Abstract
Maternal influenza during pregnancy is a controversial risk factor for schizophrenia in the child. We conducted a meta-analysis to examine whether birth during the 9-month period after the pandemic of 1957 was a risk factor for schizophrenia. Studies that compared the risk of schizophrenia among subjects born after the pandemic with that among those born in corresponding time periods in surrounding years were divided into those conducted in the United States, Europe, or Australia (type A studies, n = 8) and those from Japan, where the epidemic came in 2 waves (type B studies, n = 3). Other studies examined the risk among subjects born to mothers who were pregnant during the pandemic and reported having had influenza (type C studies, n = 2). Relative risks (RRs) were extracted or calculated for each month and/or trimester of possible exposure by 2 independent authors. All analyses were performed using a fixed-effects model. The weighted results of the type A studies did not indicate a significantly increased risk of schizophrenia among children exposed during any trimester or month of prenatal life. Not a single study found a significant first- or second-trimester effect. The mean weighted RR for subjects who were in their first, second, or third trimester of prenatal life during the pandemic (8 effect sizes) was 0.91 (95% confidence interval [CI]: 0.85–0.98), 1.00 (95% CI: 0.93–1.07), and 1.05 (95% CI: 0.98–1.12), respectively. The pooled results of the type B and type C studies were also negative. Given high infection rates during the pandemic (about 50%), these results do not support the maternal influenza hypothesis.
Keywords: psychosis, epidemiology, prenatal infection, prenatal exposure, cytokines
Introduction
Maternal influenza during pregnancy is in many circles an established risk factor for schizophrenia. The basis to support the hypothesis, however, is controversial. The first evidence came from studies of the 1957 pandemic of A2 (“Asian”) influenza. An influential study from Finland, published in 1988, reported an increased proportion of schizophrenia diagnoses in patients whose second trimester of fetal life overlapped with the influenza pandemic.1 Three years later, an investigation from England and Wales reported an 88% increased risk of schizophrenia among subjects born 5 months after the peak of the pandemic.2 Subsequent studies, however, reported inconsistent findings.3
A limitation of these so-called ecological studies is the absence of information on exposure: Subjects are considered to be exposed if they are born in a given period after an epidemic or pandemic. A more sophisticated method of documenting exposure is the assessment of influenza antibodies in pregnant mothers. The only study to compare antibody titers in pregnancies giving rise to affected and unaffected offspring involved an American cohort born during the period 1959–1966, ie, not exposed to the pandemic.4 Exposure to influenza virus during the first, but not the second or third, trimester of pregnancy was associated with an increased risk of schizophrenia. However, this result was inconclusive because the 95% confidence interval (CI) for the measure of outcome was extremely large and included 1.0 (Mantel-Haenszel odds ratio = 7.0, 95% CI: 0.7–75.3).
Thus, a correct interpretation of the ecological investigations remains important for an evaluation of the influenza hypothesis. The absence of information on exposure does not completely invalidate these studies because infection rates during pandemics are assumed to be about 50%.5,6 When interpreting these studies, it is important to bear in mind that the likelihood of research findings being true diminishes with the number of relationships tested.7 This could be relevant here because many studies tested for an association between schizophrenia risk and exposure to influenza during any of the 9 months of prenatal life. Because this method leads to 9 statistical tests, some associations will emerge by chance.
We reasoned that if maternal influenza contributes to the etiology of schizophrenia, a pandemic should increase the risk for those in utero. We therefore performed a meta-analysis to examine whether birth during the 9-month period after the 1957 pandemic, or maternal reports of influenza during pregnancy at the time of the pandemic, were risk factors for schizophrenia in the child.
Methods
Data Sources
We performed a MEDLINE search (1960 to March 2008) using the key words influenza, pandemic, psychosis, schizophrenia, and paranoid, and reviewed the reference lists of relevant articles. In order to be included a study had to report a relative risk (RR) for children born during (part of) the 9-month period after the pandemic (or for children born to mothers who reported having had influenza). Studies that provided sufficient information to allow us to calculate the RR were also included.
Study Selection
Eighteen potentially relevant studies were retrieved,1,2,8–23 5 of which were subsequently excluded.8–12 Among these was a study using data sets from England, Scotland, and Denmark that did not report patient numbers and presented the results in terms of t values, which could not be translated into RRs.8 However, the data sets from England and Scotland overlapped strongly with other data sets from these countries, described in previous publications, from which we could extract RRs.2,15 Unfortunately, the Danish data set was not available (it had not been saved). We excluded a second study, from Surinam and the Netherlands Antilles, because the lack of information on the number of live births in these countries made it difficult to estimate the RRs with any certainty.9 We also excluded a study from Palau because it examined schizophrenia risk by calendar year of birth.10 Finally, of the 3 overlapping studies investigating the impact of the pandemic on the Dutch population,11–13 we included the Selten and Slaets study because it provided information by gender.13
We distinguished 3 types of studies. Those designated type A studies included 8 ecological studies from Europe, America, and Australia that compared the risk of schizophrenia among subjects born any time in the 9 months after the pandemic (ie, index period) with that among those born during corresponding periods of time in the previous and/or subsequent year (ie, control periods).1,2,13–18 Although it was not known whether the mothers actually had influenza, these children were considered to have been exposed. Likewise, those born in the corresponding period in the previous and/or subsequent year were considered not to have been exposed. One type A study15 provided information from 2 overlapping sources: the Edinburgh Psychiatric Case Register and a data set for the whole of Scotland. We used the largest data set (Scotland). Six of the type A studies only included patients who had ever been hospitalized, while 2 included outpatients as well as inpatients.14,18
Three ecological studies from Japan were designated type B studies.19–21 We distinguished between the Japanese and other ecological studies because the course of the pandemic in Japan differed markedly from that in other countries in that it came in 2 waves, from June to July 1957 and from November to December 1957. Thus, a subject born in February 1958 could have been exposed during the first, second, or third trimester of prenatal life. Two studies examined the risk for subjects admitted to hospitals in Greater Tokyo and the southern part of Shikoku island (Kochi), respectively.19,20 A third study included a survey among a randomly selected sample of hospitals and outpatient clinics throughout the whole of Japan.21 Because the degree of overlap between the national survey and the studies in Greater Tokyo and Kochi was considered to be modest, the 3 studies were retained.
