaluating outcome over the next decades, and it was not until the mid-1970s to early 1980s that attention once again turned to the role of deficit or negative symptoms.3–5 Other symptom domains have since been detailed,6,7 but we are reminded that the earliest descriptions of schizophrenia emphasized a disturbance of volition/will as the fundamental underlying process in its pathology.
The present article revisits this notion, tracking the changes that have taken place in our understanding of negative symptoms, their description, and measurement. Evidence that these symptoms represent a distinct domain of the illness is discussed, as are their ties to other symptoms, as well as functional outcome. Returning to Kraepelin's and Bleuler's descriptions of schizophrenia, we propose that the negative symptoms represent the illness’ core and may, in fact, be the most significant factor in the impaired functional recovery associated with schizophrenia. Finally, we attempt to reframe the conceptualization of negative symptoms in the context of other lines of investigation such as apathy, drive, and motivation. Integration of these various lines of investigation is likely to add considerably to our strategies in better understanding the etiology, clinical manifestations, and treatment of schizophrenia's negative symptoms.
Negative Symptoms: Historical Perspective
Terminology distinguishing “positive” and “negative” symptoms dates back almost 150 years now and has its foundations in the field of neurology (for review, see Pearce8). Positive symptoms were identified as superimposed behaviors, eg, clonic jerking, while negative symptoms represented an absence of normal function, eg, loss of sensation.9 In psychiatry, it is the name of Hughlings Jackson, also a neurologist, that has been more closely linked to the positive-negative symptom dichotomy. Building upon Herbert Spencer's writings regarding dissolution and evolution of the nervous system, Jackson proposed that negative symptoms reflect a dissolution and loss of function of “neural arrangements,” whereas positive symptoms represent the loss of higher inhibitory control and resultant excitation or release of lower systems.10
Years later, psychiatry embraced this distinction in differentiating the symptoms of schizophrenia, with the suggestion that each represented distinct pathophysiologies.5 However, enthusiasm about the treatability of negative symptoms was tempered by a line of thinking suggesting that these features were reflective of underlying morphological changes and, as such, not amenable to pharmacological intervention.4
That said, the distinction between positive and negative symptoms represented an important turning point in the conceptualization of schizophrenia. It better reflected what was observed clinically, and as a model it set the stage for describing the illness in the context of multiple symptom domains. Efforts to better delineate the features of negative symptoms were undertaken,11 with the subsequent distinction of primary and secondary negative symptoms, the latter seen as iatrogenic and/or environmental factors that may improve with treatment, eg, extrapyramidal symptoms. In contrast, a subgroup of individuals was described who exhibit primary or idiopathic negative symptoms of a persistent nature (ie, being present for most of the preceding 12 months, including during periods of clinical stability), collectively termed the “deficit syndrome.”12 The prevalence of the deficit syndrome has since been estimated at approximately 15% in first-episode patients and 25%–30% in chronic schizophrenia.13
The seminal work leading to clozapine's reintroduction in the 1990s challenged the notion that negative symptoms cannot be treated pharmacologically.14 Indeed, the introduction of numerous “atypical” antipsychotics modeled after clozapine's unique clinical profile was accompanied by optimism that even primary negative symptoms could be diminished with this new class of antipsychotics.15,16 Methodological issues, as well as more recent effectiveness data, have called into question the degree and scope of this benefit.17,18 However, with the development of the second-generation antipsychotics there has occurred a fundamental shift in thinking regarding negative symptoms and their response to treatment.
The importance of these symptoms has recently prompted the formation of a focused National Institute of Mental Health (NIMH) initiative, using a similar strategy to that employed by the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) to enhance understanding of the cognitive domain of schizophrenia.19 It is now routine to evaluate changes in negative symptoms with treatment, and a variety of pharmacological strategies are currently being investigated in this regard.20 In line with this approach, regulatory agencies such as the Food and Drug Administration have for the first time indicated that they will entertain approval of add-on treatments proven to be effective in specific domains such as the negative symptoms.21
Definition and Assessment
The negative symptoms of schizophrenia have traditionally been considered to consist of blunted affect, poverty of speech, asociality, avolition, and anhedonia.3 The recent NIMH-MATRICS consensus statement on negative symptoms echoes this definition,19 although there is a lack of agreement regarding the relationship between individual symptoms. As will be discussed, there is also evidence to suggest that some of the terminology that is commonly used may, in fact, need modification.
There exist in the literature a number of scales for the assessment of negative symptoms, with varying degrees of overlap. Of these, Andreasen's Scale for the Assessment of Negative Symptoms (SANS)3 appears to be the one with the most extensive coverage of negative symptoms.19,22 The SANS consists of 5 subscales: affective flattening or blunting, alogia, avolition/apathy, anhedonia/asociality, and attentional impairment. Other rating scales include the Positive and Negative Syndrome Scale (PANSS),23 the Brief Psychiatric Rating Scale,24 the Schedule for the Deficit Syndrome (SDS),25 the Krawiecka-Manchester Scale,26 the Negative Symptom Scale,27 the Negative Symptom Scale of Pogue-Geile and Harrow,28 and the Emotional Blunting Scale.29 A comparison of these different measures demonstrates considerable overlap but also highlights the lack of consensus in definition (table 1).22,30 Despite good overall correlation between the scales, notable differences exist regarding inclusion of certain symptoms, in particular social isolation, anhedonia, and avolition or apathy.
Table 1.
