Abstract
Studies commonly report poor performance in psychotic patients compared with controls on tasks testing a range of cognitive functions, but, because current IQ is often not matched between these groups, it is difficult to determine whether this represents a generalized deficit or specific abnormalities. Fifty-three first-episode psychosis patients and 53 healthy controls, one-to-one matched for sex, age, and full-scale current IQ, were compared on Wechsler Adult Intelligence Scale (WAIS) subtests representing indices of perceptual organization, verbal comprehension, processing speed, and working memory as well as other tests of executive function and episodic memory. The groups showed an equivalent pattern of performance on all WAIS subtests except digit symbol processing speed, on which the patients were significantly worse. Patients were also worse on measures where performance correlated with digit symbol score, namely working and verbal memory tasks. Standardized residual scores for each subtest were calculated for each patient using the difference between their actual subtest score and a predicted subtest score based on their full-scale IQ and the performance of controls. Scaled scores and residual scores were examined for relationships with clinical measures. Digit symbol–scaled score was significantly correlated with concurrent negative syndrome score at baseline, and digit symbol residual score significantly predicted residual negative symptoms at 1-year follow-up. In summary, our comparison of patients and controls precisely matched for IQ revealed that processing speed was attenuated in recent-onset schizophrenia, contributed significantly to working and episodic memory deficits, and was a prognostic factor for poor outcome at 1 year.
Keywords: schizophrenia, cognition, intelligence
Introduction
A wide range of cognitive deficits are present at the onset of psychosis in people with schizophrenia.1,2 Large-scale studies comparing schizophrenia patients, recruited in an unbiased way as they present with their first psychotic episode, with healthy controls, recruited from the same community, find decrements in memory and executive function as well as Wechsler Adult Intelligence Scale (WAIS) IQ.3–6 When general intellectual ability is controlled by matching for WAIS IQ, impairments in memory and executive function can still be discerned in patients with first-episode and more long-term schizophrenia.7–11 Such findings suggest that impairments in these cognitive domains are central to schizophrenia.
Matching for WAIS IQ does not ensure equivalence on the cognitive components contributing to general ability. For example, schizophrenia patients tend to have higher verbal IQ relative to performance IQ when compared with IQ-matched controls.1,10–12 Wilk et al13 investigated this in more detail by comparing the performance of Wechsler Adult Intelligence Scale III (WAIS-III) IQ–matched schizophrenia patients and healthy controls on the indices of working memory, processing speed, perceptual organization, and verbal comprehension. They found that control performance was equivalent across all 4 indices, but patients were better than controls on verbal comprehension and perceptual organization and worse on working memory and processing speed. The processing speed index, made up of digit symbol coding and symbol search subtests, showed the biggest effect size for discriminating patient and control performance, a finding which applied even to a subgroup of matched pairs with IQ in the high-average range. This finding is in agreement with a recent meta-analysis,14 which showed that, out of a wide range of neuropsychological measures, performance on the digit-symbol test was by far the most sensitive index differentiating schizophrenia patients from controls. Two compatible studies15,16 have shown that schizophrenia patients with normal intellectual function are impaired on a psychophysiological measure of processing speed, inspection time. Others have found that performance on tests of processing speed are significantly correlated with neuropsychological indices of memory and executive function in schizophrenia patients.17–21
Taken together these findings suggest that patients with schizophrenia may be impaired on tests of memory and executive function when matched to controls on IQ because the tests share aspects of performance linked to processing speed rather than because they represent separable cognitive functions. To test this hypothesis, we adopted the approach of Wilk al13 and matched schizophrenia patients with healthy volunteers, one-to-one on current IQ; we additionally matched subjects for age and sex. We used a 4 subtest version of the WAIS-III that included digit symbol coding and has been validated for use with schizophrenia.22 We also compared the groups on estimated premorbid IQ and measures of memory and executive function. We predicted that, although the groups were precisely matched for IQ, differences in processing speed would still be evident and that this would be highly associated with impairments in memory and executive function in the patients. Patients were tested at the time of presentation with their first psychotic episode and 1 year later to examine the trajectory of these cognitive functions in the context of symptom change and longer term medication effects.
Methods
Participants
The patients were recruited for this study as part of a longitudinal study of first-episode psychosis in West London. Patients were screened for eligibility for the study using the World Health Organization (WHO) Psychosis Screen,23 and the inclusion criteria were as follows: aged between 16 and 50 years, presenting with a psychotic illness for the first time, and had no more than 12 weeks cumulative exposure to antipsychotic medication prior to baseline assessment. In each case, the diagnosis was ascertained using a structured interview, the diagnostic module of the Diagnostic Interview for Psychosis,24 which includes items from the Operational Criteria Checklist for Psychosis25 (OPCRIT) and the WHO Schedules for Clinical Assessment in Neuropsychiatry.26 Healthy volunteers served as control subjects and were recruited from the same catchment area as patients by advertising in local colleges and hospitals. For the controls, participants were excluded if they had a history of psychiatric illness in themselves or their first-degree relatives. For both groups, exclusion criteria were previous head injury or other neurological illness or endocrine disorder affecting brain function, such as epilepsy and thyroid disease, and drug or alcohol dependence.
Fifty-three patients and 53 controls were selected for this study on the basis that they had an initial diagnosis of schizophrenia, schizophreniform, or schizoaffective disorder; a current WAIS IQ of ≥70; and could be individually matched with controls on sex, WAIS IQ within 3 points, and age within 10 years. Twenty-three patient-control pairs had exactly the same IQ, 5 within one point, 21 within 2 points, and 4 within 3 points. The mean difference in age for the pairs was 3.45 years (see table 1). As part of the prospective, longitudinal study, the patients and the community mental health teams are routinely contacted 1 year later at which time the diagnosis is reviewed. Thirty-nine patients agreed to undergo a repeat diagnostic interview. The diagnosis of the remaining 14 patients was established by 2 psychiatrists (T.R.E.B. and E.M.J.) using the OPCRIT to compile information from the responsible psychiatrists, community psychiatric nurses, and the clinical notes. The final Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, diagnoses at follow-up were schizophrenia in 41 patients and schizoaffective disorder in 12 patients.
Table 1.