Finally, 2 studies compared the risk of schizophrenia among children whose mothers reported having had influenza during the pandemic with that among those whose mothers reported not having had influenza during the pandemic.22,23 These were designated type C studies. In most studies, the diagnosis of schizophrenia was made according to standardized criteria; however, 2 of the Japanese studies did not specify the diagnostic criteria used.19,21
Data Extraction
Type A Studies.
Table 1 provides an overview. For the purpose of this meta-analysis, we retrieved data to enable us to extract or calculate the RR of schizophrenia for those exposed (1) at any time during pregnancy, (2) in a particular trimester of pregnancy, and (3) in a particular month of pregnancy. In all studies, births were assumed to be full-term (9-month) deliveries. Where possible, the results were considered separately for men and women. Two of the authors (J.-P.S. and A.F.) independently extracted the data and calculated the RRs. Discrepancies were resolved by discussion.
Table 1.
Eight Ecological Studies from Europe, America, and Australia (Type A Studies) of Schizophrenia Risk for Subjects In Utero in the First, Second, or Third Trimester of Prenatal Life During the 1957 Pandemic of A2 Influenza
| First Author and Publication Year | Region | Na | End of Follow-up Period | Timing of Birth of Control Patients | Relative Risk by Trimester of Exposure | ||
| 1st | 2nd | 3rd | |||||
| Mednick, 1988 | Uusimaa County, Finland | 58 | 1984 | 6 previous y | 0.88 | 0.85 | 0.77 |
| Kendell, 1989 | Scotland | 170 | 1988 | 2 previous y | 0.87 | 0.81 | 0.90 |
| O’Callaghan, 1991 | Regions of England and Wales | 263 | 1984 | 2 previous and 2 subsequent y | 0.84 | 1.24 | 1.15 |
| Torrey, 1991 | 10 U.S. states | 3368 | 1987/1988 | 1 previous and 1 subsequent y | 0.95 | 1.00 | 1.07 |
| Erlenmeyer-K, 1994 | Croatia | 82 | 1990 | 2 previous and 2 subsequent y | 0.90 | 0.98 | 1.11 |
| Selten, 1994 | The Netherlands | 654 | 1991 | 2 previous and 2 subsequent y | 0.86 | 0.99 | 1.01 |
| McGrath, 1994 | Queensland, Australia | 156 | 1988 | 5 previous and 5 subsequent yb | 0.57 | 1.27 | 1.21 |
| Morgan, 1997 | Western Australia | 72 | 1996 | 1 previous and 1 subsequent yc | 0.86 | 1.00 | 1.86 |
Number of patients born in period of 9 months after pandemic.
Except 1954, 1957, and 1959.
See Morgan et al18 for details.
We used the natural logarithm of the RR. The variance V of a RR is given by the formula: V = 1/a − 1/b + 1/c − 1/d, where a is the number of preschizophrenic births in the index period, b is the number of live births in the index period, c is the number of pre-schizophrenic births in the control period, and d is the number of live births in the control period. Although 3 studies did not mention the numbers of live births,2,14,15 we estimated these numbers (see below). Because an estimation is always imperfect, we also calculated the variance of each RR using another formula, V = 1/a + 1/c, where a is the number of cases in the index period and c is the number of cases in the control period. This method of variance estimation is not influenced by the size of denominators.24
All analyses were carried out using the fixed-effects model with MetaWin 2.0 statistical software.25 A homogeneity statistic, Q, was calculated to test whether the studies could be considered to share a common population effect size. A significant Q statistic indicates heterogeneity of the individual study effect sizes, which means that a certain amount of variance cannot be attributed to sampling error. If the value of Q was statistically significant, we repeated the analysis using the random-effects model, which is more conservative and takes the extra variance into account.24,26
The arrival of A2 influenza and the peak of the epidemic differed by country. Most studies considered infants born in the first month after the peak as being exposed during the ninth month of pregnancy and classified the other months accordingly. Two studies departed from this rule and considered those born during the peak month of the epidemic as being exposed during the ninth month of pregnancy.14,17 In order to apply the same method to all studies, we reclassified exposure in these 2 studies using the approach used in the other studies. Morgan et al,18 in their study of Western Australia, increased the power of their study to find a second-trimester effect by considering those born in the period from November 1957 until March 1958 as being exposed during this trimester. This was possible because the epidemic in Western Australia lasted from July to September 1957. For the purpose of the present meta-analysis, however, we reanalyzed data from this study as for the other studies. Because the peak occurred in September 1957, people born during the periods October to December 1957, January to March 1958, and April to June 1958 were considered as being exposed during the third, second, and first trimesters, respectively.