Comparison of Content in Various Negative Symptom Scales
| SANS Items | SANS | PANSS | BPRS | KMS | SDS | EBS | NSS Lewine | NSS P,G, andH |
| Affective flattening | X | X | X | X | X | X | X | X |
| Unchanging expression | X | X | X | X | X | X | P | X |
| Decreased movements | X | X | X | P | P | P | P | X |
| Paucity of gestures | X | X | X | P | P | P | P | X |
| Poor eye contact | X | X | — | P | P | P | P | X |
| Affective nonresponsivity | X | X | X | X | X | X | P | X |
| Inappropriate affect | X | — | — | X | — | X | X | — |
| Lack of vocal inflection | X | X | X | X | P | X | — | X |
| Alogia | X | P | — | X | X | P | — | — |
| Poverty of speech | X | X | — | X | X | X | — | X |
| Poverty of content | X | P | — | P | X | — | X | X |
| Blocking | X | — | P | P | — | — | — | P |
| Increased latency response | X | — | — | P | — | — | P | X |
| Avolition/apathy | X | P | — | — | — | — | — | P |
| Poor grooming/hygiene | X | X | — | — | — | X | — | — |
| Impersistance at work/school | X | — | — | — | — | — | P | — |
| Physical anergia | X | — | — | — | — | — | — | — |
| Anhedonia/asociality | X | P | P | — | X | — | P | — |
| Few recreational activities | X | P | — | — | P | — | P | — |
| Decreased sexual interest | X | — | — | — | P | — | X | — |
| Decreased capacity for closeness | X | P | — | — | P | P | — | — |
| Few friends/prefers isolation | X | X | X | — | P | P | — | — |
| Attentional impairment | X | — | — | — | — | — | — | — |
| Social inattentiveness | X | P | — | — | — | — | — | — |
| Inattentiveness on mental status examination | X | — | — | — | — | — | — | — |
| Other items | ||||||||
| Emotional withdrawal | P | X | X | — | — | X | — | P |
| Poor rapport | P | X | — | — | — | X | — | P |
| Poor abstract thinking | — | X | — | — | — | — | — | — |
| Stereotyped thinking | X | X | — | — | — | — | — | — |
| Psychomotor retardation | P | — | X | X | — | — | X | X |
| Lack of sense of purpose | P | P | — | — | X | X | — | — |
| Fatigue | — | — | — | — | — | — | X | — |
| Slowed speech | — | — | X | — | — | — | X | X |
| Depressed appearance | — | — | — | — | — | — | X | — |
| Loose associations/incoherence | — | — | X | — | — | — | X | — |
| Low voice | — | — | P | — | — | — | — | X |
Note: Adapted from Fenton and McGlashan (1992)2 and Earnst and Kring (1997)28; SANS, Scale for the Assessment of Negative Symptoms; PANSS, Positive and Negative Syndrome Scale; BPRS, Brief Psychiatric Rating Scale; KMS, Krawiecka-Manchester Scale; SDS, Schedule for the Deficit Syndrome; EBS, Emotional Blunting Scale; NSS Lewine, Negative Symptom Scale of Lewine (1983); NSS P, G, and H, Negative Symptom Scale of Pogue-Geile and Harrow (1983); X—substantial overlap; P—partial overlap.
In the past, attentional impairment, as described in the SANS, had also been considered a negative symptom. However, more recent factor analyses have consistently shown that attentional impairment is more closely related to cognitive dysfunction rather than negative symptoms.31,32 Similar results have been reported for the inappropriate affect and poverty of content of speech items of the SANS.31–34 In light of these findings, the inclusion of the attentional impairment, inappropriate affect, and poverty of content of speech items of the SANS have been questioned and, in some studies, excluded.19,35–37
The SDS, developed primarily to assess the presence or absence of the deficit syndrome in schizophrenia, also incorporates severity ratings for negative symptoms.25 Reflecting criteria for the deficit syndrome, the SDS encompasses 6 symptoms: restricted affect, diminished emotional range, poverty of speech, curbing of interests, diminished sense of purpose, and diminished social drive. The SDS has not been broadly utilized as a measure of negative symptoms and their severity; rather, its use has been confined to defining the presence or absence of the deficit syndrome.
The PANSS, on the other hand, has gained widespread popularity for the assessment of psychopathology in schizophrenia, with a subscale specifically focused on negative symptoms.23 However, an examination of the underlying factor structure of this scale by Emsley et al. (2003),38 employing patients with recent onset of a schizophrenia-spectrum illness and minimal exposure to antipsychotic medications, suggests that the domains of symptoms assessed by the PANSS differ from the structure implied in its subscales. Specifically, the PANSS negative symptom domain consists of passive social withdrawal (N4), emotional withdrawal (N2), poor rapport (N3), active social avoidance (G16), lack of spontaneity (N6), blunted affect (N1), and disturbance of volition (G13) (numbers in parentheses indicating the subscale item [N = negative subscale; G = general psychopathology subscale]). As with the SANS, not all items in the negative subscale of the PANSS appear relevant; further, this domain also includes items from the general psychopathology subscale.
While the PANSS has been used extensively, particularly in the many pharmacological trials carried out as the second-generation antipsychotics sought regulatory approval, the MATRICS initiative focusing on negative symptoms more closely approximates the multidimensional approach outlined in the SANS.19
Relationship to Cognitive Dysfunction
That there are now 2 MATRICS initiatives focused on cognition and negative symptoms, respectively, is a telling commentary on current thinking regarding schizophrenia. Certainly not a panacea, antipsychotics nevertheless revolutionized the treatment of psychosis. However, evidence indicates that this clinical benefit has not translated into substantial gains in functional recovery.39,40 Cognitive and negative symptoms have been implicated as playing a substantial role in this regard,36,41 reinforced by findings that both are evident at the time first-episode psychosis occurs and neither is improved substantially with antipsychotic treatment.40,42,43 What remains unclear is the relationship between the negative and cognitive symptom domains, although, not surprisingly, this topic is garnering considerable attention in light of their collective impact on outcome.
In cross-sectional studies, the negative symptoms of schizophrenia have frequently been found to correlate with various measures of neuropsychological performance. For example, several domains of cognitive functioning show low-to-moderate inverse correlations with negative symptoms (reviewed in Addington44 and Harvey et al45). Although a relationship exists, there does not appear to be a clear link with specific cognitive deficits. Moreover, negative symptoms seem to account for only a small proportion of the variance in cognitive impairment (ie, approximately 10%)44 Further, based on available evidence it appears that cognitive impairment, although related to negative symptoms, is a distinct construct. A recent longitudinal study failed to establish a relationship between change in negative symptoms and neurocognitive function, leading to the conclusion that they represent semiautonomous disease processes.46 The argument is made that while they co-occur, negative symptoms do not directly cause cognitive impairment or vice versa and, as a result, they do not change in parallel over time.