Comparison of Schizophrenia and Control Groups on Demographic Details, Executive Function, and Memory
| Patients | Controls | Comparison | |
| N | 53 | 53 | |
| Sex | 34 M/19 F | 34 M/19 F | |
| Age in years: mean (SD) | 26.77 (7.75) | 26.49 (6.70) | F1,105 = 0.04, P = .841 |
| Years of education: mean (SD) | 12.79 (2.07) | 13.74 (2.14) | F1,105 = 5.32, P = .023 |
| Cognitive measures: mean (SD) | |||
| Premorbid IQ (WTAR) | 101.30 (9.27) | 97.75 (8.91) | F1,105 = 4.04, P = .047 |
| Current IQ | 98.67 (13.68) | 98.32 (13.65) | F1,105 = 0.02, P = .893 |
| Spatial Span | 5.84 (1.19) | 6.37 (1.33) | F1,103 = 4.39, P = .039 |
| Spatial Working Memory (errors) | 13.51 (9.80) | 8.90 (8,84) | F1,103 = 14.47, P < .001a |
| Tower of London (perfect solutions) | 7.49 (2.69) | 8.42 (1.91) | F1,101 = 3.13, P = .08 |
| IDED (extradimensional shift errors) | 13.51 (9.80) | 8.90 (8.83) | F1,101 = 6.22, P = .014 |
| Verbal memory (summed recall over learning trials) | 42.98 (8.32) | 48.71 (10.25) | F1,105 = 10.00, P = .002a |
| Pattern Recognition Memory (number correctly recognized) | 19.73 (3.05) | 21.44 (2.45) | F1,103 = 9.97, P = .002a |
Note: M, male; F, female; WTAR, Wechsler Test of Adult Reading; IDED, Intra/Extra Dimensional Set Shift.
Significant after Bonferroni correction (P < .005).
Of the initial 53 patient-control pairs, the same neuropsychological assessments 1 year after baseline assessment were available in 29 pairs. At the time of baseline testing, 3 patients were not being prescribed antipsychotic medication, 2 were being prescribed first-generation antipsychotics, and 48 second-generation antipsychotics. Two patients were also being prescribed anticholinergic medication. Of those who underwent neuropsychological reassessment at 1-year follow-up (n = 29), those prescribed second-generation antipsychotics at baseline continued with the same medications, one had been switched from first- to second-generation antipsychotics, and the remaining patient who was drug naive was being prescribed second-generation antipsychotics. None of this subgroup was being prescribed anticholinergic medication at either time point. Permission to conduct the study was obtained from Merton, Sutton and Wandsworth, Riverside, and Ealing Research Ethics Committees. All participants gave written informed consent and were paid an honorarium for their time.
Clinical Assessments
Psychotic symptoms were assessed in all patients with the Scales for the Assessment of Positive27 and Negative28 Symptoms. Scores for the 3 symptom-derived syndromes of schizophrenia (positive, negative, disorganization) were calculated for each patient.29 The Hamilton Rating Scale for Depression30 and The Young Mania Scale31 were used to assess affective symptoms. To establish the timing of onset of the psychotic illness, the Nottingham Onset Scale32 (NOS) was used. The NOS is a short, guided interview and rating scale that records the details of the components of onset of a psychotic illness. It defines onset as comprising (a) a prodrome of 2 phases: a period of “unease” followed by a period of “nondiagnostic” symptoms, (b) the emergence of psychotic symptoms, and (c) the buildup of diagnostic symptoms leading to a definite diagnosis. The duration of untreated psychosis (DUP) was taken as the period of time between stage b and treatment onset. Age at onset was taken as the age when stage b was reached.
Neuropsychological Assessment
Current IQ was measured using a short form of the WAIS-III validated for use with schizophrenia.22 This comprised 4 subtests from the WAIS-III,33 each representing 1 of the 4 indices: information (verbal comprehension index), arithmetic (working memory index), block design (perceptual organization index), and digit symbol (processing speed index). A prorated full-scale IQ (FSIQ) was calculated using these 4 subtests, and this method has been shown to provide a reliable measure of FSIQ in psychosis.22,34 Premorbid IQ was estimated using the Wechsler Test of Adult Reading35 (WTAR).
Memory and executive function tests were taken from the Cambridge Automated Neuropsychological Test Battery.36 The measures employed were as follows. Attentional set shifting: Taken from the Intra/Extradimensional Set Shift task. The number of errors made during the extradimensional shift stage was used. This stage measures the ability to inhibit an attentional set toward one dimension of a stimulus and switch attention to another dimension of the same stimulus. Planning: Taken from the stockings of Cambridge task. This is analogous to the Tower of London task. In a series of problems varying in difficulty, subjects plan and execute a sequence of moves of stimuli in a visual array to match a goal array. The number of moves required range from 2 to 5 with 12 trials in total. The total number of perfect solutions was measured. Working memory manipulation: Taken from the Spatial Working Memory task. This is a self-ordered search task whereby participants need to recall where previous “tokens” were found from a random array of “boxes” in order to maximize success at finding subsequent “tokens.” The number of search errors was measured. Working memory span: Taken from the Spatial Span task. This test of forward spatial span is akin to the Corsi block test. The maximum number of consecutively presented spatial locations that were successfully recalled was measured. Visual recognition memory: Taken from the Pattern Recognition Memory test. This task requires identification of previously presented abstract patterns in a 2-choice recognition paradigm. The number of patterns correctly identified was measured.
Verbal learning and memory were measured with the Rey Auditory Verbal Learning Task.37 The participant is repeatedly read a list of 15 nouns and required to recall as many as possible immediately after each trial and again after presentation of a distractor list and finally after a 25- to 30-minute delay. The sum of words recalled over the 5 learning trials was used as a measure of episodic verbal recall memory.
Analysis
Data were analyzed using SPSS version 15. Group differences were examined with analysis of variance (ANOVA) or covariance (ANCOVA) and paired t test where appropriate. Pearson r correlations were used to examine associations between measures. Stepped linear regression was used to determine whether any cognitive measures predicted clinical outcome. Wilcoxon signed ranks test was used for repeated-measures analyses of categorical scores.
In additional to scaled scores, WAIS-III subtest–standardized residual scores38 were calculated for the patients. A standardized residual score reflects the degree to which performance on each particular WAIS subtest deviates from that predicted by the FSIQ score in each individual, based on the relationship between FSIQ and the relevant subtest in the control group. The score is calculated for each subtest by regressing the relevant scaled score on FSIQ using the control group data then calculating a residual score for each patient based on this equation. Finally, the residual score was divided by the SE of the regression in the control group giving a standardized residual score for each patient on each subtest.
