Abstract
Schizophrenia is a condition that impairs higher brain functions, some of which are specific to humans. After identification of susceptibility genes for schizophrenia, many efforts have been made to generate genetics-based models for the disease. It is under debate whether behavioral deficits observed in rodents are sufficient to characterize these models. Alternatively, anatomical and neuropathological changes identified in brains of patients with schizophrenia may be utilized as translatable characteristics between humans and rodents, which are important for validation of the models. Here, we overview such anatomical and neuropathological changes in humans: enlarged ventricles, dendritic changes in the pyramidal neurons, and alteration of specific subtypes of interneurons. In this review, we will overview such morphological changes in brains from patients with schizophrenia. Then, we will describe that some of these alterations are already recapitulated even in classic nongenetic models for schizophrenia. Finally, in comparison with the changes in patients and nongenetic models, we will discuss the anatomical and neuropathological manifestation in genetic models for schizophrenia.
Keywords: brain imaging, ventricular enlargement, neuropathology, interneurons, spine density
Introduction
SZ is a condition that impairs high brain functions, some of which are specific to humans, complicating modeling the disease in mice. How can we evaluate in mice the existence of hallucination, delusion, and disorganized speech, which are characteristics of schizophrenia? Thus, behavioral deficits observed in mouse models might not serve as sufficient criteria to judge whether they are good models for schizophrenia.
For the past decade, there has been enormous progress in understanding the neurobiology of schizophrenia.1 Major progress was made by identification of susceptibility genes for schizophrenia.2 Although causality is hard to prove, these genetic factors have shed light on specific biological cascades that are linked to the pathology of the disease.3 Another major advance in schizophrenia research is identification of structural and pathological alterations that are frequently found in brains of patients with schizophrenia: Enlarged ventricles at the gross anatomy level have been reported in many brain imaging studies4; dendritic changes in the pyramidal neurons5 and alteration of specific subtypes of interneurons6 are known to be important in the pathology of schizophrenia.
Mouse models for other brain disorders, such as Alzheimer disease, have been validated by utilizing representative neuropathological hallmarks found in the brains from patients with these diseases.7 As a result, these models are accepted to be very useful for molecular understanding of the mechanisms and course of the disease, as well as promising for compound screening for translational purposes. Combined with the ability to modulate the etiology of disease directly by straightforward genetic engineering, mice provide a good resource for modeling brain disorders. In analogy to these successful cases, anatomical and neuropathological changes identified in brains of patients with schizophrenia may become good indicators to validate possible mouse models for schizophrenia.
Based on this notion, this review first summarizes anatomical and neuropathological changes in schizophrenia brains that have been reported in brain imaging and neuropathological studies. We then discuss how such hallmarks are studied in putative mouse models for schizophrenia. In this discussion, we will also overview neuropathological changes found in classic nongenetic rodent models for schizophrenia, although most of them have been generated in rats. Compared with nongenetic models, we will finally discuss how genetically engineered mice will be useful in studies of schizophrenia with both basic and translational significance. This review does not aim at covering all previous publications and models but instead proposes a useful strategy for mouse models for schizophrenia research.
Anatomical and Neuropathological Changes in Schizophrenia Patients
Gross Anatomy by Brain Imaging
Advances in brain imaging technology, especially magnetic resonance imaging, have established that there are significant, although not very robust, anatomical changes in brains of patients with schizophrenia (table 1).
Table 1.
Structural and Morphological Abnormalities in Schizophrenia Patients. (meta, meta-analysis; *, first episode)
| Brain Region | Representative References | |
| Gross anatomy (imaging) | ||
| Decreased brain volume | Whole brain | Vita et al8 meta*, Steen et al10 meta*, Wright et al9 meta |
| Temporal lobe | Shenton et al4 review | |
| Hippocampus | Vita et al8 meta*, Steen et al10 meta*, Wright et al9 meta | |
| Amygdala | Wright et al9 meta | |
| Thalamus | Ellison-Wright et al11 meta* | |
| Anterior cingulate | Ellison-Wright et al11 meta* | |
| Basal ganglia-thalamocortical circuit | Ellison-Wright et al11 meta* | |
| Enlarged ventricles | Lateral ventricles | Vita et al8 meta*, Steen et al10 meta*, Shenton et al4 review, Wright et al9 meta |
| Third ventricles | Vita et al8 meta*, Shenton et al4 review | |
| Changed asymmetry | Sommer et al19meta | |
| Decreased white matter | Whole brain | Wright et al9meta |
| Frontal & temporal cortex | Ellison-Wright 2009 meta | |
| Neurohistology | ||
| Pyramidal neurons | ||
| Reduced neuron density | PFC (no change in cell number | Selemon and Goldman-Rakic20review |
| Reduced soma size | PFC | Rajkowska et al21 |
| Reduced dendritic field | PFC | Black et al22 |
| Reduced spine density | FC | Glantz and Lewis5 |
| Interneurons | ||
| Less GAD67+ | PFC (mRNA) | Akbarian et al26 |
| Less reelin+ | PFC (mRNA) | Guidotti et al23 |
| Less parvalbumin+ | PFC (IR) | Beasley et al28 |
| Hc (IR) | Zhang and Reynolds30 | |
| No change in calretinin+ | PFC (IR) | Beasley et al28 |
| Hc (IR) | Zhang and Reynolds30 | |
| Less somatostatin+ | PFC (mRNA) | Hashimoto et al32 |
| Less cholecystokinin+ | PFC (mRNA) | Hashimoto et al32 |
| Glia | ||
| Fewer oligodendrocytes | PFC | Vostrikov et al35 |
| Thalamus | Byne et al33 | |
| Hc | Schmitt et al34 | |
Note: PFC, prefrontal cortex; IR, immunoreactivity; Hc, hippocampus.
Imaging studies of chronic schizophrenia patients have detected enlarged ventricles (figure 1A) accompanied by volume decreases in amygdala, parahippocampal gyrus, and temporal lobes.4,8,9 Enlarged lateral and third ventricles and decrease in whole brain, hippocampal, basal ganglia, and thalamic volumes are present in first-episode patients with schizophrenia.10,11 There has been a debate about how long these changes continue after the onset of the disease. Recent longitudinal studies have suggested continuously progressive decreases in volumes of brain tissue and increases in volumes of lateral ventricles up to at least 20 years after the first symptoms. Progressive volume loss after the onset of the disease seems most pronounced in the frontal and temporal gray matter areas.12 Focusing on the frontal lobe, progressive volume decreases have been repeatedly reported.13–15 The progression of cortical gray matter deficits could arise from pathological disease progression, drug effect,16 or possibly in some cases comorbidity such as alcoholism.17
Fig. 1.
