Abstract
Individuals with schizophrenia have consistently been found to exhibit cognitive deficits, which have been identified as critical mediators of psychosocial functional outcomes. Recent reviews of cognitive remediation (CRT) have concluded that these deficits respond to training. This multi-site community study examined 40 individuals with schizophrenia who underwent cognitive remediation using the Neuropsychological Educational Approach to Remediation1 (NEAR). Assessments using the same neuropsychological tests and measures of psychosocial outcome were made at four time points: baseline, before start of active intervention, end of active intervention and 4 months after end of active intervention. Dose of antipsychotic medication remained constant throughout the study period. After participating in NEAR, individuals showed significant improvements in verbal and visual memory, sustained attention and executive functioning. This effect persisted 4 months after the treatment ceased. The average effect size was mild to moderate. Social and occupational outcomes also improved from baseline to post-treatment, which persisted 4 months later. Our findings replicate those of previous studies that suggest that NEAR is effective in improving cognition in individuals with schizophrenia in a naturalistic and ecologically valid setting. Further it extends such findings to show a generalisation of effects to social/occupational outcomes and persistence of effects in the short term.
Keywords: cognitive remediation, schizophrenia
Introduction
Core to schizophrenia are cognitive impairments in attention, memory, and executive functions.1–3 These impairments are relatively independent from other symptom domains such as positive and negative psychotic symptoms4 and have been found to predict functional outcome5 as well, if not better, than do negative symptoms.6,7 Therefore, in order to improve outcome, treatment efforts should target cognitive impairments in addition to symptomatology.
Antipsychotic medications developed to date have had limited effect on cognitive functions in schizophrenia.8 Alternatively, a number of cognitive remediation programs have been developed, and randomized controlled studies have shown positive effects on cognition.9,10 One cognitive remediation approach is known as the Neuropsychological Educational Approach to Remediation (NEAR),11 an evidence-based approach to cognitive remediation that utilizes a set of carefully crafted instructional techniques that reflect an understanding of how people learn best. The NEAR program involves a combination of “drill and practice” exercises and teaching strategies to improve cognitive functioning.11 It utilizes commercially available educational software to create a rich learning environment that is intrinsically motivating and rewarding.11
Using NEAR, Medalia et al12 found that a group of 14 acutely ill psychiatric inpatients who received 6 hours of problem solving remediation showed significantly greater improvement in verbal problem solving (as measured by the Comprehension Test) than did another group of 14 individuals with psychiatric illness who just received typing instruction.12 There were also concurrent improvements in ability to cope as measured by the Symptom Checklist (Positive Symptom Distress Index). Medalia et al13 conducted another study of 18 individuals with chronic schizophrenia who participated in 10 sessions of NEAR, specifically targeting problem solving. Relative to a control group of individuals with schizophrenia who received treatment as usual and no remediation, those who participated in NEAR showed significant improvements in problem solving as reflected in better scores after treatment on an ecologically valid measure of problem solving from the Independent Living Scale. Follow-up studies have shown that gains in problem solving seen over the course of the cognitive remediation program persisted for up to 4 weeks after the program ended.14
Therefore, the NEAR program appears to hold promise in improving cognitive functions in those with schizophrenia. However, key questions remain about the treatment effect in individuals with first-episode schizophrenia. The research conducted to date often used more chronic older populations. First-episode psychosis patients generally have had a more recent decline in cognitive skills from premorbid functioning and therefore would benefit from an early intervention program that specifically aims to remediate cognitive deficits and enhance recovery and prevent relapse. In addition, studies of NEAR to date have used single cognitive measures to evaluate outcome and limited measures of psychosocial functioning. Therefore, there are some unanswered questions about the generalization of improvements to other cognitive domains and also psychosocial domains. The durability of NEAR's effects beyond 4 weeks is also unclear.
This study sought to evaluate the effects of NEAR on cognition immediately after treatment and 4 months after cessation of treatment in a community setting. It sought to use a wide range of cognitive measures in those with first-episode and chronic schizophrenia. It also aimed to examine the effects of NEAR on functional outcome measures including those related to social and occupational functioning, quality of life, and self-esteem.
The following were hypothesized:
There would be improvement in cognition following treatment with NEAR.
The improvement in cognition would remain stable over a period of follow-up.
There would be improvement in psychosocial functioning.
Methods
Participants
The multisite study initially recruited 69 individuals (41 males, 28 females) with schizophrenia, schizophreniform disorder, or schizoaffective disorder via referral from case managers within the Sydney West and Northern Sydney and Central Coast Area Health Services. There were 3 early intervention outpatient centers, 2 chronic inpatient rehabilitation centers, and 4 chronic community outpatient centers.