Results
A mixed within- and between-subjects ANOVA with the 4 WAIS subtest–scaled scores entered as the within-subjects factor and group as the between-subjects factor revealed a main effect of subtest (F3,312 = 38.99, P < .001, 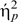 =0.27 indicating that the performance profile for both groups was not flat (see figure 1). To explore this further, post hoc within-subjects contrasts were performed across all subjects. The mean of each subtest was compared with the grand mean of all other subtests, showing that the significant main effect reflected individual subtest-scaled scores that were lower than average for arithmetic (F = 6.78, P = .005) and digit symbol (F = 67.15, P < .001) and higher than average for information (F = 94.58, P < .001) and block design (F = 8.38, P = .005).
=0.27 indicating that the performance profile for both groups was not flat (see figure 1). To explore this further, post hoc within-subjects contrasts were performed across all subjects. The mean of each subtest was compared with the grand mean of all other subtests, showing that the significant main effect reflected individual subtest-scaled scores that were lower than average for arithmetic (F = 6.78, P = .005) and digit symbol (F = 67.15, P < .001) and higher than average for information (F = 94.58, P < .001) and block design (F = 8.38, P = .005).
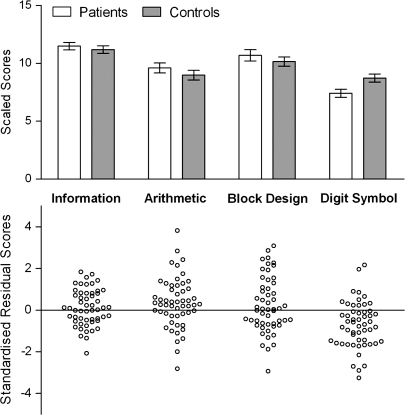
Fig. 1.
Scaled Scores and Standardized Residual Scores for Each Wechsler Adult Intelligence Scale III Subtests. Mean patient and control data are shown for scaled scores, and individual patient data are shown for the residual scores.
There was also a significant interaction between subtest and group (F3,312 = 4.10, P = .007, 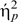 =0.04). Univariate ANOVAs revealed that the groups were not significantly different on information (F1,105 = 0.44, P = .510), arithmetic (F1,105 = 1.09, P = .300), and block design (F1,105 = 0.74, P = .393) but that the patients had significantly lower digit symbol–scaled scores than the controls (F1,105 = 7.16, P = .009) as illustrated in figure 1. Figure 1 also shows the standardized residual scores of each patient for each subtest illustrating the degree to which performance on each subtest differed from that predicted. A repeated-measures ANOVA on the standardized residual scores showed a significant effect of subtest (F3,156 = 7.30, P < .001,
=0.04). Univariate ANOVAs revealed that the groups were not significantly different on information (F1,105 = 0.44, P = .510), arithmetic (F1,105 = 1.09, P = .300), and block design (F1,105 = 0.74, P = .393) but that the patients had significantly lower digit symbol–scaled scores than the controls (F1,105 = 7.16, P = .009) as illustrated in figure 1. Figure 1 also shows the standardized residual scores of each patient for each subtest illustrating the degree to which performance on each subtest differed from that predicted. A repeated-measures ANOVA on the standardized residual scores showed a significant effect of subtest (F3,156 = 7.30, P < .001, 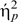 =0.12). Post hoc within-subjects contrasts comparing digit symbol residual scores to each of the other 3 scores confirmed that patient performance was significantly worse than predicted on digit symbol as compared with information (F = 13.22, P = .001), arithmetic (F = 17.43, P < .001), and block design (F = 12.25, P = .001), all of which survived Bonferroni correction (P < .016).
=0.12). Post hoc within-subjects contrasts comparing digit symbol residual scores to each of the other 3 scores confirmed that patient performance was significantly worse than predicted on digit symbol as compared with information (F = 13.22, P = .001), arithmetic (F = 17.43, P < .001), and block design (F = 12.25, P = .001), all of which survived Bonferroni correction (P < .016).
Univariate ANOVAs revealed that the patients had significantly poorer performance than controls on the measures of memory and executive function with verbal recall memory, working memory manipulation and visual recognition memory remaining significant after Bonferroni correction (see table 1).
Pearson r correlations showed IQ to be significantly correlated with all memory and executive scores in both groups (see table 2). Digit symbol performance correlated highly with working memory manipulation in both groups and with verbal learning in the patient group. Because digit symbol score differentiated patients from controls in addition to these 2 variables, we performed ANCOVAs to determine the degree to which group differences in working memory manipulation and verbal learning was associated with differences in digit symbol performance. For working memory manipulation, digit symbol was a highly significant covariate (F1,100 = 16.01, P < .001), but the difference between the 2 groups remained significant (F1,100 = 8.62, P = .004). For verbal learning, digit symbol was also a highly significant covariate (F1,106 = 31.74, P < .001), but again the difference between the 2 groups remained significant (F1,106 = 4.21, P = .043).
Table 2.
Correlations Between IQ and WAIS Subtest–Scaled Scores and Other Neuropsychological Measures in Patients and Controls
| Controls | Patients | |||||||||
| FSIQ | Information | Arithmetic | Block Design | Digit Symbol | FSIQ | Information | Arithmetic | Block Design | Digit Symbol | |
| Spatial Span | 0.28* | 0.13 | 0.32* | 0.09 | 0.23 | 0.44** | 0.30* | 0.27 | 0.33* | 0.30* |
| Spatial Working Memory (errors) | 0.46** | 0.26 | 0.44*** | 0.38** | 0.26 | 0.53*** | 0.37** | 0.42** | 0.25 | 0.50*** |
| IDED (extradimensional shift errors) | 0.45*** | 0.30* | 0.38** | 0.48*** | 0.13 | 0.40** | 0.29* | 0.31* | 0.38** | 0.12 |
| Verbal learning (summed recall over learning trials) | 0.47*** | 0.38** | 0.32* | 0.25 | 0.43** | 0.31* | 0.13 | 0.05 | 0.20 | 0.55*** |
| Pattern Recognition Memory (number correctly recalled) | 0.28* | 0.27 | 0.12 | 0.17 | 0.27 | 0.37** | 0.29* | 0.38** | 0.17 | 0.21 |
Note: WAIS, Wechsler Adult Intelligence Scale; FSIQ, full-scale IQ. Emboldened figures are significant after Bonferroni correction (P < .002).
*Significant at P < .05; **significant at P < .01; ***significant at P < .001.
To test the specificity of the relationship between digit symbol performance and other cognitive variables, we analyzed a patient subgroup that obtained a scaled score of <9 on the digit symbol subtest and were therefore in the lowest quartile of the general population according to the WAIS-III scoring manual (n = 38). As in the group as a whole, Pearson r correlations showed no significant correlation between the digit symbol score and visual recognition memory (r = 0.08), working memory span (r = 0.15), planning (r = 0.05), or set shifting (r = 0.10).