The finding that negative and cognitive symptoms are separate domains has been reinforced by Harvey et al. (2006),45 who have approached the problem from the standpoint of 4 theoretical models: (1) negative and cognitive symptoms are identical features of the illness or alternate manifestations of the same basic underlying process; (2) these features of schizophrenia are separable but share similar underlying etiological factors; (3) each of these symptom dimensions has a separate but related etiology; and (4) these symptom dimensions are distinct from each other, with separate etiologies. Correlations between them are influenced by measurement and definitional issues or “third variable” relationships with other features of the illness, such as distal outcome measures. After reviewing the available evidence, including recent path analysis studies by the same group, the authors suggest that negative and cognitive symptoms of schizophrenia appear to be related but potentially separable domains, more consistent with their third and fourth models. Of note, subsequent investigations by the same group reveal that both symptom domains impact functional outcomes, with neuropsychological performance affecting an individual's functional capacity, while negative symptoms appear more related to the likelihood of implementation of these abilities.41,45
Social cognition, a specific domain of cognitive functioning that refers to “the mental operations that underlie social interactions, including perceiving, interpreting, and generating responses to the intentions, dispositions, and behaviors of others,”47 has emerged as an important area of psychopathology in schizophrenia. This construct is believed to consist of at least 5 distinct domains: theory of mind, social perception, social knowledge, attributional bias, and emotional processing (reviewed in Green et al [2008]47). However, despite recent advances in our understanding of social cognition deficits in schizophrenia, its relationship with other important symptom domains such as neurocognition and negative symptoms remains unclear. Sergi et al (2007)37 have shed some light on this issue in a recent cross-sectional investigation; through structural equation modeling they found that social cognition and neurocognition are distinct, yet highly related, constructs. In addition, social cognition and negative symptoms, as assessed by the SANS, are also distinct constructs. A proposed 3-factor model suggests that the relationship between social cognition and negative symptoms, while significant, is weaker than that between social cognition and neurocognition.
Emerging evidence from separate lines of investigation may help to shed further light on the relationship between the cognitive and negative symptoms in schizophrenia. Heerey et al (2007)48 examined delay discounting, the impact delay in reward has on subjective value of the reward in schizophrenia. In a comparison of individuals with schizophrenia or schizoaffective disorder on stable doses of antipsychotic medication and healthy controls, the former discounted the value of future rewards at a significantly greater rate. In addition, they found a significant relationship between memory function and discounting, with individuals with better memory functioning demonstrating less severe discounting. There was also a trend toward an inverse relationship between negative symptoms and delay discounting, suggesting that those with more negative symptoms may exhibit less delay discounting.
Additional work from the same group has examined deficits in reward- and punishment-driven learning in individuals with schizophrenia on stable doses of antipsychotic medication compared with healthy controls.49 Using a probabilistic selection task, they demonstrated a selective deficit in the ability to learn from positive outcomes in individuals with schizophrenia, while finding no deficits in learning from negative outcomes. Moreover, these deficits were significantly correlated with negative symptoms but not with standard neuropsychological measures, including measures of working memory, suggesting that the observed deficits are not based on a general impairment in neuropsychological performance.
Concerns have been raised about the impact motivational deficits have on measures of cognitive dysfunction, with the possibility that some of the cognitive dysfunction seen in schizophrenia may be secondary to a lack of motivation.50 In line with these concerns, an examination of the role of effort in cognitive functioning in schizophrenia by Gorissen et al (2005)51 revealed that insufficient effort accounted for up to one-third of the variance in neuropsychological test performance, and, further, this lack of effort was correlated with negative symptom severity. The authors propose that this lack of effort is in line with a motivational deficit, reflective of avolition in the cognitive realm. Overall, results from these lines of investigation suggest a complex relationship between negative and cognitive symptoms in determining goal-directed behavior in schizophrenia, as well as a detrimental impact of motivational deficits in cognitive functioning.
Categorizing Negative Symptoms
As with positive symptoms, there have been efforts to also categorize negative symptoms into specific subdomains. To this end, investigations have incorporated the use of exploratory and confirmatory factor analysis, as well as component analysis. Most studies have focused on the SANS, in part due to its larger number of items addressing negative symptoms compared with the other commonly used rating scales.
In a sample of 207 schizophrenia patients, Mueser et al (1994)52 identified 3 underlying factors in the SANS through exploratory principal axis factor analysis: (1) affective flattening/blunting, (2) avolition/apathy and anhedonia/asociality, and (3) alogia and inattention. Of note, the poverty of speech item loaded on the affective flattening/blunting factor, rather than with the other items in the alogia subscale.
Sayers et al (1996),34 through confirmatory factor analysis on SANS ratings of 457 patients with a schizophrenia-spectrum illness, identified 3 factors: (1) diminished expression (affective flattening/blunting), (2) inattention/alogia, and (3) social amotivation (avolition/apathy and asociality/anhedonia). These results confirmed the previous findings of Mueser et al52; however, the final SANS used in this study consisted of 18 items, the inappropriate affect and poverty of content of speech items being dropped. A similar examination of SANS ratings of patients with schizophrenia or schizoaffective disorder, both while on and off haloperidol, revealed the existence of 2 primary factors: affective flattening and diminished motivation (anhedonia and apathy), and various other factors representing disorganization (including attentional impairment, alogia, and poverty of content of speech).53
Peralta and Cuesta (1999)32 conducted an item-level exploratory factor analysis of the SANS and SAPS, identifying 3 factors that reflect negative symptoms: (1) poverty of affect/speech, (2) social dysfunction, and (3) attention. The first factor, poverty of affect and speech, was comprised of the symptoms from the affective flattening subscale except inappropriate affect (which loaded on a separate factor along with thought disorder) and poverty of speech plus poverty of content of speech items. The social dysfunction factor consisted of the items from the avolition/apathy and anhedonia/asociality subscales. The third factor, attention, consisted of distractibility, blocking, increased latency of responses, and both attentional items. A second-order factor analysis resulted in a negative symptom factor that consisted of “poverty of affect/speech” and “social dysfunction.” The “attention” factor from the first-order analysis loaded moderately on both the negative and disorganization second-order factors.
There has also been a similar analysis conducted by Kimhy et al (2006)54 on a sample of individuals with deficit schizophrenia, revealing 2 distinct factors within the SDS. The first was an avolition factor, comprising symptoms of curbing of interests, diminished sense of purpose, and diminished social drive. The second factor was one of emotional expression, consisting of symptoms of restricted affect, diminished emotional range, and poverty of speech.
Deconstructing Subdomains: Avolition Vs Anhedonia
The data presented above suggest 2 subdomains of negative symptoms: (1) diminished expression, consisting of affective flattening and poverty of speech, and (2) amotivation, consisting of avolition/apathy and anhedonia/asociality. Other symptoms such as inappropriate affect, poverty of content of speech, and attentional impairment appear to be more closely related to cognitive dysfunction rather than negative symptoms. This is not unlike the present approach outlined in the MATRICS initiative, which distinguishes between 2 classes of subscales: (1) anhedonia/avolition/apathy and (2) blunted affect/alogia. In short, one category speaks to issues of involvement with the surrounding environment (ie, drive and pleasure), while the other addresses an expressive component (ie, affect and speech). However, the relationship between these subdomains or, in fact, the features within each remain in question. Further, do individuals with schizophrenia suffer from hedonic or motivational deficits?