Once contact was established with the referring center, a site inspection by the investigators was conducted to review the setup of computers and treatment room, the availability of an adequate range of computer software, adequate trained staff to complete rating scales and administer treatment, and adequate area to conduct neuropsychological assessments on site in order to establish treatment veracity across centers. In addition, to determine whether there was compliance with treatment principles, the investigators of this study reviewed the treatment plans and strategies recorded by facilitators in their progress notes. The centers that participated in this study met all the criteria mentioned above.
Inclusion criteria were as follows:
diagnosis of schizophrenia determined by trained psychiatrists;
stable on medication for a minimum of 2 months;
stable in residence;
premorbid IQ greater than 70 as determined by performance on the Wide Range Achievement Test-Revised;15
Positive and Negative Syndrome Scale (PANSS)16 conceptual disorganization score less than 5;
impairment in one cognitive domain greater than 1 SD;
no evidence of significant head injury, neurological disease, learning disorder, ECT in past 6 months, or current diagnosis of substance dependence (other than nicotine);
English speaking.
Informed consent was obtained from all subjects. This research project was approved by the ethics committees from Sydney West Area Health Service, Northern Sydney and Central Coast Area Health Service, and Macquarie University. The trial was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12605000202662).
Dropouts
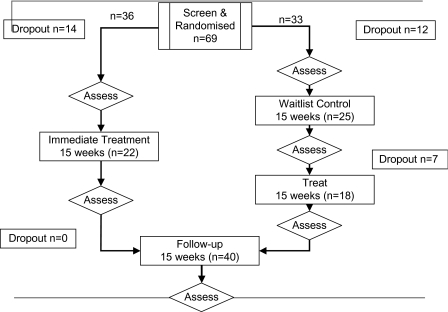
Figure 1 shows the consort diagram. Twenty-nine participants (42.0%) dropped out at different points of the study. There were a number of reasons including inpatient admission (N = 2), work commitments (N = 4), discharge from hospital (N = 1), lost contact with center (N = 5), withdrew consent (N = 2), deceased (N = 2), clinician unavailable (N = 2), wait-list (N = 7), loss of motivation to attend cognitive remediation therapy (CRT) (N = 4). Comparison of participants who dropped out of the study and those who remained on core clinical, demographic, and neuropsychological variables revealed no significant differences.
Fig. 1.
Consort Diagram.
Final Sample Used for Analysis
The final sample consisted of 40 participants. Twenty-two were randomized to treatment group (57.1%) and 18 to wait-list group (42.9%). Participants were aged between 17 and 50 years with an average of 11 years of education (see table 1).
Table 1.
Demographic and Clinical Characteristics of Sample (N = 40)
| Baseline | Posttreatment | 4-mo follow-up | |||
| Mean (SD) | Mean (SD) | F (P) | Mean (SD) | F (P) | |
| Age (y) | 31.33 (9.08) | ||||
| Gender | 24 male, 16 female | ||||
| Education (y) | 11.00 (1.16) | ||||
| Premorbid IQa | 93.72 (11.98) | ||||
| Medication dose (chlorpromazine) | 649.89 (476.49) | 615.85 (440.14) | 0.60 (.446) | 684.00 (515.55) | 1.84 (.186) |
Wide Range Achievement Test, Third Edition—standard score.
Materials
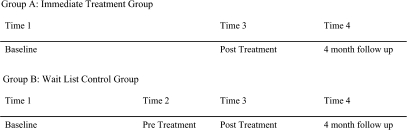
Participants from both the wait-list and immediate treatment groups were assessed at (see figure 2) baseline (time 1), posttreatment (time 3), and 4-month follow-up (time 4) by a research assistant (master's level) for all cognitive tests. Additionally the wait-list group received a second baseline assessment prior to commencement of treatment (pretreatment, time 2). Clinicians were trained in the administration of rating scales used in the battery and after attaining acceptable interrater reliability rated subjects on symptom rating scales.
Fig. 2.
Group (A: Immediate Treatment group; B: Wait-List Control Group) × Time (1: Baseline; 2: Pretreatment; 3: Posttreatment; 4: 4-mo Follow-up).
Assessment included the following measures.
Primary Outcome Measures:
Cognitive
Trail Making Test17
Part A (Trail Making Test [TMT]a): Requires an individual to sequence numbers within the format of a visual motor task. This measures processing speed.
Part B (TMTb): Requires an individual to switch back and forth between connecting numbers and letters in sequence. This measures an aspect of executive function referred to as set shifting.
Conners’ Continuous Performance Test (CCPT)18 is a computerized sustained performance measure that requires an individual to press a button on a keyboard for every letter other than “x” that appears on the computer screen. The attentiveness (d) score was used as the primary index of sustained attention.