Change Over Time
Twenty-nine pairs of participants underwent retesting after approximately 12 months. With regard to the patients, those that were tested as a follow-up pair did not differ significantly from those that were not on IQ, age at first assessment, psychotic syndrome scores, scores for depression and mania, DUP, and age at onset (range of Fs = 0.01–1.60, all P > .1).
Wilcoxon signed ranks showed that those retested experienced significant improvement in symptoms. Nineteen showed reduced negative syndrome (z = −2.10, P = .036), 22 had reduced positive syndrome (z = −3.65, P < .001), and 20 had reduced disorganization syndrome (z = −3.79, P < .001).
Table 3 shows the mean first-episode and follow-up WAIS-III IQ and subtest-scaled scores for patients and controls. For IQ, a repeated-measures ANOVA was performed with time as the within-subjects factor (baseline, 1 year) and group as the between-subjects factor. The main effect of time was not significant (F1,56 = 1.46, P = .233, 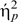 = 0.03), but there was a significant interaction between time and group (F1,56 = 7.79, P = .007,
= 0.03), but there was a significant interaction between time and group (F1,56 = 7.79, P = .007, 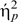 = 0.12). The individual scaled scores were therefore examined. There were no significant main effects or interactions for information (time: F1,56 = 2.73, P = .104,
= 0.12). The individual scaled scores were therefore examined. There were no significant main effects or interactions for information (time: F1,56 = 2.73, P = .104, 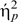 = 0.05; time × group: F1,56 = 1.28, P = .263,
= 0.05; time × group: F1,56 = 1.28, P = .263, 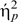 = 0.02), arithmetic (time: F1,56 = 0.34, P = .565,
= 0.02), arithmetic (time: F1,56 = 0.34, P = .565, 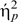 = 0.01; time × group: F1,56 = 162, P = .208,
= 0.01; time × group: F1,56 = 162, P = .208, 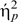 = 0.03), or block design (time: F1,56 = 0.01, P = .959,
= 0.03), or block design (time: F1,56 = 0.01, P = .959, 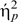 = 0.00; time × group: F1,56 = 1.17, P = .285,
= 0.00; time × group: F1,56 = 1.17, P = .285, 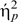 = 0.02). For digit symbol, there was no effect of time (F1,56 = 0.64, P = .426,
= 0.02). For digit symbol, there was no effect of time (F1,56 = 0.64, P = .426, 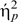 = 0.01), but there was a significant interaction between time and group (F1,56 = 8.35, P = .005,
= 0.01), but there was a significant interaction between time and group (F1,56 = 8.35, P = .005, 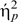 = 0.13). Paired t tests showed that this reflected a nonsignificant decline in the patients (t28 = 1.44, P = .162) and a significant improvement in the controls (t28 = 3.02, P = .005).
= 0.13). Paired t tests showed that this reflected a nonsignificant decline in the patients (t28 = 1.44, P = .162) and a significant improvement in the controls (t28 = 3.02, P = .005).
Table 3.
First-Episode and 1-Year Follow-up IQ and Scaled Scores of the Patients and Controls on Each WAIS Subtest
| WAIS Subtest: Mean (SD) | Patients, n = 29 | Controls, n = 29 | ||
| Initial | Follow-up | Initial | Follow-up | |
| FSIQ | 98.62 (11.75) | 97.10 (9.73) | 98.24 (11.82) | 102.07 (11.43) |
| Information | 11.45 (2.28) | 11.55 (2.28) | 11.21 (2.06) | 11.76 (2.40) |
| Arithmetic | 9.69 (2.84) | 9.48 (2.59) | 9.03 (2.92) | 9.59 (2.81) |
| Block design | 10.24 (2.98) | 9.90 (1.99) | 10.38 (3.14) | 10.76 (2.82) |
| Digit symbol | 7.98 (2.74) | 7.51 (2.43) | 8.52 (2.16) | 9.31 (2.17) |
Note: WAIS, Wechsler Adult Intelligence Scale; FSIQ, full-scale IQ.
Relationship With Clinical Variables
Pearson r correlations revealed that the negative syndrome score was significantly and inversely correlated with FSIQ, digit symbol–scaled score, and digit symbol–standardized residual score at initial assessment (see table 4). The disorganization syndrome score was significantly inversely correlated with block design–scaled score. The Young Mania Scale score was also inversely correlated with block design deviation score and positively correlated with arithmetic deviation score. However, only the correlation between the negative syndrome score and digit symbol–scaled score withstood Bonferroni correction for multiple comparisons (P = .0008). Pearson r correlations revealed that digit symbol score was significantly correlated with all the global subscale scores comprising the negative syndrome: affective flattening (r = −0.57), alogia (r = −0.48), and anhedonia/asociality (r = −0.44) were all correlated at P < .001; avolition/apathy (r = −0.41) at P < .01; and attention (r = −0.30) at P < .05.
Table 4.
Pearson r Correlations Between IQ, Scaled Scores, and Standardized Residual Scores for Each of the 4 WAIS-III Subtests and Clinical Measures at First Presentation
| FSIQ | Scaled Scores | Standardized Residual Scores | |||||||
| Information | Arithmetic | Block Design | Digit Symbol | Information | Arithmetic | Block Design | Digit Symbol | ||
| DUP (wk) | −0.03 | −0.02 | 0.05 | 0.02 | −0.19 | 0.01 | 0.12 | 0.06 | −0.19 |
| Age at onset of psychosis | 0.02 | −0.11 | 0.11 | −0.15 | 0.24 | −0.18 | 0.14 | −0.22 | 0.26 |
| Negative syndrome | −0.32* | −0.11 | −0.09 | −0.18 | −0.56 | 0.18 | 0.24 | 0.02 | −0.41** |
| Positive syndrome | 0.05 | 0.11 | 0.12 | −0.06 | 0.05 | 0.12 | 0.11 | −0.13 | 0.02 |
| Disorganization syndrome | −0.23 | −0.15 | 0.00 | −0.29* | −0.18 | 0.02 | 0.28* | −0.22 | −0.04 |
| Hamilton Depression Scale | 0.01 | 0.02 | 0.03 | −0.03 | 0.04 | 0.02 | 0.03 | −0.05 | 0.04 |
| Young Mania Scale | 0.06 | −0.01 | 0.26 | −0.20 | 0.16 | −0.08 | 0.32* | −0.34* | 0.14 |
Note: WAIS-III, Wechsler Adult Intelligence Scale III; FSIQ, full-scale IQ; DUP, duration of untreated psychosis.