While the extraction of RRs was straightforward in some studies, the methods used for 5 studies require further clarification. First, the study from Finland examined hospital admissions of people born in Uusimaa County, a region including Greater Helsinki.1 The authors compared the proportion of schizophrenia diagnoses among psychiatric patients born in the index period with that of schizophrenia diagnoses among patients born in the control periods. Among patients born from February 15 to May 14, 1958 (ie, exposed to the pandemic during the second trimester of fetal life), 34.6% were diagnosed with schizophrenia compared with 20.8% among patients born in the corresponding period in the previous 6 years. The authors controlled for differences between the index and the control groups in the risk period for psychiatric admission by excluding those subjects from the control group who had their first hospital admission at an older age than the maximum age at first admission for patients in the index group (ie, 26 years and 56 days). The authors reported an excess of schizophrenic births among people born in Uusimaa County who were exposed during the second trimester of pregnancy. However, the proportion of schizophrenia diagnoses among hospitalized patients is not a good outcome variable for statistical analysis because it remains unclear whether an increase in the proportion is due to an increase in schizophrenia or a decrease in other diagnoses. A more direct approach involves the calculation of the risk of hospitalization for schizophrenia among subjects born in Uusimaa County. In order to calculate population-based RRs, we obtained information from the Finnish Bureau of Statistics on the number of live births in Uusimaa County for each month during 1951–1958. Because the 3-month periods of fetal exposure began on the 15th day of a given month (eg, February 15, 1958, to May 14, 1958), the number of live births for the research periods was estimated by interpolation. Monthly figures by gender were not available but could be estimated using the proportion of male and female live births in Finland during the period 1950–1960 (51.2% and 48.8%, respectively). The calculation of population-based RRs yielded striking findings (see table 2). To begin with, the risk of hospital admission for schizophrenia was very high (1.9%–2.7%) among people born in Uusimaa County and was higher than the recently estimated lifetime risk among the Finnish population (0.87%).27 Moreover, the RR for people exposed during the second trimester was decreased rather than increased (RR = 0.85, 95% CI: 0.68–1.07). Although the statistical analysis of the Finnish study has been criticized,15 the decreased population-based RR is a novel finding. The recalculated schizophrenia risk among the Uusimaa population and the decreased population-based RR for exposure in the second trimester question the validity of the 1988 publication. For the purpose of the present meta-analysis, we used the population-based RRs given in table 2.
Table 2.
Cases of Schizophrenia That Were or Were Not Exposed in Prenatal Life to the 1957 A2 Influenza Pandemic in Uusimaa County, Finland
| Time of Birth | Trimester of Gestationa | Exposed (Born November 15, 1957, to August 14, 1958) | Not Exposed (Born in Corresponding Periods of 6 Previous Years, 1951–1957) | Relative Risk | 95% Confidence Interval | ||||
| Live Births | Cases of Schizophrenia | Risk Per 1000 | Live Births | Cases of Schizophrenia | Risk Per 1000 | ||||
| November 15 to February 14 | 3rd | 3419 | 65 | 19.0 | 18 898 | 513 | 27.1 | 0.70 | 0.54–0.90 |
| February 15 to May 14 | 2nd | 3536 | 81 | 22.9 | 20 775 | 558 | 26.9 | 0.85 | 0.68–1.07 |
| May 15 to August 14 | 1st | 3288 | 70 | 21.3 | 20 495 | 494 | 24.1 | 0.88 | 0.69–1.13 |
Trimester of gestational exposure for exposed cohorts.
Second, the study from 10 health regions of England and Wales compared the number of patients born in a particular index month with the mean number of patients born in the corresponding month in the 2 previous and 2 subsequent years.2 The authors assumed that there were no major fluctuations in the number of births in the general population. We estimated RRs by using the numbers of patients born in the index and control periods. For example, the RR for those born 5 months after the peak of the epidemic was 48 divided by 25.5 (ie, the mean for the 4 control periods) = 1.88. We used data from another publication to estimate the denominator needed (ie, number of live births) to calculate the variance of the RR. According to a follow-up study of all children born in Great Britain in the week of March 3–9, 1958, the risk of developing schizophrenia was 0.0035.22 Because the data source (hospital admissions recorded by the Mental Health Inquiry) and length of follow-up were similar for both studies, we assumed that the risk was similar among the subjects from the 10 health regions and estimated the numbers of live births and the variance of the RRs.
A third study, the study of the national registry of Scotland, compared the number of preschizophrenic births in an index month with the mean number of such births in the same month in the 2 previous years.15 The authors failed to adjust for the somewhat longer period of risk for subjects born in the control years. However, because data were collected up to 1988, most subjects had passed the period of maximum risk. The number of live births was estimated using data from another publication with the same data set in which the authors reported that the risk of hospitalization for schizophrenia and entry into the national registry was 0.00158 for people born in 1958.28
The fourth and largest study, conducted in the United States, used information from 10 states on the month and year of birth of all individuals diagnosed with schizophrenia who were receiving mental health services.14 The number of patients born from 1950 to 1959 was 43 778, with approximately 3368 patients being born in the 9-month period after the pandemic. Using the monthly numbers of live births in these states, the authors calculated monthly birth rates of future schizophrenic patients during the period 1950–1959, not stratified by gender. Because this study is larger than the other studies combined, and to prevent it from dominating the meta-analysis, we compared the birth rate in an index month with the average birth rate in the same calendar month of only 1 previous year and 1 subsequent year. For instance, the birth rate in November 1957 was 0.00444. Because the mean birth rate of schizophrenic patients in November 1956 and November 1958 was 0.00390, the RR was estimated at 44.4/39.0 = 1.138. The authors provided sufficient information to allow an estimation of denominators.
Lastly, with regard to the Dutch study,13 we obtained information about the number of live births per month from Statistics Netherlands and calculated population-based RRs. The epidemic in the Netherlands peaked between mid-September and mid-October 1957. Subjects born in October 1957 were regarded as being exposed during the ninth month of pregnancy, and subjects born in June 1958 were considered to have been exposed during the first month of pregnancy. The risk of subjects born in index periods was compared with that of subjects born in the corresponding periods in the 2 previous and 2 subsequent years.
Type B Studies.
Japanese researchers, inspired by the findings in England and Wales,2 tested the hypothesis that the risk of schizophrenia would be increased in subjects born 5 months after the epidemic, ie, in November or December 1957 or April or May 1958. Kunugi et al19 compared the number of patients, by gender, who had been born in the 4 above-mentioned index months with the mean number of patients born during the corresponding months of the 2 previous and 2 subsequent years (ie, 16 calendar months). Mino et al21 used a similar method. The only study to provide information on the number of live births was that of Izumoto et al.20 In order to apply the same method to all Japanese studies, we summed the numbers of patients born in these particular index and control months (see table 3). We then compared the number of patients born in the 4 index months with the mean number of patients born in the 16 control months and estimated RRs. The risk of schizophrenia among Japanese men and women born in the control months of the Izumoto et al study was 0.008209 and 0.006298, respectively. Assuming the same risk in the 2 other Japanese studies, we estimated the numbers of births in the control months for the 3 studies combined, by gender. Given the estimated RRs for males and females, the denominators for the index groups could be estimated, by gender.