Rey Auditory Verbal Learning Test (RAVLT)17 consists of a 15-word list given repeatedly over 5 learning trials. The total number of words learned across the learning trials was used as a measure of verbal learning (RAVLT learning). Verbal memory performance was represented by the number of words recalled spontaneously 20–30 minutes after the fifth learning trial (RAVLT delayed recall).
Rey Complex Figure Test17 examined the ability to construct a complex figure and remember it for later recall. It is used as a measure of visual memory that is represented by the total score obtained on delayed recall (Rey delayed recall).
Delis-Kaplan Executive Function System (DKEFS)19 sorting subtest: The examinee must sort sets of 6 cards into as many different categorization rules as possible according to some common verbal or perceptual attribute. The number of Free Sorting Confirmed Correct was used as a measure of reasoning/cognitive flexibility.
Alternate forms of tests were used for TMT, RAVLT, and Rey Figure and sorting subtest.
Psychosocial Functions
Social and Occupational Function Scale (SOFAS)20 was used to allow clinicians to assess patients’ social and occupational functioning independently from psychopathology. Patients are assessed on an overall rating score out of 100.
Life Skills Profile—39 (LSP-39)21 was used as a measure of those aspects of functioning which affect how successfully people with schizophrenia live in the community. There are 39 items and 5 subscales in LSP: self-care, nonturbulence, social contact, communication, and responsibility. LSP-39 items were based on observed behaviors and completed by treating clinicians who knew the subjects well. A total score was obtained by summing the responses for all items with low scores reflecting high level of skills.
Quality of life: The World Health Organization Quality-of-Life Scale-Brief Form (WHOQOL-Bref)22 instrument comprises 26 items, which provides separate scores for each of the following domains: physical health, psychological health, social relationships, and environment.
Self-esteem: The Rosenberg Self-Esteem Scale23 was used to measure adolescents’ global feelings of self-worth or self-acceptance. It includes 10 items that are scored using a 4-point response ranging from strongly disagree to strongly agree. An overall score is obtained with higher scores reflecting higher levels of self-esteem.
Positive and negative symptoms: PANSS16 is a broad based psychopathology scale used to detect overall changes in the features of schizophrenia over time. It includes 7 positive subscale items, 7 negative subscale items, and 16 general psychopathology subscale items that were aggregated to achieve the total positive, total negative, and total general psychopathology scores, respectively. All raters received PANSS training.
Depression: Calgary Depression Scale24 (CDS) is a 9-item semistructured scale used to assess depression in patients with schizophrenia. Scores for all items are aggregated to produce an overall score out of 27.
Design
The study had a randomized wait-list control design. Participants who enrolled in the study were randomized to either a group that was immediately treated or to a group that “waited” for 15 weeks before being treated. Both groups completed the above-mentioned questionnaires and participated in cognitive assessment. After 20–30 sessions of treatment (if in the immediate treatment group) or 15 weeks of waiting (if in the wait-list control), individuals were tested again with the same measures. Individuals were tested again after a further 15 weeks to determine if any benefits persisted. This design allowed all individuals to be treated eventually. It allowed for a comparison between a period of treatment and a period of no treatment. It also permitted measurement of change in performance before and after treatment and also the persistent of such effects 4 months later.
Randomization
Participants were randomized in permuted blocks of 4 patients into immediate treatment or wait-list control. The research assistant (D.S.) was responsible for the generation of the allocation sequence and was blind to this assignment at the time of treatment allocation. However, subsequent to this, the study was not blinded, and those clinicians administering the intervention and those assessing the outcome were aware of group assignment. There was no masking in this study.
Treatment Procedure
Based on the treatment protocol described in the treatment manual,25 participants attended two 1-hour sessions each week for 10–5 weeks, making a maximum total of 30 sessions. The treatment program consisted of participants working on various computer software programs with guidance/assistance from a therapist. The computer software programs were specifically selected to promote improvement and practice in specific cognitive skills that were identified as being deficient on neuropsychological testing as identified by performance more than 1 SD below the normative mean for each respective cognitive measure. The participants worked on the computer software programs at their own pace and over the treatment period. Each participant built a repertoire of software programs that addressed the following cognitive domains: sustained attention, processing speed, memory, and executive functions. The therapist guided the process using questions to enhance meta-cognition and information processing during activity performance. The therapist's goal was to facilitate the development of cognitive functions by monitoring subjects’ progress, providing instruction and feedback as necessary, and facilitating a positive learning experience. All facilitators received the same training workshops on NEAR conducted by the same 2 experienced clinicians (M.A.R.H., P.W.) trained originally by Dr Alice Medalia. Treatment was conducted on site at the various treatment centers following a satisfactory site inspection by trained clinicians in CRT. Random inspection of CRT session notes confirmed treatment fidelity across centers. The treatment sessions conformed to the treatment plan in terms of cognitive domains covered and time spent and captured the theoretically active ingredients important in enhancing learning and self-determination. In order to help ensure treatment fidelity, all clinicians were supervised fortnightly—monthly over the course of the trial. All rating scales measuring psychopathology were completed by the treating clinician and collated by the research assistant. All scores were entered into a database and were checked by 2 investigators who were blind to the group membership.