*Significant at P < .05; **significant at P < .01; ***significant at P < .001.
Predictors of 1-Year Clinical Outcome From Baseline WAIS-III Subscale Scores
We used the significant (P < .05) correlations between baseline clinical and WAIS-III scores to determine the analysis of 1-year clinical outcome in those pairs that had been successfully followed up. In each case, a linear regression was used to determine whether the relevant baseline cognitive score was a significant predictor of the 1-year clinical measure, once the equivalent baseline clinical measure was already entered as a predictor in the model. Thus, controlling for baseline symptoms, we found no effects of block design on disorganization (first step: baseline syndrome score r2 = 0.02, F1,27 = 0.37, P = .550; second step: baseline block design–scaled score r2 = 0.02, F1,26 = 0.70, P = .409), arithmetic on disorganization (first step: baseline syndrome score r2 = 0.01, F1,27 = 0.37, P = .550; second step: baseline block design–scaled score r2 = 0.05, F1,26 = 1.17, P = .290), arithmetic on mania (first step: baseline mania score r2 = 0.01, F1,27 = 0.28, P = .602; second step: baseline arithmetic-standardized residual score r2 = 0.03, F1,26 = 0.70, P = .409), or block design on mania (first step: baseline mania score r2 = 0.01, F1,27 = 0.28, P = .602; second step: baseline block design–standardized residual score r2 = 0.04, F1,26 = 1.106, P = .312). Regarding the negative syndrome, digit symbol–scaled score had no effect (first step: baseline syndrome score r2 = 0.11, F1,27 = 3.45, P = .074; second step: baseline digit symbol–scaled score r2 = 0.01, F1,26 = 0.35, P = .559), but digit symbol–standardized residual score accounted for a significant amount of the variance in the negative syndrome score once the baseline negative syndrome had been taken into account (second step: baseline digit symbol–standardized residual score r2 = 0.16, F1,26 = 5.83, P = .023).
Discussion
In this study, we compared patients with recent-onset schizophrenia and healthy controls, individually matched for sex, age, and WAIS-III IQ on scaled scores of the 4 subtests that contributed to their IQ score.22 The main aim of the study was to ascertain whether performance on a test of processing speed, digit symbol coding, is disproportionally impaired in patients with recent-onset schizophrenia who otherwise have intact intellectual function. A second aim was to examine the relationship between processing speed and memory and executive function as well as symptoms at first episode and over time. We found that the patients were significantly impaired on digit symbol coding but were no different from controls on information, arithmetic, or block design. Standardized residual scores of the patients, based on control performance, indicated that only performance on the digit symbol subtest deviated significantly from that expected given their IQ. When we retested a subgroup of patient-control pairs 1 year later, the controls showed significant improvement in digit symbol performance but the patients showed no change despite significant amelioration of symptoms with treatment, a finding compatible with a previous longitudinal study of first-episode schizophrenia.39 Our findings are also consistent with the results of a recent meta-analysis14 showing that, out of a large range of neuropsychological tests, digit symbol coding was by far the most sensitive marker of cognitive impairment in schizophrenia.14 The authors also showed that digit symbol impairment was the most sensitive indicator of cognitive impairment in nonpsychotic relatives of patients with schizophrenia and point out that performance on this task has been shown to differentiate siblings at high risk of schizophrenia who later became psychotic from those who do not.40 Taken together, these findings indicate that impaired digit symbol performance is a stable trait in schizophrenia and reflects an abnormal cognitive process central to the disorder: it is present at the onset of psychosis in the context of intact performance on other tests of general intellectual ability; it is impervious to practice effects and is unaffected by antipsychotic medication over the first year of the illness.
Digit symbol performance was also sensitive to symptom severity in our study. We correlated IQ and individual subtest scores with psychotic and affective symptoms, age of onset, and DUP. For each subtest, we considered the scaled score and the standardized residual score. The results showed several modestly significant relationships between clinical and cognitive measures, but, following correction for multiple comparisons, only an inverse relationship between the negative syndrome and digit symbol–scaled score remained significant. When we examined the predictive value of digit symbol performance in a subset of patients for whom we also had follow-up data, the digit symbol–standardized residual score was found to predict a significant amount of the remaining variance in the 1-year negative syndrome score after having accounted for the contribution of negative symptoms at presentation. Thus, the magnitude of relative impairment in digit symbol performance at the first psychotic episode was a prognostic factor for poor early outcome with respect to the development or persistence of negative symptoms. The contribution of the first-episode digit symbol–standardized residual score to the negative syndrome at follow-up was 20% after baseline negative syndrome score was already entered into the model. This suggests that the finding is not simply an artifact of measurement whereby the scales for negative syndrome and digit symbol subtest are measuring a shared characteristic. This distinction is important because digit symbol is a timed task and the negative syndrome incorporates concepts such as avolition, affective flattening, and attention that could adversely impact on reaction times.
In this study, we also found that, despite precisely matching for IQ, patients were impaired on measures of memory and executive function, replicating our findings in a different, larger group of first-episode patients5,7 and in keeping with studies of patients with more longstanding illness.8–11,15 The differences were particularly large for verbal learning, visual recognition memory, and a measure of spatial working memory manipulation. Verbal learning and spatial working memory correlated strongly with digit symbol performance in the patients and to a lesser extent in controls. Because neither the verbal learning nor working memory tests had a timed component, the association with digit symbol was not secondary to a common procedural factor. One explanation is that the cognitive processes measured by the verbal memory and spatial working memory tests were hampered in the patients by poor information processing speed.41 An alternative explanation however is that the association was due to both sets of measures being sensitive to a generalized impairment. Other first-episode studies have found that a significant proportion of the performance variance across a number of neuropsychological tests is accounted for by a single general factor in data reduction models.42,43 One of these studies included the digit symbol test and found that the weight of loading of this test score onto the general factor was similar in magnitude to that of a number of other neuropsychological tests.42 Although digit symbol was the only IQ subtest to discriminate patients and controls in our study, it is noteworthy that IQ, as a composite measure of general intellectual ability, was in both groups a better correlate of performance on the tests of memory and executive function than the IQ subtests taken individually. Thus, performance on the digit symbol test in our study may have contributed to a general ability factor underpinning performance on the verbal learning and spatial working memory tests rather than it being uniquely relevant for these cognitive functions. In addition, although further analysis showed a highly significant digit symbol covariate effect, significant differences between the groups remained on verbal learning and spatial working memory tests suggesting that, even if processing speed contributed to performance, other cognitive processes were also important in discriminating patients from controls. Finally, the correlations between digit symbol performance and measures of set shifting and visual recognition memory were small and not statistically significant, even when we examined this in a subgroup of patients with extremely poor digit symbol performance. This suggests that not all aspects of neuropsychological performance in schizophrenia can be attributed to slow information processing speed, at least as measured by this test.