In Vivo Magnetic Resonance Imaging Demonstrating Enlarged Lateral Ventricles in Both Patients With Schizophrenia and Mouse Models for Schizophrenia. A, Schizophrenia patients: shrinkage in the left temporal horn is detected (white arrow). B–D, mouse models for schizophrenia: a representative 2-dimensional image with the lateral ventricles (top in B) and a 3-dimensional construction (bottom in B). Enlarged lateral ventricles in transgenic mice expressing a truncated, dominant-negative form of DISC1 (C, conventional model in the C57 black strain; D, inducible model in a mixed genetic background). (A, reprinted with permission from The New England Journal of Medicine; 1992; B, top panel, and C, adapted from Proceedings of National Academy of Sciences United States of America; B, bottom panel, and D, reprinted by permission from Molecular Psychiatry; 2007.
Evaluation of the integrity of white matter fiber tracts by diffusion tensor imaging has shown abnormalities in the prefrontal and temporal lobes, cingulum, and corpus callosum.18
The normal human brain is anatomically and functionally asymmetrical. A meta-analysis of anatomical asymmetry in schizophrenia found abnormal brain torque and decrease of asymmetry favoring the left planum temporal and left Sylvian fissure. Because asymmetries in these areas are strongly related to cerebral dominance, the decreased temporo-parietal asymmetries may contribute to the decreased language dominance in schizophrenia.19
Neuropathology Observations
There are 3 major pathological changes in autopsied brains from patients with schizophrenia. In interpreting the neuropathological data in schizophrenia brains, it is important to be aware of the existence of confounding factors, in particular long-term medication.
Pyramidal Neurons.
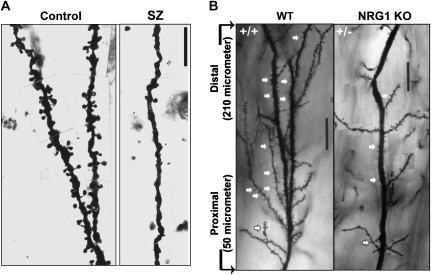
The volume changes detected by brain imaging may be explained, at least in part, by dense packing of neurons in the cortex. Morphometric analyses of the prefrontal cortex (PFC) have revealed increased density of pyramidal cells in schizophrenia brains, without alteration in total cell numbers.20,21 The more dense packing of neurons may occur due to decreased soma size and decreased neuropil, and evidence exists for both. These 2 factors are actually related because there is a correlation between soma and dendritic arbor size. The soma of pyramidal neurons in the PFC is smaller in schizophrenia brains compared with that in normal controls.20 The dendrites are shorter and less branched in schizophrenia.22,23 Furthermore, the spine density is lower in schizophrenia (figure 2A).5,24 These neuropathological changes may underlie a disturbance in neuronal connectivity in schizophrenia.
Fig. 2.
Decrease in Dendritic Spine Density in Both (A) Patients With Schizophrenia (Prefrontal Cortex) and (B) A Mouse Model for Schizophrenia (Neuregulin-1 Type III Knockout Mice Compared With Wild-Type Animals in Hippocampus). (A, Adapted from Archives of General Psychiatry; 57:65–73; B, with permission from Chen et al73 Journal of Neuroscience; 2008).
Interneurons.
For the past decade, many groups have reported molecular changes associated with interneurons in the cortex. An unbiased approach to examine gene expression profile by microarray analysis suggested the presence of molecular changes in γ-aminobutyric acid (GABA)–producing (GABAergic) neurons.25 More specifically, decrease in a GABA-synthesizing enzyme, glutamic acid decarboxylase-67 (GAD67), has been reproducibly observed.23,26,27 Reduction of calcium-binding proteins that are selectively expressed in subclasses of GABAergic interneurons in the PFC and hippocampus, such as parvalbumin and calbindin, has been reported (figure 3A).6,28–30 No changes have been found in the expression of the third class of calcium-binding proteins, calretinin. The parvalbumin-positive neurons are of special interest because they are fast spiking, synchronize pyramidal neuron firing, and give rise to the gamma oscillations, which are impaired in schizophrenia (reviewed in Lewis).31 Decrease in the expression of the neuropeptides, somatostatin, and cholecystokinin suggests that GABA neurotransmission is impaired in the Martinotti and non–fast-spiking basket cell subsets of GABAergic neurons as well.32
Fig. 3.
Decrease in the Level of Parvalbumin (PV) Expression in the Prefrontal Cortex, Observed Both in (A) Schizophrenia Patients (mRNA Level) and (B) A Mouse Model for Schizophrenia (disrupted in schizophrenia-1 [DISC1] Transgenic Mice Expressing the Truncated, Dominant-Negative Form of “DISC1”). There was decrease in PV staining but not in calbindin (CB) staining. A, reprinted by permission from Nature Reviews Neuroscience31; 2005. B, adapted from Proceedings of the National Academy of Sciences United States of America.81
Oligodendrocytes.
Fewer oligodendrocytes in various brain regions have been reported in schizophrenia.33–35 In addition, a series of gene expression studies have indicated that expression levels of myelin-related genes are decreased in schizophrenia. The most notable result of a genome-wide expression analysis of postmortem dorsolateral frontal cortex was downregulation of 5 oligodendrocyte-enriched genes that are involved in myelination.36 Another study, which focused a priori on oligodendrocyte-specific and myelination-associated genes in PFC, found a downregulation of key oligodendrocyte and myelination genes, including transcription factors that regulate these genes.37 These alterations may underlie or explain white matter deficits found in some brain imaging studies, contributing to the pathology of neuronal disconnectivity in schizophrenia.38
Structural and Anatomical Changes in Nongenetic Rodent Models of Schizophrenia
Many investigators attempted to generate models for schizophrenia even before identification of genetic susceptibility factors for schizophrenia in the past decade. Most of the efforts have been made with rats, not with mice. Such an approach is divided into 3 key strategies: the first approach is to focus on the pathophysiology of the disease, without considering its real etiologies and pathological course. Animals treated with drugs that can elicit psychotic symptoms in humans are used in this category of models. The second approach is to use environmental stressors that may play roles in the pathological course of schizophrenia. As described below, prenatal/perinatal complications and postnatal stress can elicit, in addition to behavioral deficits in adulthood, some neuropathological traits in rodents similar to those reported in brains of schizophrenia patients. The third approach is to emphasize the neurodevelopmental risks of schizophrenia in general and to make brain lesions at appropriate timing by using toxins. Here, we cover the most representative models from each category (table 2).