Statistical Analysis
Raw scores were used for all measures except for DKEFS sorting for which scaled scores were used. For all cognitive and psychosocial measures, repeated-measures analysis of variance (ANOVA) was used to compare the following:
baseline (time 1) and pretreatment (time 2) measures to determine whether there were any practice effects for each cognitive measure for all participants (N = 40);
baseline (time 1) and posttreatment (time 3) to determine whether there were any changes in cognitive and psychosocial variables after treatment within groups (N = 40);
wait-list group (N = 18, change from Time 1 to Time 2, during which they received no treatment) and immediate treatment group (N = 22, change from time 1 to time 3, during which they received treatment);
posttreatment (time 3) and 4-month follow-up (time 4) to determine whether the effects seen at posttreatment persisted.
The Bonferroni method was used to adjust for multiple comparisons of outcomes with a mean correlation of 0.41. Therefore, the α level was set at .02. To determine the magnitude of change, this study calculated effect sizes (Cohen d).
To determine whether cognitive changes seen were clinically significant, this study adopted method of Jacobson and Truax26 to calculate reliable change indices for each cognitive measure.
where σ = SD, r = test-retest correlation.
Reliability coefficients from published norms that matched the general demographics of the experimental sample were used. The 68% confidence interval was used to determine reliable change.
Results
The demographic and clinical characteristics of the group are presented in table 1. At baseline, there were no differences between treatment and wait-list groups on any of the demographic, clinical, or cognitive variables. There were no statistically significant changes from baseline to posttreatment in terms of medication dose.
There were no significant changes in medication dose (table 1) and any of the PANSS scores (table 2) over the treatment period and also 4 months after the treatment period ceased.
Table 2.
Results of Analyses of Variance for Psychosocial Functional Measures, Baseline Compared With Posttreatment and Posttreatment Compared With 4-Months Follow-up
| Baseline | Posttreatment | 4-mo Follow-up | |||
| Mean (SD) | Mean (SD) | F (P) | Mean (SD) | F (P) | |
| PANNS positive | 13.36 (5.30) | 13.92 (5.99) | 0.68 (.413) | 13.20 (5.77) | 0.450 (.486) |
| PANNS negative | 18.00 (6.74) | 17.49 (5.94) | 0.320 (.575) | 16.42 (6.70) | 0.07 (.796) |
| PANSS general | 32.79 (10.42) | 31.51 (9.10) | 0.79 (.379) | 29.97 (9.84) | 1.23 (.275) |
| CDS | 4.03 (3.22) | 3.92 (4.08) | 0.03 (.869) | 4.40 (4.04) | 0.26 (.614) |
| SOFAS | 54.97 (16.10) | 60.14 (15.38) | 8.64 (.003) | 60.50 (17.22) | 0.00 (.983) |
| LSP-39 | 20.89 (12.05) | 18.45 (13.92) | 2.10 (.079) | 13.93 (9.59) | 1.53 (.23) |
| WHOQOL-Bref psychological | 20.63 (4.41) | 20.88 (4.77) | 0.04 (.423) | 21.03 (4.27) | 0.42 (.520) |
| WHOQOL-Bref physical | 25.00 (4.84) | 24.31 (3.57) | 0.32 (.292) | 24.83 (3.52) | 0.03 (.868) |
| WHOQOL-Bref social | 10.19 (2.29) | 4.81 (2.76) | 0.25 (.314) | 10.52 (2.54) | 0.75 (.395) |
| WHOQOL-Bref environmental | 28.19 (4.00) | 27.00 (4.41) | 0.94 (.174) | 29.60 (4.59) | 2.54 (.121) |
| Rosenberg Self-Esteem Score | 27.88 (4.57) | 29.19 (5.60) | 2.26 (.072) | 29.93 (5.98) | 0.75 (.395) |
Note: PANSS, Positive and Negative Syndrome Scale; CDS, Calgary Depression Scale; SOFAS, Social and Occupational Function Scale; LSP-39, Life Skills Profile—39; WHOQOL-Bref, World Health Organization Quality-of-Life Scale-Brief Form.