In keeping with the argument that digit symbol performance is a relatively nonspecific indicator of cognitive dysfunction, deficits on this test have been demonstrated in a number of different cognitive disorders suggesting that it may be multifactorial in nature.37 Thus, the findings in schizophrenia may be explained by the psychometric properties of the test rather than it identifying a fundamental cognitive process that is impaired above all others. Joy et al44,45 suggest, with experimental support, that digit symbol performance in young healthy subjects is comprised of independent contributions from motor speed, cognitive speed, and visual scanning efficiency primarily and, to a lesser extent, from visual memory (for digit-symbol pairs) and set-shifting ability (between digits and symbols). Two further studies,46,47 also in young healthy subjects, analyzed eye movements during digit symbol performance and found that time spent inspecting the code correlated highly with performance whereas time spent writing did not. This suggests that the speed of searching and encoding information was a more important process than simple motor speed for performance. Taken together, these studies indicate that digit symbol is primarily a test of speed, particularly cognitive speed, in young healthy adults. There are indications that this is also the case in schizophrenia,48 but further studies are required to decompose the processes contributing to digit symbol performance in this disorder.14
There is also accumulating and convergent evidence from other studies that tests of processing speed are indexing something fundamental about schizophrenia. For example, several studies in addition to ours have reported specific relationships between the negative syndrome and processing speed.49–52 Of particular note is a study50 that used relatively unconfounded measures of processing speed in different sensory modalities in a large sample of patients and found that the negative syndrome, but not disorganization or positive syndromes, accounted for a significant amount of the variance in auditory reaction time and visual backward masking measures. Other studies, like ours, have found significant statistical associations between performance on tests of processing speed and other neuropsychological functions in schizophrenia,17–21 and Badcock16 has shown that the psychophysiological measure, inspection time, inversely correlated with performance IQ. However, because these are association studies, it is difficult to conclude that measures of processing speed reflect a process that mediates individual differences in performance of the more complex psychometric tasks. Experimental work has addressed this point more specifically. For example, by manipulating target exposure time or posttarget processing time, studies have shown that impaired working memory performance reflects, to a significant extent, slowing of information processing speed.53,54 We have previously shown, by analyzing eye movements during performance of a complex planning task, that patients were not generally slow and approached and executed the task in the same strategic manner as controls; the only difference between the groups explaining poorer performance in the patients was that they spent longer fixating targets suggesting that they had difficulty in encoding features essential for performance.55
Several other aspects of our study deserve comment. The first concerns the representativeness of the sample. The IQ of both the patients and controls group ranged from “borderline low” to “superior.” However, because IQ is normally lower in schizophrenia than in healthy subjects, our patient group was inevitably biased toward the higher end of the IQ spectrum for this disorder in order to obtain one-to-one IQ matching with controls. Our findings therefore may not be typical for schizophrenia. Nevertheless, this method allowed us to detect certain cognitive impairments even in patients who would be considered normal on the basis of their intellectual ability.56,57 The elucidation of cognitive impairment in the context of groups matched for normal IQ has also been a positive strategy in previous studies of schizophrenia.10,11
A second point concerns why patients scored the same IQ as controls despite being worse on digit symbol that contributed to the IQ calculation. In accordance with the study of Wilk et al,13 we had expected that any subtest impairment in patients would be “balanced” by better performance in others. There was some support for this in that patients were numerically better than controls on the other 3 subtests, but none of the differences were significant. A further indication that the patients were better than controls on some aspects of cognition comes from the finding that premorbid IQ was significantly higher in the patients. Premorbid IQ was estimated using a reading test, the WTAR, which assesses knowledge of irregular word pronunciation and is therefore a measure of vocabulary. Vocabulary in turn is a measure of crystalline intelligence that is more impervious to brain changes than fluid intelligence, reflected in problem solving ability and processing speed.58 Although the differences between premorbid and current IQ estimates were small, they suggest that the patients had undergone intellectual decline as a consequence of the illness and are thus in keeping with the findings of other studies.5–11,13,15
In summary, our findings add to a growing body of work demonstrating the importance of processing speed for cognitive function and clinical outcome in schizophrenia. We found that an index of processing speed, digit symbol coding, was disproportionately impaired early in the course of the illness and that this was highly associated with working memory and verbal learning impairment; cognitive domains frequently reported to be abnormal in schizophrenia. This processing speed measure was also uniquely related to symptoms, in that it was inversely correlated with the severity of current negative symptoms and the relative level of underperformance in processing speed predicted the severity of negative symptoms 1 year later. Further work is required to ascertain whether processing speed is specific and fundamental to higher order cognitive processes in schizophrenia or whether it is one component of a more generalized deficit.
Funding
Wellcome Trust (064607); The Raymond Way Fund to E.M.J.
Acknowledgments
We are grateful to the consultants and nurses of West London and South West London and St George's Mental Health National Health Service Trusts for greatly facilitating the study. We thank the 2 anonymous reviewers for their helpful suggestions on an earlier draft of this article. Barnes has acted as a consultant for Servier, Johnson & Johnson, and Bristol-Myers Squibb. Leeson, M. Harrison, Matheson, I. Harrison, Mutsutsa, Ron, and Joyce have no biomedical financial interests or potential conflicts of interest.