Table 2.
Structural and Morphological Abnormalities in Nongenetic Rodent Schizophrenia Models (Poly I:C, Polyinosinic-Polycytidylic Acid; PCP, Phencyclidine; BLA, Basoleteral Amygdala; DG, Dentate Gyrus; GFAP, Glial Fibrillary Acidic Protein; Hc, Hippocampus; IR, Immunoreactivity; mPFC, Medial Prefrontal Cortex)
| Neuropathohistology |
||||||
| Model | Imaging | Gross Anatomy | Cytoarchitecture | Pyramidal Neurons | Interneurons | Glia |
| N-methyl-D-aspartic acid receptor antagonists | ||||||
| PCP (rat) | Fewer PFC synapses (Hajszan et al43) | Less parvalbumin + IR in Hc (Jenkins et al42) | Increased astroglia process density w/o change in glia number (Hajszan et al43) | |||
| PCP (mouse) | Fewer parvalbumin + mRNA cells in PFC (Thomsen et al41) | |||||
| Ketamine (rat) | Fewer parvalbumin + IR cells in Hc (Keilhoff et al44) | |||||
| MK-801 (rat) | Less parvalbumin + IR, no change in calretinin in Hc (Braun et al45) | |||||
| Dopamine enhancement | ||||||
| Amphetamine (rat) | Reduced GAD67 IR in Hc, PFC, thalamus, amygdala (Peleg-Raibstein et al47) | No change in GFAP in caudate-putamen (Peleg-Raibstein et al47) | ||||
| Prenatal/perinatal environmental insults | ||||||
| Cesarean +/− anoxia (rat) | Changes in spine density and dendrite length at the PFC and CA1 (Juarez et al50) | |||||
| Poly I:C (mouse G9) | Decreased Hc myelination (Makinodan et al51) | Increased GABAA α2 IR at ventral DG and BLA (Nyffeler et al52) | No change in oligodendrocyte number (Makinodan et al51) | |||
| Influenza (mouse G9) | Enlarged brain (Fatemi et al53) | Increased pyramidal and nonpyramidal cell density (Fatemi et al53) | Increased GFAP IR in cortex and Hc (Fatemi et al53 Mol) | |||
| Influenza (mouse G18) | Smaller brain volume, white matter atrophy at the corpus callosum (Fatemi et al54) | |||||
| LPS (rat G15–16) | Reduced dendrite length and spine density in mPFC and CA1 (Baharnoori et al55) | |||||
| Postnatal stress | ||||||
| Isolation rearing (rat) | Smaller mPFC w/o change in cell number (Day-Wilson et al56) | Reduced dendritic length at CA1 and decreased spine density at mPFC, Hc (Silva-Gomez et al57) | ||||
| Chronic corticosteroids (rat) | Neuronal loss and atrophy of PFC layer2 (Cerqueira et al58) | |||||
| Developmental lesions | ||||||
| Neonatal Ventral Hc (rat) | Shorter and less branched basilar dendrites and reduced spine density at the mPFC (Flores et al60) | Decreaced GAD67 at the PFC (Lipska et al61) | ||||
| MAM (rat) | Thinning of the entorhinal cortex, abnormal temporal asymmetry (Talamini et al63) | Disorganized cortical layering (Talamini et al63) | Decreased density of parvalbumin neurons at the mPFC, Hc (Lodge et al64) | |||
Pharmacological Models
N-Methyl-D-Aspartic Acid Receptor Antagonists.
Exposure of humans to N-methyl-D-aspartic acid (NMDA)– type glutamate receptor antagonists, such as phencyclidine (PCP), causes schizophrenia-like symptoms.39 Thus, this drug has been administered also to rodents, attempting to build a model for schizophrenia. In rodents treated subchronically with PCP in adulthood, in addition to behavioral manifestations similar to the endophenotypes of schizophrenia,40 an important histological trait in human schizophrenia has been reproduced: decreased parvalbumin in the PFC (in mice)41 and the hippocampus (in rats).42 Furthermore, subchronic PCP treatment of rats decreases the number of synaptic spines in the PFC, detected by electron microscopy.43 The same article reported increased density of astrocyte processes without change in the number of astrocytes in PCP-treated rats, which has not been reported in schizophrenia patients. Taken together, signs of interneuron deficits and synaptic spine changes support the idea that PCP induces, at least in part, schizophrenia-like pathophysiology. At present, the subchronic PCP model is widely used, especially in compound screening for schizophrenia treatment. In addition to PCP, treatment with other NMDA receptor antagonists also results in schizophrenia-like pathophysiology. For example, chronic administration of ketamine in rats reduces the density of parvalbumin-immunoreactive hippocampal interneurons.44 Chronic MK-801 treatment in rats has a similar effect in the hippocampus: decreased immunoreactivity of parvalbumin without change in that for calretinin but no effect in the PFC.45
Amphetamine.
Amphetamine can mimic mainly the positive symptoms of schizophrenia by increasing the dopamine concentration in the synaptic cleft.46 Escalating amphetamine injection results in decreased GAD67 immunoreactivity in the hippocampus, PFC, thalamus, and amygdala. This was not accompanied by enhanced neurotoxicity or reactive gliosis.47
Environmental Stress Models
Prenatal/Perinatal Environmental Insults.