Was There Any Change in Cognitive Scores From Baseline to Pretreatment During the Period of No CRT?
Repeated-measures ANOVA revealed no significant changes in all the cognitive test scores from baseline to pretreatment (table 3). These results suggest no evidence of significant practice effects.
Table 3.
Results of Analyses of Variance for Cognitive Domains, Baseline Performance Compared With Posttreatment and Posttreatment Compared With 4-Months Follow-up for N = 40 Participants (From Wait-List and Immediate Treatment Groups) That Completed the Cognitive Remediation Program
| Baseline | Posttreatment | 4-mo Follow-Up | |||
| Mean (SD) | Mean (SD) | F (P) | Mean (SD) | F (P) | |
| RAVLT learning | 36.6 (13.95) | 39.07 (12.90) | 3.52 (.034) | 41.83 (11.79) | 4.38 (.043) |
| RAVLT delayed recall | 6.38 (3.48) | 7.25 (3.26) | 4.93 (.016) | 6.95 (3.60) | 0.81 (.373) |
| Rey delayed recall | 9.28 (6.23) | 14.83 (7.58) | 37.78 (.000) | 16.01 (7.63) | 0.83 (.368) |
| CCPT d | 23.60 (27.09) | 43.84 (34.33) | 19.85 (.000) | 42.01 (33.49) | 0.17 (.686) |
| TMTa | 45.79 (28.29) | 39.64 (23.22) | 3.15 (.042) | 35.42 (17.44) | 0.96 (.334) |
| TMTb | 121.48 (91.72) | 100.73 (50.94) | 4.58 (.020) | 44.56 (44.87) | 1.16 (.288) |
| DKEFS sorting | 7.28 (2.42) | 7.56 (3.05) | 0.312 (.292) | 9.13 (2.37) | 0.08 (.777) |
Note: RAVLT, Rey Auditory Verbal Learning Test; CCPT, Conners’ Continuous Performance Test; TMT, Trail Making Test; DKEFS, Delis-Kaplan Executive Function System.
Was There Significant Improvement in Any of the Cognitive Scores From Baseline to Posttreatment?
Within-Group Comparisons.
Inspection of cognitive scores (see table 3) revealed significant improvement in scores from baseline to posttreatment after correction for multiple comparisons. This was evident in RAVLT delayed recall (F = 4.93, P < .02), Rey delayed recall (F = 37.78, P < .02), CCPTd, (F = 19.85, P < .02), and TMTb (F = 4.58, P < .02). The effect sizes for these changes were 0.18, 0.26, 0.65, and 0.28, respectively.
The change seen in Rey delayed recall, CCPTd, and TMTb was reliable at 68% confidence using formula of Jacobson and Truax23 for determining reliable change.
Between-Group Comparisons.
Repeated-measures ANOVA (table 4) revealed no significant interaction between time (pretreatment to posttreatment) and group (wait-list and immediate treatment) on any of the cognitive measures. However, effect sizes ranged from 0.08 (RAVLT delayed recall) to 0.77 (Rey delayed recall) to 0.90 (CCPTd and sorting).
Table 4.
Results of Analysis of Variance Comparing N = 18 Wait-List Control Group and N = 22 Immediate Treatment Group Across the Cognitive Domains
| Baseline | Posttreatment | |||||
| Wait-List Control | Immediate Treatment | Wait-List Control | Immediate Treatment | F | P | |
| RAVLT learning | 34.00 ± 14.30 | 40.45 ± 11.20 | 33.04 ± 11.78 | 39.50 ± 13.21 | 0.000 | .998 |
| RAVLT delayed recall | 5.32 ± 2.90 | 7.36 ± 3.36 | 5.32 ± 3.08 | 7.09 ± 3.27 | 0.148 | .702 |
| Rey delayed recall | 7.66 ± 4.96 | 11.32 ± 6.42 | 11.44 ± 6.40 | 16.64 ± 7.33 | 0.631 | .431 |
| CCPT d | 20.01 ± 27.78 | 25.22 ± 27.93 | 33.51 ± 34.18 | 50.96 ± 30.07 | 2.57 | .117 |
| TMTa | 52.01 ± 32.56 | 39.06 ± 15.63 | 51.57 ± 35.53 | 34.28 ± 10.99 | 0.768 | .386 |
| TMTb | 130.60 ± 103.09 | 110.31 ± 61.75 | 135.57 ± 78.10 | 98.45 ± 37.60 | 1.293 | .262 |
| DKEFS sorting | 5.96 ± 3.42 | 7.35 ± 3.36 | 7.32 ± 2.43 | 9.90 ± 2.22 | 2.136 | .151 |
Note: RAVLT, Rey Auditory Verbal Learning Test; CCPT, Conners’ Continuous Performance Test; TMT, Trail Making Test; DKEFS, Delis-Kaplan Executive Function System.