Table 3.
Three Ecological Studies From Japan (Type B Studies) Showing Cases of Schizophrenia That Were or Were Not Exposed in the Fifth Month of Prenatal Life to the 1957 A2 Influenza Pandemic
| First Author and Publication Year | Region | Year of Follow-Up | Exposeda (N) | Average for Not Exposedb (N) | Exposed Divided by Average for Not Exposed |
| Males | |||||
| Kunugi, 1995 | Greater Tokyo | 1994 | 50 | 52.25 | |
| Izumoto, 1999 | Kochi | 1996 | 19 | 19.50 | |
| Mino, 2000 | Japan | 1993 | 224 | 247.75 | |
| Total | 293 | 319.50 | 0.92 | ||
| Females | |||||
| Kunugi, 1995 | Greater Tokyo | 1994 | 37 | 32.50 | |
| Izumoto, 1999 | Kochi | 1996 | 20 | 14.25 | |
| Mino, 2000 | Japan | 1993 | 142 | 138.00 | |
| Total | 199 | 184.75 | 1.08 | ||
| Both sexes | |||||
| Kunugi, 1995 | Greater Tokyo | 1994 | 87 | 84.75 | |
| Izumoto, 1999 | Kochi | 1996 | 39 | 33.75 | |
| Mino, 2000 | Japan | 1993 | 366 | 385.75 | |
| Total | 492 | 504.25 | 0.98 | ||
Exposed: Born November 1957, December 1957, April 1958, or May 1958.
Not Exposed: Born in same calendar months of 2 previous and 2 subsequent years.
Type C Studies.
There were 2 studies of this type. One study concerned all children born in Great Britain in the week of March 3–9, 1958.22 Influenza during pregnancy was documented by an interview with the mother conducted by the midwife. The children were followed up by a search in the Mental Health Inquiry records.
A second study was a follow-up of individuals who had been identified originally for a prospective investigation of the association between prenatal viral infection and congenital abnormalities.23 The epidemic reached Dublin in October 1957. All women who attended antenatal clinics in the 3 principal maternity hospitals between October 1, 1957, and June 1, 1958, were asked: “Have you had influenza during pregnancy?” Using the published data, we calculated RRs for children born to mothers who reported having had influenza during the pandemic (vs the risk for children whose mothers reported not having had influenza). We also pooled the results of both studies.
Data Synthesis
The American study was larger than all other type A studies combined.14 Consequently, before conducting any meta-analyses, we used meta-analysis of variance to examine whether its effect sizes, with regard to birth in any of the 3 trimesters after the pandemic, differed significantly from those in the other studies.26 The differences were found to be statistically not significant, and we included the American study in the meta-analysis. The latter study did not provide information by gender and was not included in the separate analyses of risks for males and females.
Results
Type A Studies
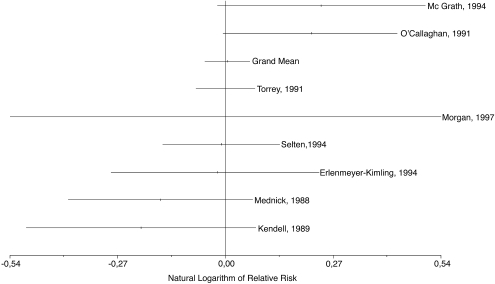
Eight type A studies met our inclusion criteria yielding 8 effect sizes for birth in any of the 3 trimesters (or the whole 9-month period) after the pandemic and 6 effect sizes for birth in any of the 9 months. The mean weighted RR of schizophrenia among subjects exposed during the second trimester or the fifth month of prenatal life, considered risk periods in some studies, was not significantly increased. Figure 1 shows that the distribution of effect sizes across studies was random and that not a single study found a significant second-trimester effect. The mean weighted RR for subjects exposed during the first trimester of prenatal life was significantly decreased, but the other results were not significant (table 4). The results of the separate analyses for males and females also failed to show a significantly increased risk of schizophrenia (table 5). As shown in table 4, the 2 methods to calculate the variance of the effect sizes yielded virtually identical results. (The results obtained with the 2 methods to estimate variance in males and females were also similar and are available from the authors upon request.)
Fig. 1.
Natural Logarithm of Relative Risks (and 95% Confidence Intervals) for Subjects Included in Eight Ecological Studies from Europe, America, and Australia (Type A Studies) of Risk for Schizophrenia Associated with Exposure to 1957 Influenza Pandemic During Second Trimester of Prenatal Life. Studies are identified by first author and year of publication. Figure shows natural logarithm of all effect sizes and natural logarithm of grand mean.
Table 4.