Closer inspection of the subdomains of negative symptoms reveals some interesting relationships. In addition to highlighting 2 subdomains of negative symptoms, these same studies have demonstrated that these 2 subdomains exhibit a moderate interrelationship (interfactor correlation coefficients between 0.47 and 0.57).32,34,52 An examination of the separate subscales of the SANS also noted moderate interrelationships for affective flattening with avolition-apathy and anhedonia-asociality subscales (r = 0.49 and 0.48, respectively), as well as for alogia with avolition-apathy and anhedonia-asociality subscales (r = 0.61 and 0.53, respectively).31 These findings suggest that the negative symptom domains and symptoms that comprise them, although distinct phenomenological entities, may reflect a common underlying process.
Anhedonia, often identified as a feature of schizophrenia, has been defined as a diminished capacity to experience pleasant emotions55 or, alternatively, difficulty in experiencing interest or pleasure.3 Current evidence from experimental paradigms using diverse emotion-eliciting stimuli, including films, pictures, sounds, and drinks, indicates individuals with schizophrenia report intact experiences of both pleasant and unpleasant emotions in the moment with at least equal intensity compared with healthy controls.56–60 This is true in spite of their diminished capacity for outward expression of emotion and regardless of medication status. Similar results have been obtained in comparisons of deficit and nondeficit schizophrenia with healthy controls, where there was no significant reduction in the experience of emotion, despite a reduction in emotional expressivity in individuals with deficit schizophrenia.61 These findings suggest that individuals with schizophrenia, despite impairments in outward emotional expression, do not have deficits in the internal experience of emotions. That is, they do not appear to have a hedonic deficit, as implied by the terminology used in commonly accepted definitions and rating scales for negative symptoms.
A recent review of the anhedonia construct in schizophrenia by Horan et al (2006)62 also highlights some of the issues contributing to the difficulties in distinguishing anhedonia from amotivation in patients with schizophrenia. With the SANS being the current standard for quantifying negative symptoms, the authors note that the anhedonia/asociality ratings are not only based solely on patients’ capacity to experience pleasant emotions but also on the frequency, quality, and level of interest and engagement in recreational and social activities, therefore measuring several conceptually distinct processes. While decreased interest and engagement in such activities are possible consequences of anhedonia, they may also be the result of various other emotional, motivational, and social factors. Thus, by incorporating actual performance measures, this scale may reflect a social performance deficit more than a fundamental hedonic capacity deficit.62
The concept of anhedonia in schizophrenia, supported by numerous studies over the past 25 years, has been based largely on results using the Chapman physical and social anhedonia scales.62 The majority of these studies have revealed elevated levels of self-reported anhedonia in individuals with schizophrenia, including both social and physical anhedonia in deficit compared with nondeficit schizophrenia,63 while studies using these and other measures of anhedonia have revealed mixed results.62,64 However, there have been concerns about the construct and discriminant validity of the Chapman physical and social anhedonia scales.65–68 These studies raise questions about the underlying construct that is measured by the scales, ie, whether they measure hedonic capacity. Given that much of the support for hedonic deficits in schizophrenia is based on findings using these scales, as well as the contradictory findings between these trait measures of anhedonia and experimental paradigms that have shown intact hedonic capacity,56–58 the presence of anhedonia in this illness remains questionable.
Further examination in schizophrenia of the discrepancy between self-reported trait measures of diminished experience of pleasure and the aforementioned objective findings of intact abilities to experience emotions in the moment suggests that there may be separable components of pleasure. Horan et al (2006)62 draw attention to Klein's (1984)69 distinction between anticipatory pleasure (ie, pleasure derived from anticipating that an activity will be enjoyable) and consummatory pleasure (ie, pleasure derived from engaging in enjoyable activities). Further work in this area by the same group has revealed that patients with schizophrenia, compared with healthy controls, report lower anticipatory pleasure but similar consummatory pleasure.70 In particular, those with schizophrenia report significantly less anticipatory pleasure for goal-directed activities (making dinner, doing errands, working/studying) vs non–goal-directed activities (eating, watching TV, smoking, sleeping). Patients were also significantly less often engaged in goal-directed activities compared with controls; further to this point, anticipatory pleasure scale scores were significantly correlated with clinical ratings of anhedonia and impaired community functioning.70
Recent work by Heerey and Gold (2007)58 has also provided some insight into the experiential and motivational deficits in schizophrenia through exploration of the coupling of affective experience and behavior. Using an experimental paradigm assessing self-reported ratings of pleasure and arousal, as well as degree of effort exerted to seek or avoid exposure to slides of varying affective valence in the present and future, several interesting findings emerged. Individuals with schizophrenia exhibit deficits in their ability to couple their behavior to the motivational properties of a stimulus despite equivalent subjective in the moment pleasantness and arousal ratings for these stimuli compared with healthy controls. Furthermore, significant correlations were noted between these deficits and working memory impairment, particularly for those situations requiring the maintenance of an internal representation for the stimulus. The authors conclude that motivational deficits in schizophrenia reflect impairment in the ability to translate experience into action.
Returning to our question, current evidence suggests that individuals with schizophrenia do not have a hedonic deficit in the strictest sense. Rather, it seems that they experience a diminished capacity to anticipate that pursuit or achievement of a goal will be pleasurable, in addition to impairment in the translation of subjective experience into action, with a resultant decrease in goal-directed behavior. This concept of anticipatory pleasure has been suggested to be more closely related to motivation and goal-directed behavior,69 as well as to the concept of “wanting.”71 Overall, these findings suggest that individuals with schizophrenia experience amotivation rather than anhedonia. Moreover, the interrelationship between diminished expression and amotivation suggests that these subdomains of symptoms may represent differential expressions of a common underlying process.