Prenatal/antenatal environmental insults, especially birth hypoxia and congenital virus/pathogen infection, are well-established environmental risk factors for schizophrenia.48 Maternal infections, especially during the first and second trimester increase the risk for schizophrenia. It still remains debatable whether and/or why infection at certain gestation periods may confer maximal risk for neurodevelopmental disturbances.49 A rat model of delayed cesarean section shows decreased spine density of the pyramidal neurons of the PFC and the hippocampal cornus ammonis 1 (CA1) at postnatal day 35 (P35). When Cesarean section is combined with anoxia, the decrease in spine density of the PFC is further augmented. Increase in the length of dendrites of medium spiny neurons is also observed at P35 but is normalized by P70.50 Injection of double-stranded RNA polyinosinic-polycytidylic acid (Poly I:C) mimics the immune response elicited by viral infection. Poly I:C injection to a mouse dam around gestational day 9 (G9, late first trimester) results in decreased myelination and axonal diameters in the hippocampus of juvenile offspring, without loss of oligodendrocytes.51 Immunohistochemistry for the GABAA receptor α2 subunit detects increases in the ventral (but not in the dorsal) dentate gyrus and basolateral amygdala in adult offspring after poly I:C at G9.52 Infection of mice with influenza virus at G9 results in increased pyramidal and nonpyramidal cell densities and increased brain size in offspring in adulthood.53 When the influenza infection is carried out in the late second trimester (G18), the offspring display smaller brain volume and fractional anisotropy of the corpus callosum, as well as white matter atrophy at P35.54 Prenatal challenge with the bacterial immune activator lipopolysaccharide in rats reduces the dendritic arbor and spine density in the medial PFC and CA1 pyramidal neurons and affects spine structure at CA1.55
Postnatal Stress.
Rats normally live in social groups. When reared in isolation after weaning, various neurobehavioral abnormalities emerge. The following behavioral abnormalities have been reported: volume loss of the medial PFC without change in neuron number,56 as well as decreased dendritic spine density on PFC and hippocampal pyramidal neurons with reduced dendritic length only at the hippocampus.57 Stress can be mediated by corticosteroids. Thus, chronic corticosteroid treatment in rats results in neuronal loss and atrophy specifically of layer II of the infralimbic, prelimbic, and cingulate cortices.58
Developmental Lesion Models
Neonatal Hippocampal Lesion.
A classic neurodevelopmental schizophrenia model is generated by excitotoxic lesion of the ventral hippocampal formation in rats at P7.59 When analyzed in adulthood, the basilar dendrites of the PFC layer 3 pyramidal neurons are shorter and less branched and have decreased spine density, similar to findings from schizophrenia patients.60 GAD67 mRNA is decreased in the PFC in these rats implying a deficit in GABAergic interneurons.61
Methylazoxymethanol.
Administration of a mitotoxin, methylazoxymethanol (MAM), to pregnant rats interferes with development of the embryonic brain region in which progenitor cells proliferate.62 When administered once during gestational days 9–12 (G9–12), the entorhinal cortex shows cortical thinning, disorganized cortical layering, and abnormal temporal asymmetries.63 The abnormalities are more evident the later the lesion. When MAM is administered at G17, adult offspring display decreased density of parvalbumin-positive interneurons at the medial PFC and ventral subiculum of the hippocampus.64
Structural and Anatomical Changes in Genetic Mouse Models of Schizophrenia
Schizophrenia susceptibility genes identified by human genetic studies have been found only recently, finally enabling generation of mouse models on the basis of genetic etiology. Because causal mutations per se have not been identified, there is still debate on the significance of each gene. Nonetheless, many of the genetically engineered models for these genes display behavioral abnormalities and morphological/anatomical alterations that may be relevant to schizophrenia. Here, we discuss morphological/anatomical changes in these mice. Among promising candidate genes for schizophrenia, as far as we are aware, no mouse models for regulator of G protein signaling 4 and carboxyl-terminal PDZ ligand of neuronal nitric oxide synthase have been published yet. In knockout (KO) mice deficient in neuronal nitric oxide synthase, serine racemase, calcineurin, and metabotropic glutamate receptor, published data do not include anatomical and morphological assessment.65–69 Thus, in this section, we will introduce genetic models for dysbindin, neuregulin-1 (NRG1), ErbB4, disrupted in schizophrenia-1 (DISC1), Akt1, and genes found in chromosomal region 22q11 (table 3).
Table 3.
Structural and Morphological Abnormalities in Genetic Schizophrenia Models
| Neuropathohistology |
||||||
| Model | Imaging | Gross Anatomy | Cytoarchitecture | Pyramidal Neurons | Interneurons | Glia |
| Dysbindin | Morphological changes in asymmetrical synapses in CA1 (Chen et al71) | |||||
| NRG1 | Increased lateral ventricles (Chen et al71), Normal myelin (Brinkmann et al74) | Reduced spine density in Hc (Chen et al71, Barros et al75) | ||||
| ErbB4 | Normal myelin (Brinkmann et al74) | Reduced spine density in cortex, Hc (Barros et al75) | Reduced parvalbumin IR (Fisahn et al76) Reduced calbindin IR (Flames et al77) | Increased oligodendrocyte number, thinner myelin (Roy et al78) | ||
| Disc1 | Smaller brain volume, Enlarged lateral ventricles (Hikida et al81, Pletnikov et al) | Enlarged lateral ventricles partial agenesis of corpus callosum, thinner cortex (Shen et al83) | Altered organization of dentate granule cells (Kvajo et al86) | Shorter dendrites (Pletnikov et al, Li et al85, Shen et al83) | Reduced parvalbumin IR at mPFC, Hc (Hikida et al81, Shen et al83) | |
| Akt-1 | Altered pyramidal cell morphology at PFC (Lai et al87) | No change in parvalbumin and calbindin density in the PFC (Lai et al87) | ||||
| Δ22q11 (COMT, GNB1L, PRODH, ZDHHC8) | Decreased spine density and dendritic complexity (Mukai et al89) | |||||
Note: CA1, cornus ammonis 1; DISC1, disrupted in schizophrenia-1; Hc, hippocampus; IR, immunoreactivitiy; mPFC, medial prefontal cortex.