Mean Weighted RRs of Schizophrenia for Subjects Born in 9-Month Period After 1957 Pandemic of A2 Influenza By Gestational Age at Exposure
| Gestational Age at Exposure | Number of Effect Sizes | Fixed-Effects Model | Random-Effects Model | |||||||
| Method of Variance Estimation Aa | Method of Variance Estimation Bb | Method of Variance Estimation Aa | ||||||||
| RR | 95% CI | Q | RR | 95% CI | Q | RR | 95% CI | Q | ||
| Month 1 | 6 | 0.89 | 0.78–1.02 | 0.91 | 0.89 | 0.78–1.02 | 0.91 | |||
| Month 2 | 6 | 0.87 | 0.77–1.00 | 2.69 | 0.88 | 0.79–1.00 | 2.68 | |||
| Month 3 | 6 | 0.98 | 0.86–1.13 | 7.28 | 0.98 | 0.86–1.13 | 7.27 | |||
| Month 4 | 6 | 1.02 | 0.89–1.16 | 4.69 | 1.02 | 0.89–1.16 | 4.68 | |||
| Month 5 | 6 | 1.06 | 0.93–1.20 | 14.44* | 1.06 | 0.93–1.20 | 14.39* | 1.17 | 0.89–1.54 | 4.62 |
| Month 6 | 6 | 0.96 | 0.84–1.10 | 2.97 | 0.96 | 0.84–1.10 | 2.95 | |||
| Month 7 | 6 | 1.03 | 0.90–1.17 | 3.59 | 1.03 | 0.90–1.17 | 3.58 | |||
| Month 8 | 6 | 1.04 | 0.91–1.18 | 2.65 | 1.04 | 0.91–1.18 | 2.64 | |||
| Month 9 | 6 | 1.14 | 1.00–1.29 | 2.32 | 1.14 | 1.00–1.30 | 2.32 | |||
| Trimester 1 | 8 | 0.91 | 0.85–0.98 | 3.93 | 0.91 | 0.85–0.98 | 3.82 | |||
| Trimester 2 | 8 | 1.00 | 0.93–1.07 | 10.93 | 1.00 | 0.94–1.07 | 10.87 | |||
| Trimester 3 | 8 | 1.05 | 0.98–1.12 | 16.85* | 1.05 | 0.98–1.12 | 16.65* | 1.04 | 0.90–1.19 | 10.33 |
| 9-mo period | 8 | 0.99 | 0.96–1.03 | 15.69* | 0.99 | 0.96–1.03 | 15.54* | 0.98 | 0.90–1.05 | 8.69 |
Note: RRs, relative risks; CI, confidence interval.
Formula for variance estimation was 1/a − 1/b + 1/c − 1/d, where a is the number of preschizophrenic patients in index period, b is the number of live births in index period, c is the number of preschizophrenic births in control period, and d is the number of live births in control period.
Formula for variance estimation was 1/a + 1/c.
*Significant value of Q (P < .05).
Table 5.
Mean Weighted RRs of Schizophrenia for Subjects Born in 9-Month Period After 1957 Pandemic of A2 Influenza By Gestational Age at Exposure and Gender
| Gestational Age | Number of Effect Sizes | Fixed-Effects Modela | Random-Effects Modela | ||||
| RR | 95% CI | Q | RR | 95% CI | Q | ||
| Males | |||||||
| Month 1 | 4 | 1.00 | 0.69−1.45 | 0.39 | |||
| Month 2 | 4 | 0.97 | 0.67−1.41 | 2.29 | |||
| Month 3 | 4 | 0.74 | 0.48−1.14 | 2.08 | |||
| Month 4 | 4 | 1.06 | 0.75−1.51 | 7.15 | |||
| Month 5 | 4 | 1.18 | 0.82−1.68 | 4.77 | |||
| Month 6 | 4 | 0.82 | 0.56−1.21 | 1.58 | |||
| Month 7 | 4 | 0.92 | 0.61−1.38 | 1.68 | |||
| Month 8 | 4 | 1.18 | 0.83−1.68 | 2.79 | |||
| Month 9 | 4 | 1.05 | 0.73−1.50 | 2.24 | |||
| Trimester 1 | 6 | 0.88 | 0.75−1.04 | 1.37 | |||
| Trimester 2 | 6 | 0.99 | 0.85−1.16 | 11.60* | 1.00 | 0.75−1.25 | 6.01 |
| Trimester 3 | 6 | 0.99 | 0.84−1.16 | 10.61 | |||
| 9-mo period | 6 | 0.95 | 0.87−1.04 | 11.66* | 0.95 | 0.81−1.11 | 5.52 |
| Females | |||||||
| Month 1 | 4 | 0.55 | 0.29−1.05 | 1.08 | |||
| Month 2 | 4 | 0.84 | 0.49−1.41 | 0.66 | |||
| Month 3 | 4 | 0.91 | 0.53−1.57 | 2.08 | |||
| Month 4 | 4 | 0.84 | 0.50−1.42 | 0.88 | |||
| Month 5 | 4 | 1.50 | 0.95−2.36 | 7.05 | |||
| Month 6 | 4 | 1.22 | 0.75−1.98 | 4.47 | |||
| Month 7 | 4 | 1.01 | 0.61−1.66 | 3.40 | |||
| Month 8 | 4 | 0.93 | 0.56−1.52 | 0.31 | |||
| Month 9 | 4 | 1.30 | 0.79−2.15 | 3.16 | |||
| Trimester 1 | 6 | 0.80 | 0.63−1.00 | 3.16 | |||
| Trimester 2 | 6 | 1.07 | 0.87−1.30 | 9.51 | |||
| Trimester 3 | 6 | 1.04 | 0.85−1.28 | 7.59 | |||
| 9-mo period | 6 | 0.96 | 0.85−1.09 | 3.60 | |||
Note: Abbreviations are explained in the first footnote to table 4.
Formula for variance estimation was 1/a − 1/b + 1/c − 1/d, where a is the number of preschizophrenic patients in index period, b is the number of live births in index period, c is the number of preschizophrenic births in control period, and d is the number of live births in control period.
*Significant value of Q (P < .05).
Of 39 Q values calculated, 4 were statistically significant. For subjects exposed during the fifth month of prenatal life, this heterogeneity was due to a difference between the American study,14 which reported a decreased risk (RR = 0.96), and the other studies, which reported an increased risk. When the analysis was conducted without the American study, the mean weighted RR was not significantly increased (RR = 1.24, 95% CI: 1.00–1.55). The significant heterogeneity in the 3 other meta-analyses was not solely due to the American study.