References
- 1.Bilder RM, Lipschutz-Broch L, Reiter G, Geisler SH, Mayerhoff DI, Lieberman JA. Intellectual deficits in first-episode schizophrenia: evidence for progressive deterioration. Schizophr Bull. 1992;18:437–448. doi: 10.1093/schbul/18.3.437. [DOI] [PubMed] [Google Scholar]
- 2.Hoff AL, Riordan H, O'Donnell DW, Morris L, DeLisi LE. Neuropsychological functioning of first-episode schizophreniform patients. Am J Psychiatry. 1992;149:898–903. doi: 10.1176/ajp.149.7.898. [DOI] [PubMed] [Google Scholar]
- 3.Addington J, Brooks BL, Addington D. Cognitive functioning in first episode psychosis: initial presentation. Schizophr Res. 2003;62:59–64. doi: 10.1016/s0920-9964(02)00340-7. [DOI] [PubMed] [Google Scholar]
- 4.Bilder RM, Goldman RS, Robinson D, et al. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry. 2000;157:549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- 5.Joyce EM, Hutton SB, Mutsatsa SH, Barnes TRE. Cognitive heterogeneity in first-episode schizophrenia. Br J Psychiatry. 2005;187:516–522. doi: 10.1192/bjp.187.6.516. [DOI] [PubMed] [Google Scholar]
- 6.Mohamed S, Paulsen JS, O'Leary D, Arndt S, Andreasen N. Generalized cognitive deficits in schizophrenia: a study of first-episode patients. Arch Gen Psychiatry. 1999;56:749–754. doi: 10.1001/archpsyc.56.8.749. [DOI] [PubMed] [Google Scholar]
- 7.Hutton SB, Huddy V, Barnes TRE, et al. The relationship between antisaccades, smooth pursuit, and executive dysfunction in first-episode schizophrenia. Biol Psychiatry. 2004;56:553–559. doi: 10.1016/j.biopsych.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Elliott R, McKenna PJ, Robbins TW, Sahakian BJ. Neuropsychological evidence for frontostriatal dysfunction in schizophrenia. Psychol Med. 1995;25:619–630. doi: 10.1017/s0033291700033523. [DOI] [PubMed] [Google Scholar]
- 9.Weickert TW, Goldberg TE, Gold JM, Bigelow LB, Egan MF, Weinberger DR. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Arch Gen Psychiatry. 2000;57:907–913. doi: 10.1001/archpsyc.57.9.907. [DOI] [PubMed] [Google Scholar]
- 10.Kremen WS, Seidman LJ, Faraone SV, Tsuang MT. Intelligence quotient and neuropsychological profiles in patients with schizophrenia and in normal volunteers. Biol Psychiatry. 2001;50:453–462. doi: 10.1016/s0006-3223(01)01099-x. [DOI] [PubMed] [Google Scholar]
- 11.Kremen WS, Seidman LJ, Faraone SV, Tsuang MT. IQ decline in cross-sectional studies of schizophrenia: methodology and interpretation. Psychiatry Res. 2008;158:181–194. doi: 10.1016/j.psychres.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Heaton RK, Drexler M. Clinical Neuropsychological Findings in Schizophrenia and Aging. New York, NY: Guildford; 1987. [Google Scholar]
- 13.Wilk CM, Gold JM, McMahon RP, Humber K, Iannone VN, Buchanan RW. No, it is not possible to be schizophrenic yet neuropsychologically normal. Neuropsychology. 2005;19:778–786. doi: 10.1037/0894-4105.19.6.778. [DOI] [PubMed] [Google Scholar]
- 14.Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64:532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- 15.Badcock JC, Dragovic M, Waters FAV, Jablensky A. Dimensions of intelligence in schizophrenia: evidence from patients with preserved, deteriorated and compromised intellect. J Psychiatr Res. 2005;39:11–19. doi: 10.1016/j.jpsychires.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Badcock JC, Willison JR, Anderson M, Jablensky A. Speed of processing and individual differences in IQ in schizophrenia: general or specific cognitive deficits. Cognit Neuropsychiatry. 2004;9:233–247. doi: 10.1080/13546800344000228. [DOI] [PubMed] [Google Scholar]
- 17.Holthausen EA, Wiersma D, Sitskoorn MM, Dingemans PM, Schene AH, van den Bosch RJ. Long-term memory deficits in schizophrenia: primary or secondary dysfunction? Neuropsychology. 2003;17:539–547. doi: 10.1037/0894-4105.17.4.539. [DOI] [PubMed] [Google Scholar]
- 18.Brebion G, Amador X, Smith MJ, Gorman JM. Memory impairment and schizophrenia: the role of processing speed. Schizophr Res. 1998;30:31–39. doi: 10.1016/s0920-9964(97)00123-0. [DOI] [PubMed] [Google Scholar]
- 19.Brebion G, David AS, Bressan RA, Pilowsky LS. Processing speed: a strong predictor of verbal memory performance in schizophrenia. J Clin Exp Neuropsychol. 2006;28:370–382. doi: 10.1080/13803390590935390. [DOI] [PubMed] [Google Scholar]
- 20.Morrens M, Hulstijn W, Van Hecke J, Peuskens J, Sabbe BGC. Sensorimotor and cognitive slowing in schizophrenia as measured by the Symbol Digit Substitution Test. J Psychiatr Res. 2006;40:200–206. doi: 10.1016/j.jpsychires.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Sánchez JM, Crespo-Facorro BENE, González-Blanch CESA, Perez-Iglesias R, Vázquez-Barquero JL, PAFIP Group Study Cognitive dysfunction in first-episode psychosis: the processing speed hypothesis. Br J Psychiatry. 2007;191:s107–s110. doi: 10.1192/bjp.191.51.s107. [DOI] [PubMed] [Google Scholar]
- 22.Blyler CR, Gold JM, Iannone VN, Buchanan RW. Short form of the WAIS-III for use with patients with schizophrenia. Schizophr Res. 2000;46:209–215. doi: 10.1016/s0920-9964(00)00017-7. [DOI] [PubMed] [Google Scholar]
- 23.Jablensky A, Sartorius N, Ernberg G, et al. Schizophrenia: manifestations, incidence and course in different cultures. A World Health Organization ten-country study. Psychol Med Monogr Suppl. 1992;20:1–97. doi: 10.1017/s0264180100000904. [DOI] [PubMed] [Google Scholar]
- 24.Jablensky A, McGrath J, Herrman H, et al. Psychotic disorders in urban areas: an overview of the Study on Low Prevalence Disorders. Aust N Z J Psychiatry. 2000;34:221–236. doi: 10.1080/j.1440-1614.2000.00728.x. [DOI] [PubMed] [Google Scholar]
- 25.McGuffin P, Farmer A, Harvey I. A polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT system. Arch Gen Psychiatry. 1991;48:764–770. doi: 10.1001/archpsyc.1991.01810320088015. [DOI] [PubMed] [Google Scholar]
- 26.Wing JK, Babor T, Brugha T, et al. SCAN. Schedules for Clinical Assessment in Neuropsychiatry. Arch Gen Psychiatry. 1990;47:589–593. doi: 10.1001/archpsyc.1990.01810180089012. [DOI] [PubMed] [Google Scholar]