Negative Symptoms and Functional Outcome: Further Evidence for Avolition
Negative symptoms and functional outcomes have consistently been linked, with several studies reporting worse functional outcomes in individuals with more prominent negative symptoms.36,42,72,73 More specifically, these studies have demonstrated correlations between negative symptoms and impairments in occupational functioning, household integration, relationships, and recreational activities. Similar associations have been found for the enduring primary symptoms that characterize the deficit syndrome, with these individuals consistently demonstrating poorer functional outcomes.13 Given the subdomain structure of negative symptoms, an interesting question arising is the impact these domains have on functional outcomes. Studies examining the association between anhedonia and functional outcomes in individuals with schizophrenia have produced inconsistent results. Significant relationships have been demonstrated between anhedonia and functional outcomes, both in short-term and long-term follow-up studies.74,75 However, others have failed to replicate these findings.76 Of note, these studies have depended on the Chapman physical and social anhedonia scales for their measurement of hedonic capacity, and the concerns discussed previously about the validity of these scales, as well as the mounting evidence of intact affective experiences in schizophrenia, suggests cautious interpretation of these associations with functioning. This is reinforced by the findings of Gard et al (2007),70 noting that consummatory pleasure was not associated with community functioning.
Conversely, the study by Sayers et al (1996)34 demonstrated the amotivation factor to be moderately and consistently positively associated with patients’ social dysfunction. This included measures of instrumental role performance, household adjustment, extended family functioning, social/leisure functioning, and general adjustment, as measured by the Social Adjustment Scale—Patient Version. Similarly, a study by Kiang et al (2003)78 examining apathy in a sample of patients with schizophrenia found a significant correlation between the degree of clinician-rated apathy, assessed by Marin's Apathy Evaluation Scale, and worse functional outcome, measured by the Independent Living Skills Survey. The recent work of Gard et al (2007)70 adds to these findings, noting deficits in anticipatory pleasure in schizophrenia to be significantly correlated with worse community functioning.
Several studies have also examined the impact of affective flattening, as well as the subdomain of diminished expression, on functional outcomes of individuals with schizophrenia. Gur et al (2006)79 examined individuals with schizophrenia with and without prominent affective flattening, demonstrating a significant relationship between affective flattening and functioning at initial assessment and 1-year follow-up. However, those subjects in the affective flattening group also exhibited significantly more severe negative symptoms overall, making conclusions about the role of affective flattening difficult. A similar examination between affective flattening and social skills, using a role-play method, demonstrated that affective flattening and social skills deficits were not correlated.80 In looking at the relationship between the negative symptom subdomain of diminished expression and social functioning, Sayers et al (1996)34 found no significant relationship, in contrast to the significant relationship observed for the amotivation domain (described above).
Taken together, the results of these studies reinforce the relationship between negative symptoms and functional outcomes in schizophrenia and suggest that amotivation may be the key contributor to this relationship. This is not to suggest that other factors do not play important roles in determining the functional outcomes of individuals with schizophrenia. In fact, cognitive dysfunction has also been repeatedly demonstrated to contribute to functional outcomes in schizophrenia, with different studies finding varying degrees of contribution for cognitive and negative symptoms.36,41,43,81 However, the influence of motivation on cognitive functioning,51 the relationship between negative symptoms and reward-driven learning,49 the increased delay discounting in schizophrenia,48 and the deficits in coupling of subjective internal experiences with motivated behavior58 suggest a complex relationship between motivation and cognition in effecting the functional impairment seen in schizophrenia.
Reconceptualizing Negative Symptoms: Building on the Evidence
Through our review of historical and contemporary notions of negative symptoms in schizophrenia, there emerge several important conclusions:
Negative symptoms in schizophrenia comprise an independent symptom domain, distinct from positive symptoms, neurocognition, and social cognition.
Within the negative symptom construct, current evidence demonstrates the existence of 2 subdomains:
i. Diminished expression
ii. Amotivation
Attentional impairment, inappropriate affect, and poverty of content of speech, concepts historically believed to be negative symptoms, are more closely related to the neurocognitive or disorganized symptoms of schizophrenia.
Anhedonia is not a core symptom of the negative symptom construct in the strictest definition of the term. Investigations have failed to demonstrate diminished emotional experience despite expressive deficits. Rather, the deficits seem to lie in the realm of anticipatory pleasure, a concept related to the engagement of motivational processes and the promotion of goal-directed behavior.
Amotivation (ie, avolition) is a core negative symptom that has a direct impact on functional outcomes in schizophrenia. Further, it plays an additional indirect role through its influence on neurocognitive dysfunction.
We highlight the parallel between this position and the earliest descriptions by Kraepelin and Bleuler, who identified avolition as central to schizophrenia. In doing so, we also acknowledge a semantic complexity that cannot be ignored as we “split hairs.” Is avolition synonymous with decreased drive, amotivation, or apathy? We choose to stay with “avolition” at this point only because it is in line with the original description, although at face value we see the terms as interchangeable. There is a striking similarity, for example, between avolition and the syndrome of apathy, defined as a lack of motivation that is not attributable to intellectual impairment, emotional distress, or diminished level of consciousness.82 It is reflected in decreased goal-directed behavior, goal-directed cognition, and the emotional concomitants (ie, emotional expressivity). It will be for the purists to tease apart the nuances; fundamental to our proposition, regardless of terminology, is a decrease in goal-directed behaviors.
Occam's Razor and Diagnostic Parsimony
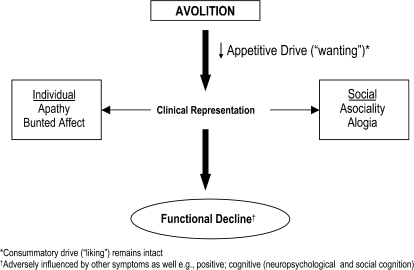
Occam's Razor, or the Law of Parsimony, is an axiom in philosophical and scientific traditions attributed to William of Occam (Ockham).83,84 It states that “it is better, in explaining something, to use as few assumptions as possible,”84 thus reflecting a principle of economy in the development of explanatory theories. In keeping with this principle, we propose that while negative symptoms are varied and broad in both their clinical presentation and longitudinal course, they can all be subsumed under the concept of avolition. As the primary construct, avolition translates to decreased functional performance, a hallmark of schizophrenia's longer term outcome but identifiable in its earliest stages. Asociality and alogia represent phenotypic expressions of this pervasive decrease in drive and are observed clinically as increased social withdrawal, also a cardinal feature in schizophrenia's prodrome.85 Blunted affect parallels loss of appetitive drive but is distinguishable from hedonic capacity, which remains intact within the confines of consummatory (vs appetitive) drive. This hypothetical model is outlined in figure 1.
Fig. 1.
Schematic Representation of Negative or Deficit Symptoms, With Avolition Representing the Primary Construct. Loss of appetitive drive is associated with clinical features that are observable in altered individual/social behaviors and rapidly translates to a gradual functional deterioration that can be observed during schizophrenia's prodrome and in advance of psychotic symptoms. While affective features may be present, this is not synonymous with anhedonia. Individuals can experience pleasure; however, what is perceived as pleasurable may no longer be in line with the individual's premorbid capacity for goal seeking and related value system.