Individual Data.
Inspection of individual performances across the cognitive domains revealed that up to 38% (15/40) of the sample made reliable changes based on formula of Jacobson and Truax.23
Was There Significant Change in Any of the Psychosocial Measures From Baseline to Posttreatment?
From baseline to posttreatment, the PANSS, CDS, LSP-39, WHOQOL-Bref, and Rosenberg Self-Esteem Scale scores did not change significantly (table 2). In contrast, the SOFAS score increased from baseline to posttreatment significantly (F = 8.64, P < .005). The effect size for this change was 0.14. There were positive trends for improvement in the LSP-39 and the Rosenberg Self-Esteem ratings. SOFAS change scores from pre- to posttreatment were significantly correlated with treatment-related changes on Rey delayed recall (r = 0.36, P < .05) but were not correlated with those changes in RAVLT delayed recall (r = 0.03, P > .05), CCPTd (r = −0.03, P > .05), or TMTb (r = 0.05, P > .05).
Did the Improvements in Cognitive and Psychosocial Functioning Persist at 4-Months Follow-up?
Comparison of performance at posttreatment and 4-month follow-up revealed no significant changes on any of the cognitive or psychosocial variables. In fact, there was a trend toward continued improvement in RAVLT total learning. These results suggest that the effects seen immediately posttreatment are at least durable up to 4 months later.
Discussion
This randomized controlled multisite study has demonstrated that the NEAR technique is associated with broad cognitive improvement after 15 weeks of cognitive remediation. This cognitive improvement was accompanied by a significant improvement in psychosocial functioning.
Practice effect factors were minimized by the use of alternate forms of cognitive tests. The observed improvements were greater than practice effects usually seen on repeat testing as indicated by reliable change analyses. This is further supported by data showing that changes seen in those treated were greater than those made by a subgroup of individuals who received no NEAR treatment between 2 separate testing occasions.
This provides the first evidence to our knowledge of the effectiveness of NEAR in individuals with schizophrenia. This is also the first examination to date of the NEAR approach using an extensive battery of cognitive and psychosocial outcome measures with a longer posttreatment follow-up. The present study found improvements in visual and verbal memory, sustained attention, and set shifting that are core cognitive deficits in schizophrenia.5 The cognitive performances (particularly for sustained attention and visual memory) at posttreatment normalized to within 1 SD of the usual reference age group (20–29 years old). These effects were maintained for up to 4 months after treatment ceased. The average effect sizes were low to moderate and somewhat compatible with those obtained in previous CRT trials in schizophrenia.27 Trends toward improvement were also seen in most other cognitive domains. Further, the effects of NEAR generalized to yield an improvement in social and occupational functioning, which previously had only been measured in a small group of studies in CRT. The group of individuals in this study improved on SOFAS scores from 54 at baseline to 60–61 at posttreatment, which reflect movement from being rated as having “moderate difficulty” to “some difficulty … but generally functioning well,” which is a clinically meaningful change. Because the participants had been on a stable dose of medication for at least 2 months prior to entering the study and there were no significant changes in medication dose over the period of the study including the 4-month follow-up, this is unlikely to be due to changes in medication. Further, there were trends toward improvement in LSP and self-esteem. The latter is consistent with feedback given by participants about enhanced sense of self-worth while attending the CRT program.
The findings are in line with those found by Medalia et al28 and also other CRT work by Ueland and Rund29 that has shown that targeted repeated programs can partially remediate attention functions in schizophrenia. The results are also similar to work of Medalia et al30 in relation to observed improvements in verbal memory following NEAR. The group's performance on posttreatment moved from the impaired range to the low average range. The present results also extend Medalia's work to show NEAR's additional benefit to visual memory.
A number of limitations may potentially limit conclusions of the study. First, the sample size was small. The small size of each of the groups (wait-list, N = 18, and immediate treatment group, N = 22) meant that the repeated-measures (between groups) analyses yielded nonsignificant improvements in most cognitive domains, probably reflecting reduced power. On a positive note, effect sizes of some of the changes were relatively large (up to 0.90) suggesting that larger numbers within each group would have yielded more significant findings. Another limitation is that the raters were not blind to the treatment group, and therefore, the resulting increase in psychosocial ratings from pre- to posttreatment could be due to bias. Analysis of correlations between changes in subjective SOFAS ratings and objective measures of cognitive functioning yielded a significant correlation in only one domain. In addition, the functional assessment was brief. A more detailed assessment of function and how these improvements transferred to successful completion of actual daily activities would be important. Fourth, the design of the study only allowed for a short-term follow-up. Follow-up of function over 12 months would determine whether effects are sustainable and generalize to real-world functions. It may be that the full gains to be obtained from this treatment will require further treatment using more traditional psychosocial rehabilitation techniques. Further work needs to examine whether the gains found with cognitive remediation therapy are synergistic, complement, or replace these forms of treatment.