Type B Studies
The pooled results of the type B studies show a 2.4% decreased risk of schizophrenia among Japanese subjects born 5 months after a wave of the A2 influenza pandemic (table 3). The estimated RR for Japanese men was 0.92 (95% CI: 0.81–1.04) and that for Japanese women was 1.08 (95% CI: 0.92–1.26).
Type C Studies
The results of both studies and the pooled results were negative (table 6).
Table 6.
Studies of Schizophrenia Risk for Children In Utero During 1957 Influenza Pandemic by Maternal Report of Influenza during Pregnancy
| First Author and Publication Year | Influenza Reported by Mother | Influenza Denied by Mother | Relative Risk | 95% Confidence Interval | ||||
| Live Births | Cases of Schizophrenia | Risk Per 1000 | Live Births | Cases of Schizophrenia | Risk Per 1000 | |||
| Crow, 1991 | 1851a | 7b | 3.8 | 14 153 | 50 | 3.5 | 1.07 | 0.49–2.36 |
| Cannon, 1996 | 238c | 2d | 8.4 | 287 | 2 | 7.0 | 1.21 | 0.17–8.50 |
| Total | 2089 | 9 | 4.3 | 14 440 | 52 | 3.6 | 1.20 | 0.59–2.42 |
Mothers exposed in first trimester (n = 231), second trimester (n = 945), and third trimester (n = 675).
Cases exposed in second (n = 3) or third trimester (n = 4).
Mothers exposed in first trimester (n = 22), second trimester (n = 80), third trimester (n = 131), or time of exposure not known (n = 5).
Cases exposed in second (n = 1) or third trimester (n = 1).
Discussion
The purpose of this meta-analysis was to examine whether birth in the 9-month period after the 1957 influenza pandemic, or maternal reports of influenza during pregnancy at the time of the pandemic, was a risk factor for schizophrenia in the child. While many of the investigations included in this meta-analysis were well-designed retrospective cohort studies with sufficient power to detect an effect of the pandemic, we failed to find such an association. This is not due to the exclusion of a data set from Denmark,8 which showed an increased risk among females who were in the sixth or seventh month of prenatal life at the time of the pandemic, months that were not indicated as being high risk by other studies. Nor were the negative results accounted for by the exclusion of studies from Surinam and the Netherlands Antilles9 or Palau10 because these samples were extremely small and the results were considered negative. It could be argued that the diagnoses recorded on psychiatric registries have a poor validity and thus obscured the effect of the pandemic. However, diagnoses on a number of registries have been validated,29,30 and there is ample alternative confirmation of their usefulness for research. Using data from the Dutch registry, eg, Susser et al31 were able to demonstrate that exposure to the Dutch Hunger Winter during the first trimester of prenatal life doubled the risk of schizophrenia. This effect of malnutrition was confirmed by 2 subsequent studies in China.32,33 One could raise the question to what extent the results may have been influenced by publication bias, but in the absence of an association, the issue seems to be less relevant. One could suggest that positive findings have remained unpublished, but this is speculative. Because we performed multiple analyses, the reduced risk of schizophrenia among subjects exposed during the first trimester of prenatal life is probably a chance finding.
Limitations
The underreporting of influenza by mothers who were pregnant during the pandemic is a likely limitation of the type C studies. However, even if one assumes that all mothers who failed to report influenza had a subclinical infection, the risk of schizophrenia among exposed cohorts was well within the expected range. Because the length of the follow-up periods was limited, we cannot exclude, on the basis of this meta-analysis, the possibility that maternal influenza may be a risk factor for late-onset schizophrenia.
Origins of Influenza Hypothesis
The original report on which the influenza hypothesis of schizophrenia was based had a small sample size and used an inappropriate statistical method.1 Our reanalysis of the Finnish data showed that the risk of schizophrenia was not increased among subjects exposed to influenza during the second trimester. The increased risk among subjects born in England and Wales 5 months after the peak of the pandemic may be due to chance.2 It is curious that, although the epidemic lasted several months, the plot of schizophrenic births showed a spike that was limited to 1 month.34
Studies Not Related to 1957 Pandemic
Studies from Scotland and Denmark found no evidence that subjects born shortly after the pandemic of 1918–1919 were at increased risk of subsequently developing schizophrenia.8,15 Multiple-year studies of the relationship between monthly variations in the prevalence of influenza and the risk of schizophrenia among subjects who were in utero at the time of the influenza outbreak have yielded inconsistent findings. A study of first admissions to hospitals in England and Wales before 1980 found an association between mortality from influenza during the third to the seventh month of prenatal life and risk of schizophrenia in adulthood.35 However, the association was modest and explained 1%–2% of the variance in the risk of schizophrenia. A second study, of first admissions after 1980, failed to replicate the previous finding. The authors reported a 14% increase in the risk of schizophrenia among females born 5 months after influenza epidemics and no such increased risk among males.36 In the Netherlands, the risk of schizophrenia was found to be associated with mortality from influenza 3 months prior to birth, but this association was present in typical schizophrenics but not in less typical cases.37 A study from Western Australia found a second-trimester effect among females exposed to the 1951 influenza epidemic but no such effect among males or females exposed to epidemics occurring in 1950, 1953, 1954, or 1959.18 Finally, 4 studies examined the relationship between the prevalence of influenza and the risk of schizophrenia in Denmark.8,38–40 Each study used a different method and covered a different birth period (1911–1950, 1911–1965, 1915–1970, and 1950–1984, respectively). Barr et al38 reported an association between schizophrenia risk and relatively high levels of influenza exposure for the season during the sixth month of gestation but did not mention a RR. A larger study by Adams et al,8 which included the birth cohort studied by Barr et al, found no such association. Takei et al39 found an increased risk for subjects exposed 4 months prior to birth (RR = 1.12, 95% CI: 1.00–1.21), but Westergaard et al40 could not replicate the findings of Takei et al even when they employed their methods and concluded that there was no association between schizophrenia risk and influenza prevalence during any month of prenatal life.