Dysbindin
“Sandy” mice have a spontaneous mutation in the schizophrenia susceptibility gene dysbindin. Because of this mutation, the homozygotes do not express dysbindin protein.70 These mice have morphological changes in excitatory asymmetrical synapses on hippocampal CA1 dendritic spines: presynaptically bigger but fewer glutamatergic vesicles, narrower synaptic cleft, and broader postsynaptic density.71 Dysbindin is involved in neurotransmitter release, which may account for the various abnormal behaviors displayed by the Sandy mice.72
Neuregulin-1 and ErbB4
Both NRG1 and one of its receptors, ErbB4, are strongly implicated in schizophrenia.3 Many mouse models with manipulated expression levels of the different NRG1 isoforms have been generated. Adult heterozygous mice with a targeted disruption for type III NRG1 have enlarged lateral ventricles and decreased density of dendritic spines on hippocampal pyramidal neurons (figure 2B). Interestingly, in vivo imaging detected hypofunction in the medial PFC and hippocampus, and behavioral analysis found cognition-related abnormalities.73 NRG1 type III is essential for myelination in the peripheral nervous system, but surprisingly conditional KO of NRG1 in cortical projection neurons from embryonic day 12 (E12) or postnatally and double KO of ErbB3/4 result in normal myelination in the central nervous system.74 Interestingly, transgenic overexpression of NRG1 results in hypermyelination.74 Mice lacking both ErbB2 and ErbB4 specifically in the central nervous system from early embryonic stages have normal brain morphology but decreased spine density in the cortex and hippocampus. The decreased spine density is expected to disturb the function of neuronal circuits, and indeed, ErbB2/4 KO displayed increased aggression and a PPI deficit.75 ErbB4 KO display reduced density of parvalbumin-positive cells in the hippocampus, resulting in reduced power of kainate-induced gamma oscillations,76 as well as reduced density of calbindin-positive and GABAergic interneurons in the cortex.77 Expression of dominant-negative ErbB4 in oligodendrocytes and myelinating Schwann cells from E15 results in thinning of the myelin sheath of the corpus callosum, altered oligodendrocyte morphology, and a surprising increase in the number of cells expressing a differentiated oligodendrocyte marker. It was suggested that the dopaminergic abnormalities seen in these mice mice might result from the defective myelin.78
Disrupted in Schizophrenia-1
Most DISC1 mouse models are based on the fact that the DISC1 gene was originally identified as truncated by a translocation that segregated with psychiatric diseases. Although there is debate whether such truncated DISC1 product exists at protein levels, the putative truncated protein acts as dominant negative.79 Thus, regardless that the Scottish genetic mutation results in haploinsufficiency or dominant-negative mutant effect or both, an overall defect is postulated to be a partial loss of DISC1 function.80 Based on this idea, several transgenic models expressing this truncated protein have been generated.81–83 Very interestingly, a major common phenotype observed in these transgenic mice is enlarged lateral ventricles, an important hallmark for schizophrenia.81–83 In addition, in a transgenic mouse expressing truncated DISC1 generated using a bacterial artificial chromosome vector, reduced cortical thickness and partial agenesis of the corpus callosum are also observed.83 Postnatal expression of this mutant DISC1 in a set of cells in the forebrain may be sufficient to lead to enlargement of lateral ventricles, which has been indicated by 2 types of transgenic mice under the temporal and spatial control by the α-calmodulin kinase II promoter.81,82 In addition, reduced brain volume is also found in mice with missense mutations L100P or Q31L of DISC1.84 Another important hallmark for schizophrenia is reduced immunoreactivity of parvalbumin.31 In 2 transgenic mice expressing truncated DISC1, reduced immunoreactivity of parvalbumin is detected in the medial PFC (figure 3B)81,83 and hippocampus.83 Abnormalities of hippocampus may underlie the pathophysiology of schizophrenia. In a transgenic model expressing a dominant-negative DISC1 (a C-terminal fragment of DISC1) transiently at P7, reduction of hippocampal dendritic complexity is reported, resulting in reduced hippocampal synaptic transmission.85 In another type of genetically engineered DISC1 mice, hippocampal granule cells display misorientated and shorter dendrites and decrease in numbers of synaptic spines. These dendritic abnormalities may cause the reduced short-term potentiation at CA3/CA1 synapses and indirectly the working memory deficit found in these mice.86
Akt1
Association studies of Akt1 with schizophrenia have yielded mixed results, but it remains an interesting candidate. Comprehensive morphological analysis of layer V pyramidal neurons in the medial PFC of Akt1 KO reveals mostly normal neuronal densities but abnormal dendritic architecture.87
22q11.2
Microdeletion at 22q11.2 causes velocardiofacial syndrome, which consists of congenital abnormalities affecting several tissues and organs. About 25% develop schizophrenia or schizoaffective disorder.88 Of the many genes in this region, the involvement of Catechol-O-methyl transferase (COMT), proline dehydrogenase (PRODH), zinc finger, DHHC-type containing 8 (ZDHHC8), and guanine nucleotide-binding protein (G protein), beta polypeptide 1-like (GNB1L) in schizophrenia has been independently supported. A mouse model with a deletion syntenic to the human microdeletion displays decreased density of dendritic spines and decreased dendritic complexity of CA1 pyramidal neurons,89 which may underlie the prepulse inhibition and fear conditioning deficits in this model.90
Perspectives
There is still debate about whether it is possible to use rodents to model psychiatric disorders in which high brain functions that are probably in part unique to humans are impaired. Nonetheless, rodent models, especially genetically engineered mice in which disease-associated etiologies (causal or susceptibility genes) are modified, have potential advantages over human studies. In order to understand disease mechanisms in depth, it is very important to characterize how the disease etiologies develop over time until development of full-blown disease. In the case of schizophrenia, initial risks for the disease occur during neurodevelopment, whereas the disease onset is in young adulthood, with almost 2 decades for the full development of pathology to the onset. Thus, it is very difficult to address this mechanism by human studies. Better understanding of the disease mechanisms and time course, therefore, is expected with use of genetically engineered mouse models. Another major advantage of mouse models is their usefulness for compound screening in drug development. In comparison to primates, rodents are much easier for preclinical drug screening from both economical and ethical viewpoints. Mouse models can provide us with an opportunity to identify novel therapeutic strategies that are directly linked to the disease mechanisms. Mouse models may not be so useful for understanding functions of primate specific schizophrenia candidate genes, such as D-amino acid oxidase activator (DAOA/G72).91
In this short review, we have tried to establish a series of similarities between pathology in humans (patients with schizophrenia) and rodent models (nongenetic rodent models and genetically engineered mice). It seems clear that the multiple similarities indicate the potential for studying these in more depth, which would provide a firm basis for clarifying the mechanisms underlying some of the characteristics of schizophrenia and useful tools for translation.