Alternative study designs are necessary to further support the utility of cognitive remediation in schizophrenia. This may include randomized controlled trials focusing on (a) demonstration of cognitive gains that extend to actual performance of activities of daily living; (b) blinding raters to treatment group membership; (c) its longer term durability (>4 months); (d) adequacy of the social control condition that matches for the therapist's time, contact, and enthusiasm; and (e) examination of the dose of treatment is required. Replication of findings in early psychosis with larger sample sizes is warranted. The applicability of NEAR to other clinical populations with similar cognitive deficits should also be explored. Recent research has shown promising results for cognitive remediation in anorexia nervosa31 and depression.32
It has been hypothesized that in part CRT works by training basic processes “via proliferation and refining of basic neural connections.”33 Indeed, a functional imaging study by Wykes et al33 has demonstrated enhanced neural activation postcognitive remediation. Future efficacy studies that incorporate functional neuroimaging methodology may help address such questions regarding the underpinnings of cognitive remediation.
In sum, these preliminary observations (1) support the NEAR framework (a readily available, motivating, time-effective group intervention) to guide cognitive interventions in individuals with schizophrenia, (2) provide information about which specific cognitive functions could be targeted by this cognitive intervention, (3) demonstrate that the remediation of basic cognitive deficits does appear to persist and that these effects appear to generalize to social and occupational functions.
Funding
Eli Lilly Australia “Answers that Matter” Mental Health Award fund; Perpetual Trustees JS Love Trust; Trust fund of the Schizophrenia Fellowship of New South Wales.
Acknowledgments
The authors of this article gratefully acknowledge the contribution of clinicians from the Northern Sydney and Central Coast Area Health Service and Sydney West Area Health Service. Thank you to Dr David Cairns (Macquarie University) for providing statistical advice. We are also grateful to Professor Alice Medalia for training and ongoing supervision.
References
- 1.Saykin AJ, Gur RC, Gur RE, et al. Neuropsychological function in schizophrenia. Selective impairment in memory and. learning. Arch Gen Psychiatry. 1991;48:618–624. doi: 10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- 2.Fitzgerald D, Lucas S, Redoblado MA, et al. Cognitive functioning in young people with first episode psychosis: relationship to diagnosis and clinical characteristics. Aust N Z J Psychiatry. 2004;38:501–510. doi: 10.1080/j.1440-1614.2004.01403.x. [DOI] [PubMed] [Google Scholar]
- 3.Addington J, Addington D. Neurocognitive and social functioning in schizophrenia. Schizophr Bull. 1999;25:173–182. doi: 10.1093/oxfordjournals.schbul.a033363. [DOI] [PubMed] [Google Scholar]
- 4.Bell MD, Mishara AL. Does negative symptom change relate to neurocognitive change in schizophrenia? Implications for targeted treatments. Schizophr Res. 2006;81:17–27. doi: 10.1016/j.schres.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 6.Green M, Kern R, Heaton R. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72:41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Velligan DI, Mahurin RK, Diamond PL, Hazleton BC, Eckert SL, Miller AL. The functional significance of symptomatology and cognitive function in schizophrenia. Schizophr Res. 1997;25:21–31. doi: 10.1016/S0920-9964(97)00010-8. [DOI] [PubMed] [Google Scholar]
- 8.Marder SR. Initiatives to promote the discovery of drugs to improve cognitive function in severe mental illness. J Clin Psychiatry. 2006;67:31–35. doi: 10.4088/jcp.0706e03. [DOI] [PubMed] [Google Scholar]
- 9.Wexler BE, Bell MD. Cognitive remediation and vocational rehabilitation for schizophrenia. Schizophr Bull. 2005;31:931–941. doi: 10.1093/schbul/sbi038. [DOI] [PubMed] [Google Scholar]
- 10.Wykes T, Reeder C, Landau S, et al. Cognitive remediation therapy in schizophrenia: randomised controlled trial. Br J Psychiatry. 2007;190:421–427. doi: 10.1192/bjp.bp.106.026575. [DOI] [PubMed] [Google Scholar]