Future Challenges
The past century has seen significant advances in our understanding and treatment of schizophrenia. Despite these, substantially altering the course of this illness and the functional disability it imparts has remained an elusive goal. The negative symptoms of schizophrenia and, in particular the avolition that forms their core, have been demonstrated to play a significant role. However, the lack of clear and consistent definitions of these symptoms, as well as reliable and valid instruments aimed at measuring the core components, has hindered our progress. Further exploration and clarification of the core deficits in schizophrenia is necessary to guide both our search for the neurobiological underpinnings of this complex illness, as well as the interventions that will ultimately effect changes in functional outcomes.
Funding
None.
Acknowledgments
Conflict of interest: None.
References
- 1.Kraepelin E. Dementia Praecox and Paraphrenia. Edinburgh, UK: Livingstone; 1919. [Google Scholar]
- 2.Bleuler E. Dementia Praecox or the Group of Schizophrenias. New York: International Universities Press; 1950. [Google Scholar]
- 3.Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Arch Gen Psychiatry. 1982;39:784–788. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- 4.Crow T. Molecular pathology of schizophrenia: more than one disease process? BMJ. 1980;280:1–9. doi: 10.1136/bmj.280.6207.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strauss JS, Carpenter WT, Jr, Bartko JJ. The diagnosis and understanding of schizophrenia. Part III. Speculations on the processes that underlie schizophrenic symptoms and signs. Schizophr Bull. 1974;11:61–69. doi: 10.1093/schbul/1.11.61. [DOI] [PubMed] [Google Scholar]
- 6.Bilder RM, Mukherjee S, Rieder RO, Pandurangi AK. Symptomatic and neuropsychological components of defect states. Schizophr Bull. 1985;11:409–419. doi: 10.1093/schbul/11.3.409. [DOI] [PubMed] [Google Scholar]
- 7.Liddle PF. The symptoms of chronic schizophrenia. A re-examination of the positive-negative dichotomy. Br J Psychiatry. 1987;151:145–151. doi: 10.1192/bjp.151.2.145. [DOI] [PubMed] [Google Scholar]
- 8.Pearce JM. Positive and negative cerebral symptoms: the roles of Russell Reynolds and Hughlings Jackson. J Neurol Neurosurg Psychiatry. 2004;75:1148. doi: 10.1136/jnnp.2004.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reynolds JR. Epilepsy: Its Symptoms, Treatment, and Relation to Other Chronic Convulsive Diseases. London, UK: John Churchill; 1861. [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson JH. Selected Writings. New York, NY: Basic Books; 1958. [Google Scholar]
- 11.Andreasen NC, Olsen S. Negative v positive schizophrenia. Definition and validation. Arch Gen Psychiatry. 1982;39:789–794. doi: 10.1001/archpsyc.1982.04290070025006. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter WT, Jr, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145:578–583. doi: 10.1176/ajp.145.5.578. [DOI] [PubMed] [Google Scholar]
- 13.Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT., Jr A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001;58:165–171. doi: 10.1001/archpsyc.58.2.165. [DOI] [PubMed] [Google Scholar]
- 14.Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 15.Moller HJ, Muller H, Borison RL, Schooler NR, Chouinard G. A path-analytical approach to differentiate between direct and indirect drug effects on negative symptoms in schizophrenic patients. A re-evaluation of the North American risperidone study. Eur Arch Psychiatry Clin Neurosci. 1995;245:45–49. doi: 10.1007/BF02191543. [DOI] [PubMed] [Google Scholar]
- 16.Tollefson GD, Sanger TM. Negative symptoms: a path analytic approach to a double-blind, placebo- and haloperidol-controlled clinical trial with olanzapine. Am J Psychiatry. 1997;154:466–474. doi: 10.1176/ajp.154.4.466. [DOI] [PubMed] [Google Scholar]
- 17.Jones PB, Barnes TR, Davies L. Randomized controlled trial of the effect on Quality of Life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1) Arch Gen Psychiatry. 2006;63:1079–1087. doi: 10.1001/archpsyc.63.10.1079. [DOI] [PubMed] [Google Scholar]
- 18.Lieberman JA, Stroup TS, McEvoy JP. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 19.Kirkpatrick B, Fenton WS, Carpenter WT, Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32:214–219. doi: 10.1093/schbul/sbj053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erhart SM, Marder SR, Carpenter WT., Jr Treatment of schizophrenia negative symptoms: future prospects. Schizophr Bull. 2006;32:234–237. doi: 10.1093/schbul/sbj055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laughren T, Levin R. Food and Drug Administration perspective on negative symptoms in schizophrenia as a target for a drug treatment claim. Schizophr Bull. 2006;32:220–222. doi: 10.1093/schbul/sbi039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fenton WS, McGlashan TH. Testing systems for assessment of negative symptoms in schizophrenia. Arch Gen Psychiatry. 1992;49:179–184. doi: 10.1001/archpsyc.1992.01820030011002. [DOI] [PubMed] [Google Scholar]
- 23.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 24.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 25.Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT., Jr The Schedule for the Deficit syndrome: an instrument for research in schizophrenia. Psychiatry Res. 1989;30:119–123. doi: 10.1016/0165-1781(89)90153-4. [DOI] [PubMed] [Google Scholar]
- 26.Krawiecka M, Goldberg D, Vaughan M. A standardized psychiatric assessment scale for rating chronic psychotic patients. Acta Psychiatr Scand. 1977;55:299–308. doi: 10.1111/j.1600-0447.1977.tb00174.x. [DOI] [PubMed] [Google Scholar]
- 27.Lewine RR, Fogg L, Meltzer HY. Assessment of negative and positive symptoms in schizophrenia. Schizophr Bull. 1983;9:368–376. doi: 10.1093/schbul/9.3.368. [DOI] [PubMed] [Google Scholar]