Conclusions
In order to appreciate the implications of this meta-analysis for the influenza hypothesis, it is important to be aware of the impact of the pandemic. A serological study from Sheffield, United Kingdom, found that 43% of subjects aged 20–39 years had A2 antibodies immediately after the pandemic.41 Recent publications on influenza pandemics assume that about 50% of the population is infected.5,6 If maternal influenza contributes to the etiology of schizophrenia, it is difficult to understand why a pandemic infecting such a high proportion of pregnant women failed to increase the risk among their children and why there is no consistent relationship between influenza epidemics during a particular period of prenatal life and the risk of schizophrenia.
We conclude that the evidence to support the maternal influenza hypothesis is insufficient. One could argue that there is a body of literature from animal studies, indicating that prenatal exposure to influenza is associated with certain changes in brain development and behavior,42 but this does not constitute proof that this exposure is a cause of schizophrenia in humans. According to the fetal brain cytokine imbalance hypothesis, the maternal induction of proinflammatory cytokines due to a wide variety of bacterial or viral pathogens may influence the development of the fetal brain and increase the risk for schizophrenia.43 The results of the present study do not support this hypothesis.
Acknowledgments
The authors thank Jane Sykes for her help with the preparation of the manuscript.
References
- 1.Mednick SA, Machón RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1988;45:189–192. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- 2.O’Callaghan E, Sham P, Takei N, Glover G, Murray RM. Schizophrenia after prenatal exposure to 1957 A2 influenza epidemic. Lancet. 1991;337:1248–1250. doi: 10.1016/0140-6736(91)92919-s. [DOI] [PubMed] [Google Scholar]
- 3.Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull. 2006;32:200–202. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown AS, Begg MD, Gravenstein S, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson NM, Cummings DAT, Cauchemez S, et al. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature. 2005;437:209–214. doi: 10.1038/nature04017. [DOI] [PubMed] [Google Scholar]
- 6.Glass RJ, Glass LM, Beyeler WE, Min HJ. Targeted social distancing design for pandemic influenza. Emerg Infect Dis. 2006;12:1671–1681. doi: 10.3201/eid1211.060255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2:696–701. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams W, Kendell RE, Hare EH, Munk-Jørgensen P. Epidemiological evidence that maternal influenza contributes to the aetiology of schizophrenia. Br J Psychiatry. 1993;163:522–534. doi: 10.1192/bjp.163.4.522. [DOI] [PubMed] [Google Scholar]
- 9.Selten JP, Slaets JP, Kahn RS. Prenatal exposure to influenza and schizophrenia in Surinamese and Dutch Antillean immigrants to The Netherlands. Schizophr Res. 1998;30:101–103. doi: 10.1016/s0920-9964(97)00105-9. [DOI] [PubMed] [Google Scholar]
- 10.Allen JS, Nero KL. Schizophrenia and influenza in Palau. Med J Aust. 1998;168:421–422. doi: 10.5694/j.1326-5377.1998.tb139013.x. [DOI] [PubMed] [Google Scholar]
- 11.Susser ES, Lin SP, Brown AS, Lumey LH, Erlenmeyer-Kimling L. No relation between risk of schizophrenia and prenatal exposure to influenza in Holland. Am J Psychiatry. 1994;151:922–924. doi: 10.1176/ajp.151.6.922. [DOI] [PubMed] [Google Scholar]
- 12.Selten JP, Brown AS, Moons KG, Slaets JP, Susser ES, Kahn RS. Prenatal exposure to the 1957 influenza pandemic and non-affective psychosis in The Netherlands. Schizophr Res. 1999;38:85–91. doi: 10.1016/s0920-9964(99)00005-5. [DOI] [PubMed] [Google Scholar]
- 13.Selten JP, Slaets JP. Evidence against maternal influenza as a risk factor for schizophrenia. Br J Psychiatry. 1994;164:674–676. doi: 10.1192/bjp.164.5.674. [DOI] [PubMed] [Google Scholar]
- 14.Torrey EF, Bowler AE, Rawlings R. An influenza epidemic and the seasonality of schizophrenic births. In: Kurstak E, editor. Second World Congress on Viruses and Mental Health. New York, NY: Plenum; 1991. pp. 109–116. [Google Scholar]
- 15.Kendell RE, Kemp IW. Maternal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 1989;46:878–882. doi: 10.1001/archpsyc.1989.01810100020004. [DOI] [PubMed] [Google Scholar]
- 16.Erlenmeyer-Kimling L, Folnegović Z, Hrabak-Zerjavić V, Borcić B, Folnegović-Smalc V, Susser ES. Schizophrenia and prenatal exposure to the 1957 A2 influenza epidemic in Croatia. Am J Psychiatry. 1994;151:1496–1498. doi: 10.1176/ajp.151.10.1496. [DOI] [PubMed] [Google Scholar]
- 17.McGrath JJ, Pemberton MR, Welham JL, Murray RM. Schizophrenia and the influenza epidemics of 1954, 1957 and 1959: a southern hemisphere study. Schizophr Res. 1994;14:1–8. doi: 10.1016/0920-9964(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 18.Morgan V, Castle D, Page A, et al. Influenza epidemics and incidence of schizophrenia, affective disorders and mental retardation in Western Australia: no evidence of a major effect. Schizophr Res. 1997;26:25–39. doi: 10.1016/S0920-9964(97)00033-9. [DOI] [PubMed] [Google Scholar]