Supplementary Material
Color versions of figures 1–3 are available as Supplementary Material at http://schizophreniabulletin.oxfordjournals.org.
Funding
MH-084018; MH-069853; MH-088753; Stanley; Cure Huntington's Disease Initiative; HighQ; S & R foundation; RUSK; Johns Hopkins Brain Science Institute (to A.S.); NARSAD (to A.S., M.V.P., H.J-.P); National Alliance for Autism Research (NAAR) (to M.V.P.).
Supplementary Material
Acknowledgments
We thank Ms. Y. Lema and Dr. P. Talalay for help with manuscript preparation. Dr Sawa reports consultation for Pfizer and Taisho and speaking for Eli Lilly, Sanofi Aventis, and Taisho.
References
- 1.Sawa A, Snyder SH. Schizophrenia: diverse approaches to a complex disease. Science. 2002;296:692–695. doi: 10.1126/science.1070532. [DOI] [PubMed] [Google Scholar]
- 2.Owen MJ, Craddock N, O'Donovan MC. Schizophrenia: genes at last? Trends Genet. 2005;21:518–525. doi: 10.1016/j.tig.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends Neurosci. 2009;32:485–495. doi: 10.1016/j.tins.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 6.Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry. 2001;158:1411–1422. doi: 10.1176/appi.ajp.158.9.1411. [DOI] [PubMed] [Google Scholar]
- 7.Richardson JA, Burns DK. Mouse models of Alzheimer’s disease: a quest for plaques and tangles. ILAR J. 2002;43:89–99. doi: 10.1093/ilar.43.2.89. [DOI] [PubMed] [Google Scholar]
- 8.Vita A, De Peri L, Silenzi C, Dieci M. Brain morphology in first-episode schizophrenia: a meta-analysis of quantitative magnetic resonance imaging studies. Schizophr Res. 2006;82:75–88. doi: 10.1016/j.schres.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 10.Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- 11.Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hulshoff Pol HE, Kahn RS. What happens after the first episode? A review of progressive brain changes in chronically ill patients with schizophrenia. Schizophr Bull. 2008;34:354–366. doi: 10.1093/schbul/sbm168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gur RE, Cowell P, Turetsky BI, et al A follow-up magnetic resonance imaging study of schizophrenia. Relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry. 1998;55:145–152. doi: 10.1001/archpsyc.55.2.145. [DOI] [PubMed] [Google Scholar]
- 14.Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:148–157. doi: 10.1001/archpsyc.58.2.148. [DOI] [PubMed] [Google Scholar]
- 15.van Haren NE, Hulshoff Pol HE, Schnack HG, et al. Focal gray matter changes in schizophrenia across the course of the illness: a 5-year follow-up study. Neuropsychopharmacology. 2007;32:2057–2066. doi: 10.1038/sj.npp.1301347. [DOI] [PubMed] [Google Scholar]
- 16.Lieberman JA, Tollefson GD, Charles C, et al. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62:361–370. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- 17.Deshmukh A, Rosenbloom MJ, De Rosa E, Sullivan EV, Pfefferbaum A. Regional striatal volume abnormalities in schizophrenia: effects of comorbidity for alcoholism, recency of alcoholic drinking, and antipsychotic medication type. Schizophr Res. 2005;79:189–200. doi: 10.1016/j.schres.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 18.Kubicki M, McCarley R, Westin CF, et al. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41:15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sommer I, Ramsey N, Kahn R, Aleman A, Bouma A. Handedness, language lateralisation and anatomical asymmetry in schizophrenia: meta-analysis. Br J Psychiatry. 2001;178:344–351. doi: 10.1192/bjp.178.4.344. [DOI] [PubMed] [Google Scholar]
- 20.Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 21.Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry. 1998;55:215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- 22.Black JE, Kodish IM, Grossman AW, et al. Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. Am J Psychiatry. 2004;161:742–744. doi: 10.1176/appi.ajp.161.4.742. [DOI] [PubMed] [Google Scholar]
- 23.Guidotti A, Auta J, Davis JM, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 24.Garey LJ, Ong WY, Patel TS, et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- 26.Akbarian S, Kim JJ, Potkin SG, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 27.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 28.Beasley CL, Zhang ZJ, Patten I, Reynolds GP. Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry. 2002;52:708–715. doi: 10.1016/s0006-3223(02)01360-4. [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto T, Volk DW, Eggan SM, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res. 2002;55:1–10. doi: 10.1016/s0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]
- 31.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto T, Arion D, Unger T, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byne W, Kidkardnee S, Tatusov A, Yiannoulos G, Buchsbaum MS, Haroutunian V. Schizophrenia-associated reduction of neuronal and oligodendrocyte numbers in the anterior principal thalamic nucleus. Schizophr Res. 2006;85:245–253. doi: 10.1016/j.schres.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 34.Schmitt A, Steyskal C, Bernstein HG, et al. Stereologic investigation of the posterior part of the hippocampus in schizophrenia. Acta Neuropathol. 2009;117:395–407. doi: 10.1007/s00401-008-0430-y. [DOI] [PubMed] [Google Scholar]
- 35.Vostrikov VM, Uranova NA, Orlovskaya DD. Deficit of perineuronal oligodendrocytes in the prefrontal cortex in schizophrenia and mood disorders. Schizophr Res. 2007;94:273–280. doi: 10.1016/j.schres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 36.Hakak Y, Walker JR, Li C, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci U S A. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tkachev D, Mimmack ML, Ryan MM, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- 38.Davis KL, Haroutunian V. Global expression-profiling studies and oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:758. doi: 10.1016/S0140-6736(03)14297-3. [DOI] [PubMed] [Google Scholar]
