Background
Sulpiride may be used in combination with other antipsychotic drugs in the hope of augmenting effectiveness—especially for those whose schizophrenia has proved resistant to treatment.
Objective
To evaluate the effects of Sulpiride augmentation vs monotherapy for people with schizophrenia.
Search Strategy
We searched the Cochrane Schizophrenia Groups Trials Register (July 2009).
Selection Criteria
All relevant randomized clinical trials (RCTs).
Data Collection and Analysis
We extracted data independently. For dichotomous data, we calculated relative risks (RRs) and their 95% confidence intervals (CIs) based on a fixed-effect model. For continuous data, we calculated weighted mean differences again based on a fixed-effect model.
Main Results
We included 3 short-term and 1 long-term trial (total N = 221). All participants had schizophrenia that was either treatment resistant or with prominent negative symptoms. All studies compared Sulpiride plus clozapine with clozapine (± placebo), were small, and were at considerable risk of bias.
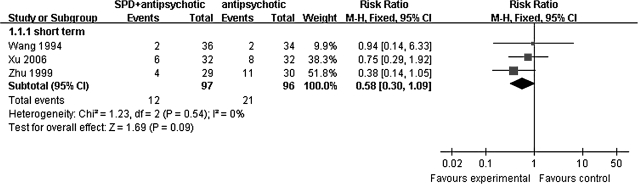
Short-term data of “no clinically significant response” in global state tended to favor Sulpiride augmentation of clozapine compared with clozapine alone (n = 193, 3 RCTs, RR 0.58 [95% CI, 0.3–1.09]; figure 1).
Fig. 1.
Sulpiride (SPD) + Antipsychotic (clozapine) vs Antipsychotic (clozapine). Outcome: no clinically significant response—short term.
People allocated to Sulpiride plus clozapine had more movement disorders (n = 70, 1 RCT, RR 48.24 [95% CI, 3.05–762.56]) and an increase in serum prolactin (skewed data, 1 RCT), but less incidence of hypersalivation (n = 162, 3 RCTs, RR 0.49 [95% CI, 0.29–0.83]) and less weight gain (n = 64, 1 RCT, RR 0.30 [95% CI, 0.09–0.99]). The augmentation of clozapine by Sulpiride also caused less appetite loss (n = 70, 1 RCT, RR 0.09 [95% CI, 0.01–0.70], number needed to treat [NNT] 4 [95% CI, 4–12], Z = 2.31, P = .02) and less abdominal distension (n = 70, 1 RCT, RR 0.10 [95% CI, 0.01–0.78], NNT 5 [95% CI, 4–19], Z = 2.20, P = .03).
Long-term data showed no significant difference in global state (n = 70, 1 RCT, RR 0.67 [95% CI, 0.42–1.08]) and relapse (n = 70, 1 RCT, RR 0.85 [95% CI, 0.5–1.3]).
Reviewers’ Conclusions
Sulpiride plus clozapine probably is more effective than clozapine alone in producing clinical improvement in some treatment-resistant schizophrenics. However, the evidence is still weak and prone to various biases. More robust data are needed.
Implications for Practice
For people with schizophrenia, if the response is not ideal to clozapine, in particular, there are some data to support use of Sulpiride as an augmentation treatment. The data, however, are weak. The profile of adverse effects after the addition of Sulpiride to clozapine is complicated. It might evoke aggravation of movement disorders and increase the serum prolactin level but improvement of some side effects often seen with clozapine medication may also occur—such as hypersalivation and weight gain.
Implications for Research
All included trials are small and of poor quality. The findings of this review are potently of important clinical relevance and indicate the need for both more studies investigating the exact mechanisms of Sulpiride augmentation and high-grade real-world clinical trials. This systematic review is fully reported elsewhere.1
Funding
National Natural Science Foundations of China (30770773); National Scientific and Technological 863 Program of China (2008AA02Z412, 2007AA02Z420); National Basic Research Program of China (973 Program, 2007CB512306).
Disclosures
None relevant.
Acknowledgments
We would like to thank the Cochrane Schizophrenia Group for their help.
References
- 1.Wang J, Omori IM, Fenton M, Soares B. Sulpiride augmentation for schizophrenia. Cochrane Database Syst Rev. 2009;(4) doi: 10.1002/14651858.CD008125.pub2. Art. No.: CD008125. doi: 10.1002/14651858.CD008125. [DOI] [PubMed] [Google Scholar]