- 27.Andreasen N. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, Iowa: University of Iowa; 1984. [Google Scholar]
- 28.Andreasen N. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City, Iowa: University of Iowa; 1983. [PubMed] [Google Scholar]
- 29.Liddle PF, Barnes TRE. Syndromes of chronic schizophrenia. Br J Psychiatry. 1990;157:558–561. doi: 10.1192/bjp.157.4.558. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 32.Singh SP, Cooper JE, Fisher HL, et al. Determining the chronology and components of psychosis onset: the Nottingham Onset Schedule (NOS) Schizophr Res. 2005;80:117–130. doi: 10.1016/j.schres.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 33.Wechsler D. Wechsler Adult Intelligence Scale-3rd Edition (WAIS-3) San Antonio, Tex: Harcourt Assessment; 1997. [Google Scholar]
- 34.Missar CD, Gold JM, Goldberg TE. WAIS-R short forms in chronic schizophrenia. Schizophr Res. 1994;12:247–250. doi: 10.1016/0920-9964(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 35.Wechsler D. The Wechsler Test of Adult Reading (WTAR) San Antonio, Tex: The Psychological Corporation; 2001. [Google Scholar]
- 36.Sahakian BJ, Owen AM. Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J R Soc Med. 1992;85:399–402. [PMC free article] [PubMed] [Google Scholar]
- 37.Lezak MD. Neuropsychological Assessment. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 38.Chapman LJ, Chapman JP. Strategies for resolving the heterogeneity of schizophrenics and their relatives using cognitive measures. J Abnorm Psychol. 1989;98:357–366. doi: 10.1037//0021-843x.98.4.357. [DOI] [PubMed] [Google Scholar]
- 39.Hill SK, Keshavan MS, Thase ME, Sweeney JA. Neuropsychological dysfunction in antipsychotic-naive first-episode unipolar psychotic depression. Am J Psychiatry. 2004;161:996–1003. doi: 10.1176/appi.ajp.161.6.996. [DOI] [PubMed] [Google Scholar]
- 40.Niendam TA, Bearden CE, Rosso IM, et al. A prospective study of childhood neurocognitive functioning in schizophrenic patients and their siblings. Am J Psychiatry. 2003;160:2060–2062. doi: 10.1176/appi.ajp.160.11.2060. [DOI] [PubMed] [Google Scholar]
- 41.Anderson M. Conceptions of intelligence. J Child Psychol Psychiatry. 2001;42:287–298. [PubMed] [Google Scholar]
- 42.Keefe RSE, Seidman LJ, Christensen BK, et al. Comparative effect of atypical and conventional antipsychotic drugs on neurocognition in first-episode psychosis: a randomized, double-blind trial of olanzapine versus low doses of haloperidol. Am J Psychiatry. 2004;161:985–995. doi: 10.1176/appi.ajp.161.6.985. [DOI] [PubMed] [Google Scholar]
- 43.Addington J, Saeedi H, Addington D. The course of cognitive functioning in first episode psychosis: changes over time and impact on outcome. Schizophr Res. 2005;78:35–43. doi: 10.1016/j.schres.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 44.Joy S, Kaplan E, Fein D. Speed and memory in the WAIS-III Digit Symbol–Coding subtest across the adult lifespan. Arch Clin Neuropsychol. 2004;19:759–767. doi: 10.1016/j.acn.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 45.Joy S, Fein D, Kaplan E. Decoding digit symbol: speed, memory, and visual scanning. Assessment. 2003;10:56–65. doi: 10.1177/0095399702250335. [DOI] [PubMed] [Google Scholar]
- 46.Stephens R, Sreenivasan B. Analysis of substitution test performance using eye movement and video data. Appl Neuropsychol. 2002;9:179–182. doi: 10.1207/S15324826AN0903_6. [DOI] [PubMed] [Google Scholar]
- 47.Stephens R. Age-related decline in Digit-Symbol performance: eye-movement and video analysis. Arch Clin Neuropsychol. 2006;21:101–107. doi: 10.1016/j.acn.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Jogems-Kosterman BJM, Zitman FG, Van Hoof JJM, Hulstijn W. Psychomotor slowing and planning deficits in schizophrenia. Schizophr Res. 2001;48:317–333. doi: 10.1016/s0920-9964(00)00097-9. [DOI] [PubMed] [Google Scholar]
- 49.Berman I, Viegner B, Merson A, Allan E, Pappas D, Green AI. Differential relationships between positive and negative symptoms and neuropsychological deficits in schizophrenia. Schizophr Res. 1997;25:1–10. doi: 10.1016/S0920-9964(96)00098-9. [DOI] [PubMed] [Google Scholar]
- 50.Cadenhead KS, Geyer MA, Butler RW, Perry W, Sprock J, Braff DL. Information processing deficits of schizophrenia patients: relationship to clinical ratings, gender and medication status. Schizophr Res. 1997;28:51–62. doi: 10.1016/s0920-9964(97)00085-6. [DOI] [PubMed] [Google Scholar]
- 51.Braff DL. Sensory input deficits and negative symptoms in schizophrenic patients. Am J Psychiatry. 1989;146:1006–1011. doi: 10.1176/ajp.146.8.1006. [DOI] [PubMed] [Google Scholar]
- 52.Green M, Walker E. Susceptibility to backward masking in schizophrenic patients with positive or negative symptoms. Am J Psychiatry. 1984;141:1273–1275. doi: 10.1176/ajp.141.10.1273. [DOI] [PubMed] [Google Scholar]
- 53.Hartman M, Steketee MC, Silva S, Lanning K, McCann H. Working memory and schizophrenia: evidence for slowed encoding. Schizophr Res. 2003;59:99–113. doi: 10.1016/s0920-9964(01)00366-8. [DOI] [PubMed] [Google Scholar]
- 54.Fuller R, Luck SJ, McMahon RP, Gold JM. Working memory consolidation is abnormally slow in schizophrenia. J Abnorm Psychol. 2005;114:279–290. doi: 10.1037/0021-843X.114.2.279. [DOI] [PubMed] [Google Scholar]
- 55.Huddy V, Hodgson TL, Kapasi M, et al. Gaze strategies during planning in first-episode psychosis. J Abnorm Psychol. 2007;116:589–598. doi: 10.1037/0021-843X.116.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heinrichs RW, Awad AG. Neurocognitive subtypes of chronic schizophrenia. Schizophr Res. 1993;9:49–58. doi: 10.1016/0920-9964(93)90009-8. [DOI] [PubMed] [Google Scholar]
- 57.Palmer BW, Heaton RK, Paulsen JS, et al. Is it possible to be schizophrenic yet neuropsychologically normal? Neuropsychology. 1997;11:437–446. doi: 10.1037//0894-4105.11.3.437. [DOI] [PubMed] [Google Scholar]
- 58.Deary IJ. Individual differences in cognition: British contributions over a century. Br J Psychol. 2001;92:217–237. [PubMed] [Google Scholar]