- 39.Krystal JH, Karper LP, Seibyl JP, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 40.Enomoto T, Noda Y, Nabeshima T. Phencyclidine and genetic animal models of schizophrenia developed in relation to the glutamate hypothesis. Methods Find Exp Clin Pharmacol. 2007;29:291–301. doi: 10.1358/mf.2007.29.4.1075358. [DOI] [PubMed] [Google Scholar]
- 41.Thomsen MS, Christensen DZ, Hansen HH, Redrobe JP, Mikkelsen JD. alpha(7) Nicotinic acetylcholine receptor activation prevents behavioral and molecular changes induced by repeated phencyclidine treatment. Neuropharmacology. 2009;56:1001–1009. doi: 10.1016/j.neuropharm.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Jenkins TA, Harte MK, McKibben CE, Elliott JJ, Reynolds GP. Disturbances in social interaction occur along with pathophysiological deficits following sub-chronic phencyclidine administration in the rat. Behav Brain Res. 2008;194:230–235. doi: 10.1016/j.bbr.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 43.Hajszan T, Leranth C, Roth RH. Subchronic phencyclidine treatment decreases the number of dendritic spine synapses in the rat prefrontal cortex. Biol Psychiatry. 2006;60:639–644. doi: 10.1016/j.biopsych.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 44.Keilhoff G, Becker A, Grecksch G, Wolf G, Bernstein HG. Repeated application of ketamine to rats induces changes in the hippocampal expression of parvalbumin, neuronal nitric oxide synthase and cFOS similar to those found in human schizophrenia. Neuroscience. 2004;126:591–598. doi: 10.1016/j.neuroscience.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 45.Braun I, Genius J, Grunze H, Bender A, Moller HJ, Rujescu D. Alterations of hippocampal and prefrontal GABAergic interneurons in an animal model of psychosis induced by NMDA receptor antagonism. Schizophr Res. 2007;97:254–263. doi: 10.1016/j.schres.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annu Rev Pharmacol Toxicol. 1993;33:639–677. doi: 10.1146/annurev.pa.33.040193.003231. [DOI] [PubMed] [Google Scholar]
- 47.Peleg-Raibstein D, Knuesel I, Feldon J. Amphetamine sensitization in rats as an animal model of schizophrenia. Behav Brain Res. 2008;191:190–201. doi: 10.1016/j.bbr.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 48.Mittal VA, Ellman LM, Cannon TD. Gene-environment interaction and covariation in schizophrenia: the role of obstetric complications. Schizophr Bull. 2008;34:1083–1094. doi: 10.1093/schbul/sbn080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meyer U, Yee BK, Feldon J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse? Neuroscientist. 2007;13:241–256. doi: 10.1177/1073858406296401. [DOI] [PubMed] [Google Scholar]
- 50.Juarez I, Gratton A, Flores G. Ontogeny of altered dendritic morphology in the rat prefrontal cortex, hippocampus, and nucleus accumbens following cesarean delivery and birth anoxia. J Comp Neurol. 2008;507:1734–1747. doi: 10.1002/cne.21651. [DOI] [PubMed] [Google Scholar]
- 51.Makinodan M, Tatsumi K, Manabe T, et al. Maternal immune activation in mice delays myelination and axonal development in the hippocampus of the offspring. J Neurosci Res. 2008;86:2190–2200. doi: 10.1002/jnr.21673. [DOI] [PubMed] [Google Scholar]
- 52.Nyffeler M, Meyer U, Yee BK, Feldon J, Knuesel I. Maternal immune activation during pregnancy increases limbic GABAA receptor immunoreactivity in the adult offspring: implications for schizophrenia. Neuroscience. 2006;143:51–62. doi: 10.1016/j.neuroscience.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 53.Fatemi SH, Earle J, Kanodia R, et al. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell Mol Neurobiol. 2002;22:25–33. doi: 10.1023/a:1015337611258. [DOI] [PubMed] [Google Scholar]
- 54.Fatemi SH, Reutiman TJ, Folsom TD, et al. Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: implications for genesis of neurodevelopmental disorders. Schizophr Res. 2008;99:56–70. doi: 10.1016/j.schres.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baharnoori M, Brake WG, Srivastava LK. Prenatal immune challenge induces developmental changes in the morphology of pyramidal neurons of the prefrontal cortex and hippocampus in rats. Schizophr Res. 2009;107:99–109. doi: 10.1016/j.schres.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 56.Day-Wilson KM, Jones DN, Southam E, Cilia J, Totterdell S. Medial prefrontal cortex volume loss in rats with isolation rearing-induced deficits in prepulse inhibition of acoustic startle. Neuroscience. 2006;141:1113–1121. doi: 10.1016/j.neuroscience.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 57.Silva-Gomez AB, Rojas D, Juarez I, Flores G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res. 2003;983:128–136. doi: 10.1016/s0006-8993(03)03042-7. [DOI] [PubMed] [Google Scholar]
- 58.Cerqueira JJ, Pego JM, Taipa R, Bessa JM, Almeida OF, Sousa N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci. 2005;25:7792–7800. doi: 10.1523/JNEUROSCI.1598-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- 60.Flores G, Alquicer G, Silva-Gomez AB, et al Alterations in dendritic morphology of prefrontal cortical and nucleus accumbens neurons in post-pubertal rats after neonatal excitotoxic lesions of the ventral hippocampus. Neuroscience. 2005;133:463–470. doi: 10.1016/j.neuroscience.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 61.Lipska BK, Lerman DN, Khaing ZZ, Weinberger DR. The neonatal ventral hippocampal lesion model of schizophrenia: effects on dopamine and GABA mRNA markers in the rat midbrain. Eur J Neurosci. 2003;18:3097–3104. doi: 10.1111/j.1460-9568.2003.03047.x. [DOI] [PubMed] [Google Scholar]
- 62.Cattabeni F, Di Luca M. Developmental models of brain dysfunctions induced by targeted cellular ablations with methylazoxymethanol. Physiol Rev. 1997;77:199–215. doi: 10.1152/physrev.1997.77.1.199. [DOI] [PubMed] [Google Scholar]
- 63.Talamini LM, Koch T, Ter Horst GJ, Korf J. Methylazoxymethanol acetate-induced abnormalities in the entorhinal cortex of the rat; parallels with morphological findings in schizophrenia. Brain Res. 1998;789:293–306. doi: 10.1016/s0006-8993(98)00020-1. [DOI] [PubMed] [Google Scholar]