- 28.Pogue-Geile MF, Harrow M. Negative symptoms in schizophrenia: their longitudinal course and prognostic importance. Schizophr Bull. 1985;11:427–439. doi: 10.1093/schbul/11.3.427. [DOI] [PubMed] [Google Scholar]
- 29.Abrams R, Taylor MA. A rating scale for emotional blunting. Am J Psychiatry. 1978;135:226–229. doi: 10.1176/ajp.135.2.226. [DOI] [PubMed] [Google Scholar]
- 30.Earnst KS, Kring AM. Construct validity of negative symptoms: an empirical and conceptual review. Clin Psychol Rev. 1997;17:167–189. doi: 10.1016/s0272-7358(96)00052-9. [DOI] [PubMed] [Google Scholar]
- 31.Peralta V, Cuesta MJ. Negative symptoms in schizophrenia: a confirmatory factor analysis of competing models. Am J Psychiatry. 1995;152:1450–1457. doi: 10.1176/ajp.152.10.1450. [DOI] [PubMed] [Google Scholar]
- 32.Peralta V, Cuesta MJ. Dimensional structure of psychotic symptoms: an item-level analysis of SAPS and SANS symptoms in psychotic disorders. Schizophr Res. 1999;38:13–26. doi: 10.1016/s0920-9964(99)00003-1. [DOI] [PubMed] [Google Scholar]
- 33.Miller DD, Arndt S, Andreasen NC. Alogia, attentional impairment, and inappropriate affect: their status in the dimensions of schizophrenia. Compr Psychiatry. 1993;34:221–226. doi: 10.1016/0010-440x(93)90002-l. [DOI] [PubMed] [Google Scholar]
- 34.Sayers SL, Curran PJ, Mueser KT. Factor structure and construct validity of the scale for the assessment of negative symptoms. Psychol Assessment. 1996;8:269–280. [Google Scholar]
- 35.Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr Bull. 2006;32:238–245. doi: 10.1093/schbul/sbj013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milev P, Ho BC, Arndt S, Andreasen NC. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162:495–506. doi: 10.1176/appi.ajp.162.3.495. [DOI] [PubMed] [Google Scholar]
- 37.Sergi MJ, Rassovsky Y, Widmark C, et al. Social cognition in schizophrenia: relationships with neurocognition and negative symptoms. Schizophr Res. 2007;90:316–324. doi: 10.1016/j.schres.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 38.Emsley R, Rabinowitz J, Torreman M. The factor structure for the Positive and Negative Syndrome Scale (PANSS) in recent-onset psychosis. Schizophr Res. 2003;61:47–57. doi: 10.1016/s0920-9964(02)00302-x. [DOI] [PubMed] [Google Scholar]
- 39.Robinson DG, Woerner MG, McMeniman M, Mendelowitz A, Bilder RM. Symptomatic and functional recovery from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry. 2004;161:473–479. doi: 10.1176/appi.ajp.161.3.473. [DOI] [PubMed] [Google Scholar]
- 40.Schooler NR. Relapse prevention and recovery in the treatment of schizophrenia. J Clin Psychiatry. 2006;67(suppl 5):19–23. [PubMed] [Google Scholar]
- 41.Bowie CR, Reichenberg A, Patterson TL, Heaton RK, Harvey PD. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. Am J Psychiatry. 2006;163:418–425. doi: 10.1176/appi.ajp.163.3.418. [DOI] [PubMed] [Google Scholar]
- 42.Ho BC, Nopoulos P, Flaum M, Arndt S, Andreasen NC. Two-year outcome in first-episode schizophrenia: predictive value of symptoms for quality of life. Am J Psychiatry. 1998;155:1196–1201. doi: 10.1176/ajp.155.9.1196. [DOI] [PubMed] [Google Scholar]
- 43.Perlick DA, Rosenheck RA, Kaczynski R, Bingham S, Collins J. Association of symptomatology and cognitive deficits to functional capacity in schizophrenia. Schizophr Res. 2008;99:192–199. doi: 10.1016/j.schres.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Addington J. Cognitive functioning and negative symptoms in schizophrenia. In: Sharma T, Harvey PD, editors. Cognition in Schizophrenia. New York, NY: Oxford University Press; 2000. pp. 193–209. [Google Scholar]
- 45.Harvey PD, Koren D, Reichenberg A, Bowie CR. Negative symptoms and cognitive deficits: what is the nature of their relationship? Schizophr Bull. 2006;32:250–258. doi: 10.1093/schbul/sbj011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bell MD, Mishara AL. Does negative symptom change relate to neurocognitive change in schizophrenia? Implications for targeted treatments. Schizophr Res. 2006;81:17–27. doi: 10.1016/j.schres.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 47.Green MF, Penn DL, Bentall R. Social cognition in schizophrenia: an NIMH Workshop on definitions, assessment, and research opportunities. Schizophr Bull. 2008 doi: 10.1093/schbul/sbm145. [published online ahead of print January 08, 2008]. doi:10.1093/schbul/sbm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heerey EA, Robinson BM, McMahon RP, Gold JM. Delay discounting in schizophrenia. Cognit Neuropsychiatry. 2007;12:213–221. doi: 10.1080/13546800601005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waltz JA, Frank MJ, Robinson BM, Gold JM. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol Psychiatry. 2007;62:756–764. doi: 10.1016/j.biopsych.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barch DM. The relationships among cognition, motivation, and emotion in schizophrenia: how much and how little we know. Schizophr Bull. 2005;31:875–881. doi: 10.1093/schbul/sbi040. [DOI] [PubMed] [Google Scholar]
- 51.Gorissen M, Sanz JC, Schmand B. Effort and cognition in schizophrenia patients. Schizophr Res. 2005;78:199–208. doi: 10.1016/j.schres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 52.Mueser KT, Sayers SL, Schooler NR, Mance RM, Haas GL. A multisite investigation of the reliability of the Scale for the Assessment of Negative Symptoms. Am J Psychiatry. 1994;151:1453–1462. doi: 10.1176/ajp.151.10.1453. [DOI] [PubMed] [Google Scholar]
- 53.Kelley ME, van Kammen DP, Allen DN. Empirical validation of primary negative symptoms: independence from effects of medication and psychosis. Am J Psychiatry. 1999;156:406–411. doi: 10.1176/ajp.156.3.406. [DOI] [PubMed] [Google Scholar]
- 54.Kimhy D, Yale S, Goetz RR, McFarr LM, Malaspina D. The factorial structure of the schedule for the deficit syndrome in schizophrenia. Schizophr Bull. 2006;32:274–278. doi: 10.1093/schbul/sbi064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kring AM, Germans MK. In: Encyclopedia of Psychology. Kazdin AE, editor. New York, NY: Oxford University Press; 2000. pp. 174–175. [Google Scholar]