- 19.Kunugi H, Nanko S, Takei N, Saito K, Hayashi N, Kazamatsuri H. Schizophrenia following in utero exposure to the 1957 influenza epidemics is Japan. Am J Psychiatry. 1995;152:450–452. doi: 10.1176/ajp.152.3.450. [DOI] [PubMed] [Google Scholar]
- 20.Izumoto Y, Inoue S, Yasuda N. Schizophrenia and the influenza epidemics of 1957 in Japan. Biol Psychiatry. 1999;46:119–124. doi: 10.1016/s0006-3223(98)00359-x. [DOI] [PubMed] [Google Scholar]
- 21.Mino Y, Oshima I, Tsuda T, Okagami K. No relationship between schizophrenic birth and influenza epidemics in Japan. J Psychiatr Res. 2000;34:133–138. doi: 10.1016/s0022-3956(00)00003-0. [DOI] [PubMed] [Google Scholar]
- 22.Crow TJ, Done DJ, Johnstone EC. Schizophrenia and influenza. Lancet. 1991;338:116–118. [Google Scholar]
- 23.Cannon M, Cotter D, Coffey VP, et al. Prenatal exposure to the 1957 influenza epidemic and adult schizophrenia: a follow-up study. Br J Psychiatry. 1996;168:368–371. doi: 10.1192/bjp.168.3.368. [DOI] [PubMed] [Google Scholar]
- 24.Lipsey MW, Wilson DB. Practical Meta-analysis. London, UK: Sage; 2001. [Google Scholar]
- 25.Rosenberg MS, Adams DC, Gurevitch J. MetaWin: Statistical Software for Meta-analysis(version 2.0) Sunderland, MA: Sinauer; 2000. [Google Scholar]
- 26.Cooper H, Hedges LV. The Handbook of Research Synthesis. New York, NY: Russell Sage Foundation; 1994. [Google Scholar]
- 27.Perala J, Suvisaari J, Saarni SI, et al. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry. 2007;64:19–28. doi: 10.1001/archpsyc.64.1.19. [DOI] [PubMed] [Google Scholar]
- 28.Adams W, Kendell RE. Annual variation in birth rate of people who subsequently develop schizophrenia. Br J Psychiatry. 1999;175:522–527. doi: 10.1192/bjp.175.6.522. [DOI] [PubMed] [Google Scholar]
- 29.Jablensky AV, Morgan V, Zubrick SR, Bower C, Yellachich LA. Pregnancy, delivery and neonatal complications in a population cohort of women with schizophrenia and major affective disorders. Am J Psychiatry. 2005;162:79–91. doi: 10.1176/appi.ajp.162.1.79. [DOI] [PubMed] [Google Scholar]
- 30.Hulshoff Pol HE, Hoek HW, Susser ES, et al. Prenatal exposure to famine and brain morphology in schizophrenia. Am J Psychiatry. 2000;157:1170–1172. doi: 10.1176/appi.ajp.157.7.1170. [DOI] [PubMed] [Google Scholar]
- 31.Susser ES, Neugebauer R, Hoek HW, et al. Schizophrenia after prenatal famine: further evidence. Arch Gen Psychiatry. 1996;53:25–31. doi: 10.1001/archpsyc.1996.01830010027005. [DOI] [PubMed] [Google Scholar]
- 32.St Clair D, Xu M, Wang P, et al. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959-1961. JAMA. 2005;294:557–562. doi: 10.1001/jama.294.5.557. [DOI] [PubMed] [Google Scholar]
- 33.Xu MQ, Sun WS, Liu BX, et al. Prenatal malnutrition and adult schizophrenia: further evidence form the 1959-1961 Chinese famine. Schizophr Bull. 2009;35:568–576. doi: 10.1093/schbul/sbn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crow TJ, Done DJ. Prenatal exposure to schizophrenia does not cause schizophrenia. Br J Psychiatry. 1992;161:390–393. doi: 10.1192/bjp.161.3.390. [DOI] [PubMed] [Google Scholar]
- 35.Sham PC, O’Callaghan E, Takei N, Murray GK, Hare EH, Murray RM. Schizophrenia following prenatal exposure to influenza epidemics between 1939 and 1960. Br J Psychiatry. 1992;160:461–466. doi: 10.1192/bjp.160.4.461. [DOI] [PubMed] [Google Scholar]
- 36.Takei N, Sham PC, O’Callaghan E, Murray GK, Glover G, Murray RM. Prenatal exposure to influenza and the development of schizophrenia: is the effect confined to females? Am J Psychiatry. 1994;151:117–119. doi: 10.1176/ajp.151.1.117. [DOI] [PubMed] [Google Scholar]
- 37.Takei N, van Os J, Murray RM. Maternal exposure to influenza and risk of schizophrenia: a 22-year study from the Netherlands. J Psychiatr Res. 1995;29:435–445. doi: 10.1016/0022-3956(95)00031-3. [DOI] [PubMed] [Google Scholar]
- 38.Barr CE, Mednick SA, Munk-Jorgensen P. Exposure to influenza epidemics during gestation and adult schizophrenia. Arch Gen Psychiatry. 1990;47:869–874. doi: 10.1001/archpsyc.1990.01810210077012. [DOI] [PubMed] [Google Scholar]
- 39.Takei N, Mortensen PB, Klaening U, et al. Relationship between in utero exposure to influenza epidemics and risk of schizophrenia. Biol Psychiatry. 1996;152:450–452. doi: 10.1016/0006-3223(95)00592-7. [DOI] [PubMed] [Google Scholar]
- 40.Westergaard T, Mortensen PB, Pedersen CB, Wohlfahrt J, Melbye M. Exposure to prenatal and childhood infections and the risk of schizophrenia. Arch Gen Psychiatry. 1999;56:993–998. doi: 10.1001/archpsyc.56.11.993. [DOI] [PubMed] [Google Scholar]
- 41.Clarke SK, Heath RB, Sutton RN, Stuart-Harris CH. Serological studies with Asian strain of influenza A. Lancet. 1958;1:814–818. doi: 10.1016/s0140-6736(58)91739-2. [DOI] [PubMed] [Google Scholar]
- 42.Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 2009;35:528–548. doi: 10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer U, Feldon J, Yee BK. A review of the fetal brain cytokine imbalance hypothesis of schizophrenia. Schizophr Bull. 2009;35:959–972. doi: 10.1093/schbul/sbn022. [DOI] [PMC free article] [PubMed] [Google Scholar]