- 64.Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Inoue R, Hashimoto K, Harai T, Mori H. NMDA- and beta-amyloid1-42-induced neurotoxicity is attenuated in serine racemase knock-out mice. J Neurosci. 2008;28:14486–14491. doi: 10.1523/JNEUROSCI.5034-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Labrie V, Fukumura R, Rastogi A, et al. Serine racemase is associated with schizophrenia susceptibility in humans and in a mouse model. Hum Mol Genet. 2009;18:3227–3243. doi: 10.1093/hmg/ddp261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tanda K, Nishi A, Matsuo N, et al. Abnormal social behavior, hyperactivity, impaired remote spatial memory, and increased D1-mediated dopaminergic signaling in neuronal nitric oxide synthase knockout mice. Mol Brain. 2009;2:19. doi: 10.1186/1756-6606-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yokoi M, Kobayashi K, Manabe T, et al. Impairment of hippocampal mossy fiber LTD in mice lacking mGluR2. Science. 1996;273:645–647. doi: 10.1126/science.273.5275.645. [DOI] [PubMed] [Google Scholar]
- 69.Zeng H, Chattarji S, Barbarosie M, et al. Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell. 2001;107:617–629. doi: 10.1016/s0092-8674(01)00585-2. [DOI] [PubMed] [Google Scholar]
- 70.Li W, Zhang Q, Oiso N, et al. Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1) Nat Genet. 2003;35:84–89. doi: 10.1038/ng1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen XW, Feng YQ, Hao CJ, et al. DTNBP1, a schizophrenia susceptibility gene, affects kinetics of transmitter release. J Cell Biol. 2008;181:791–801. doi: 10.1083/jcb.200711021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feng YQ, Zhou ZY, He X, et al. Dysbindin deficiency in sandy mice causes reduction of snapin and displays behaviors related to schizophrenia. Schizophr Res. 2008;106:218–228. doi: 10.1016/j.schres.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 73.Chen YJ, Johnson MA, Lieberman MD, et al. Type III neuregulin-1 is required for normal sensorimotor gating, memory-related behaviors, and corticostriatal circuit components. J Neurosci. 2008;28:6872–6883. doi: 10.1523/JNEUROSCI.1815-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brinkmann BG, Agarwal A, Sereda MW, et al. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron. 2008;59:581–595. doi: 10.1016/j.neuron.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barros CS, Calabrese B, Chamero P, et al. Impaired maturation of dendritic spines without disorganization of cortical cell layers in mice lacking NRG1/ErbB signaling in the central nervous system. Proc Natl Acad Sci U S A. 2009;106:4507–4512. doi: 10.1073/pnas.0900355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fisahn A, Neddens J, Yan L, Buonanno A. Neuregulin-1 modulates hippocampal gamma oscillations: implications for schizophrenia. Cereb Cortex. 2009;19:612–618. doi: 10.1093/cercor/bhn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Flames N, Long JE, Garratt AN, et al. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004;44:251–261. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 78.Roy K, Murtie JC, El-Khodor BF, et al. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc Natl Acad Sci U S A. 2007;104:8131–8136. doi: 10.1073/pnas.0702157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kamiya A, Kubo K, Tomoda T, et al. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat Cell Biol. 2005;7:1167–1178. doi: 10.1038/ncb1328. [DOI] [PubMed] [Google Scholar]
- 80.Sawa A, Snyder SH. Genetics. Two genes link two distinct psychoses. Science. 2005;310:1128–1129. doi: 10.1126/science.1121114. [DOI] [PubMed] [Google Scholar]
- 81.Hikida T, Jaaro-Peled H, Seshadri S, et al. Dominant-negative DISC1 transgenic mice display schizophrenia-associated phenotypes detected by measures translatable to humans. Proc Natl Acad Sci U S A. 2007;104:14501–14506. doi: 10.1073/pnas.0704774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pletnikov MV, Ayhan Y, Nikolskaia O, et al. Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry. 2008;13:173–186. doi: 10.1038/sj.mp.4002079. 115. [DOI] [PubMed] [Google Scholar]
- 83.Shen S, Lang B, Nakamoto C, et al. Schizophrenia-related neural and behavioral phenotypes in transgenic mice expressing truncated Disc1. J Neurosci. 2008;28:10893–10904. doi: 10.1523/JNEUROSCI.3299-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clapcote SJ, Lipina TV, Millar JK, et al. Behavioral phenotypes of Disc1 missense mutations in mice. Neuron. 2007;54:387–402. doi: 10.1016/j.neuron.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 85.Li W, Zhou Y, Jentsch JD, et al. Specific developmental disruption of disrupted-in-schizophrenia-1 function results in schizophrenia-related phenotypes in mice. Proc Natl Acad Sci U S A. 2007;104:18280–18285. doi: 10.1073/pnas.0706900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kvajo M, McKellar H, Arguello PA, et al. A mutation in mouse Disc1 that models a schizophrenia risk allele leads to specific alterations in neuronal architecture and cognition. Proc Natl Acad Sci U S A. 2008;105:7076–7081. doi: 10.1073/pnas.0802615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lai WS, Xu B, Westphal KG, et al. Akt1 deficiency affects neuronal morphology and predisposes to abnormalities in prefrontal cortex functioning. Proc Natl Acad Sci U S A. 2006;103:16906–16911. doi: 10.1073/pnas.0604994103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bassett AS, Chow EW, Husted J, et al. Clinical features of 78 adults with 22q11 Deletion Syndrome. Am J Med Genet A. 2005;138:307–313. doi: 10.1002/ajmg.a.30984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mukai J, Dhilla A, Drew LJ, et al. Palmitoylation-dependent neurodevelopmental deficits in a mouse model of 22q11 microdeletion. Nat Neurosci. 2008;11:1302–1310. doi: 10.1038/nn.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paylor R, McIlwain KL, McAninch R, et al. Mice deleted for the DiGeorge/velocardiofacial syndrome region show abnormal sensorimotor gating and learning and memory impairments. Hum Mol Genet. 2001;10:2645–2650. doi: 10.1093/hmg/10.23.2645. [DOI] [PubMed] [Google Scholar]
- 91.Chumakov I, Blumenfeld M, Guerassimenko O, et al. Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc Natl Acad Sci U S A. 2002;99:13675–13680. doi: 10.1073/pnas.182412499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.