- 11.Medalia A, Freilich B. The Neuropsychological Educational Approach to Cognitive Remediation (NEAR) model: practice principles and outcome studies. Am J Psychiatr Rehabil. In press. [Google Scholar]
- 12.Medalia A, Dorn H, Watras-Gans S. Treating problem-solving deficits on an acute psychiatric inpatient unit. Psychiatry Res. 2000;97:79–88. doi: 10.1016/s0165-1781(00)00214-6. [DOI] [PubMed] [Google Scholar]
- 13.Medalia A, Revheim N, Casey M. The remediation of problem solving skills in schizophrenia. Schizophr Bull. 2001;27:259–267. doi: 10.1093/oxfordjournals.schbul.a006872. [DOI] [PubMed] [Google Scholar]
- 14.Medalia A, Revheim N, Casey M. Remediation of problem solving skills in schizophrenia: evidence of a persistent effect. Schizophr Res. 2002;57:165–171. doi: 10.1016/s0920-9964(01)00293-6. [DOI] [PubMed] [Google Scholar]
- 15.Wilkinson G. Wilmington, DE: Wide Range; 1993. Wide Range Achievement Test-Third Edition. [Google Scholar]
- 16.Kay S. Positive and Negative Syndromes in Schizophrenia. New York, NY: Brunner/Mazel; 1991. [Google Scholar]
- 17.Spreen O, Strauss E. A Compendium of Neuropsychological Tests—Second Edition: Administration, Norms and Commentary. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 18.Conners K. The Conners Continuous Performance Test. Toronto, ON: Multi Health Systems; 1995. [computer program]. Version. [Google Scholar]
- 19.Delis DC, Kaplan E, Kramer JH. Delis Kaplan Executive Function System. Toronto, ON: Psychological Corporation; 2001. [Google Scholar]
- 20.Goldman NH, Skodol AE, Lave TR. Revising axis V for DSM-IV; a review of measures of social functioning. Am J Psychiatry. 1992;49:1148–1156. doi: 10.1176/ajp.149.9.1148. [DOI] [PubMed] [Google Scholar]
- 21.Rosen A, Hadzi-Pavlovic D, Parker G. The life skills profile: a measure assessing function and disability in schizophrenia. Schizophr Bull. 1989;15:325–337. doi: 10.1093/schbul/15.2.325. [DOI] [PubMed] [Google Scholar]
- 22.Murphy B, Herrman H, Hawthorne G, Pinzone T, Evert H. Australian WHOQoL Instruments: User's Manual and Interpretation Guide. Melbourne, Australia: Australian WHOQoL Field Study Centre; 2000. [Google Scholar]
- 23.Rosenberg M. Society and the Adolescent Self-image. Princeton, NJ: Princeton University Press.; 1965. [Google Scholar]
- 24.Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3:247–251. doi: 10.1016/0920-9964(90)90005-r. [DOI] [PubMed] [Google Scholar]
- 25.Medalia A, Revheim N, Herlands T. Remediation of Cognitive Deficits in Psychiatric Patients: A Clinician's Manual. 2002. In press. [Google Scholar]
- 26.Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 27.McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164:1791–1802. doi: 10.1176/appi.ajp.2007.07060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medalia A, Aluma M, Tryon W, Merriam AE. Effectiveness of attention training in schizophrenia. Schizophr Bull. 1998;24:147–152. doi: 10.1093/oxfordjournals.schbul.a033306. [DOI] [PubMed] [Google Scholar]
- 29.Ueland T, Rund B. Cognitive remediation for adolescents with early onset psychosis: a 1-year follow-up study. Acta Psychiatr Scand. 2005;111:193–201. doi: 10.1111/j.1600-0447.2004.00503.x. [DOI] [PubMed] [Google Scholar]
- 30.Medalia A, Revheim N. Casey M Remediation of memory disorders in schizophrenia. Psychol Med. 2000;30:1451–1459. doi: 10.1017/s0033291799002913. [DOI] [PubMed] [Google Scholar]
- 31.Tchanturia K, Davies H, Campbell I. Cognitive Remediation for patients with Anorexia Nervosa: preliminary findings. Arch Gen Psychiatry. 2007;14:1–6. doi: 10.1186/1744-859X-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elgamal S, McKinnon MC, Ramakrishnan K, Joffe RT, MacQueen G. Successful computer-assisted cognitive remediation therapy in patients with unipolar depression: a proof of principle study. Psychol Med. 2007;37:1229–1238. doi: 10.1017/S0033291707001110. [DOI] [PubMed] [Google Scholar]
- 33.Wykes T, Brammer M, Mellers J, et al. Effects on the brain of a psychological treatment: cognitive remediation therapy. Functional magnetic resonance imaging in schizophrenia. Br J Psychiatry. 2002;181:144–152. doi: 10.1017/s0007125000161872. [DOI] [PubMed] [Google Scholar]