- 56.Berenbaum H, Oltmanns TF. Emotional experience and expression in schizophrenia and depression. J Abnorm Psychol. 1992;101:37–44. doi: 10.1037//0021-843x.101.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burbridge JA, Barch DM. Anhedonia and the experience of emotion in individuals with schizophrenia. J Abnorm Psychol. 2007;116:30–42. doi: 10.1037/0021-843X.116.1.30. [DOI] [PubMed] [Google Scholar]
- 58.Heerey EA, Gold JM. Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. J Abnorm Psychol. 2007;116:268–278. doi: 10.1037/0021-843X.116.2.268. [DOI] [PubMed] [Google Scholar]
- 59.Kring AM, Kerr SL, Smith DA, Neale JM. Flat affect in schizophrenia does not reflect diminished subjective experience of emotion. J Abnorm Psychol. 1993;102(4):507–517. doi: 10.1037//0021-843x.102.4.507. [DOI] [PubMed] [Google Scholar]
- 60.Kring AM, Neale JM. Do schizophrenic patients show a disjunctive relationship among expressive, experiential, and psychophysiological components of emotion? J Abnorm Psychol. 1996;105:249–257. doi: 10.1037//0021-843x.105.2.249. [DOI] [PubMed] [Google Scholar]
- 61.Earnst KS, Kring AM. Emotional responding in deficit and non-deficit schizophrenia. Psychiatry Res. 1999;88:191–207. doi: 10.1016/s0165-1781(99)00083-9. [DOI] [PubMed] [Google Scholar]
- 62.Horan WP, Kring AM, Blanchard JJ. Anhedonia in schizophrenia: a review of assessment strategies. Schizophr Bull. 2006;32:259–273. doi: 10.1093/schbul/sbj009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kirkpatrick B, Buchanan RW. Anhedonia and the deficit syndrome of schizophrenia. Psychiatry Res. 1990;31:25–30. doi: 10.1016/0165-1781(90)90105-e. [DOI] [PubMed] [Google Scholar]
- 64.Loas G, Boyer P, Legrand A. Anhedonia and negative symptomatology in chronic schizophrenia. Compr Psychiatry. 1996;37:5–11. doi: 10.1016/s0010-440x(96)90043-7. [DOI] [PubMed] [Google Scholar]
- 65.Germans MK, Kring AM. Hedonic deficit in anhedonia: support for the role of approach motivation. Pers Indiv Differ. 2000;28:659–672. [Google Scholar]
- 66.Leak GK. An examination of the construct validity of the Social Anhedonia Scale. J Pers Assess. 1991;56:84–95. doi: 10.1207/s15327752jpa5601_8. [DOI] [PubMed] [Google Scholar]
- 67.Leventhal AM, Chasson GS, Tapia E, Miller EK, Pettit JW. Measuring hedonic capacity in depression: a psychometric analysis of three anhedonia scales. J Clin Psychol. 2006;62:1545–1558. doi: 10.1002/jclp.20327. [DOI] [PubMed] [Google Scholar]
- 68.Peterson CA, Knudson RM. Anhedonia: a construct validation approach. J Pers Assess. 1983;47:539–551. doi: 10.1207/s15327752jpa4705_16. [DOI] [PubMed] [Google Scholar]
- 69.Klein D. Depression and anhedonia. In: Clark DC, JF, editors. Anhedonia and Affect Deficit States. New York, NY: PMA Publishing; 1984. pp. 1–14. [Google Scholar]
- 70.Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93:253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 72.Blanchard JJ, Horan WP, Collins LM. Examining the latent structure of negative symptoms: is there a distinct subtype of negative symptom schizophrenia? Schizophr Res. Sep 15. 2005;77:151–165. doi: 10.1016/j.schres.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 73.Rosenheck R, Leslie D, Keefe R. Barriers to employment for people with schizophrenia. Am J Psychiatry. 2006;163:411–417. doi: 10.1176/appi.ajp.163.3.411. [DOI] [PubMed] [Google Scholar]
- 74.Blanchard JJ, Mueser KT, Bellack AS. Anhedonia, positive and negative affect, and social functioning in schizophrenia. Schizophr Bull. 1998;24:413–424. doi: 10.1093/oxfordjournals.schbul.a033336. [DOI] [PubMed] [Google Scholar]
- 75.Herbener ES, Harrow M, Hill SK. Change in the relationship between anhedonia and functional deficits over a 20-year period in individuals with schizophrenia. Schizophr Res. 2005;75:97–105. doi: 10.1016/j.schres.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 76.Katsanis J, Iacono WG, Beiser M, Lacey L. Clinical correlates of anhedonia and perceptual aberration in first-episode patients with schizophrenia and affective disorder. J Abnorm Psychol. 1992;101:184–191. doi: 10.1037//0021-843x.101.1.184. [DOI] [PubMed] [Google Scholar]
- 77.Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38:143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- 78.Kiang M, Christensen BK, Remington G, Kapur S. Apathy in schizophrenia: clinical correlates and association with functional outcome. Schizophr Res. 2003;63:79–88. doi: 10.1016/s0920-9964(02)00433-4. [DOI] [PubMed] [Google Scholar]
- 79.Gur RE, Kohler CG, Ragland JD, et al. Flat affect in schizophrenia: relation to emotion processing and neurocognitive measures. Schizophr Bull. 2006;32:279–287. doi: 10.1093/schbul/sbj041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salem JE, Kring AM. Flat affect and social skills in schizophrenia: evidence for their independence. Psychiatry Res. 1999;87:159–167. doi: 10.1016/s0165-1781(99)00068-2. [DOI] [PubMed] [Google Scholar]
- 81.Kurtz MM, Moberg PJ, Ragland JD, Gur RC, Gur RE. Symptoms versus neurocognitive test performance as predictors of psychosocial status in schizophrenia: a 1- and 4-year prospective study. Schizophr Bull. 2005;31:167–174. doi: 10.1093/schbul/sbi004. [DOI] [PubMed] [Google Scholar]
- 82.Marin RS. Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci. 1991;3:243–254. doi: 10.1176/jnp.3.3.243. [DOI] [PubMed] [Google Scholar]
- 83.Baker A. Simplicity. In: Zalta EN, editor. The Stanford Encyclopedia of Philosophy. 2004. [Google Scholar]
- 84.Moncrief JW, William Ockham. In: Science and Its Times: understanding the Social Significance of Scientific Discovery. Lauer J, Schlager N, editors. Detroit, Mich: Gale; 2001. pp. 302–303. Vol 2. [Google Scholar]
- 85.Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22:353–370. doi: 10.1093/schbul/22.2.353. [DOI] [PubMed] [Google Scholar]