Abstract
Background
Tumor-derived cell lines are widely used to study the mechanisms involved in thyroid carcinogenesis but recent studies have reported redundancy among thyroid cancer cell lines and identification of some “thyroid cell lines” that are likely not of thyroid origin.
Summary
In this review, we have summarized the uses, the limitations, and the existing problems associated with the available follicular cell-derived thyroid cancer cell lines. There are some limitations to the use of cell lines as a model to “mimic” in vivo tumors. Based on the gene expression profiles of thyroid cell lines originating from tumors of different types it has become apparent that some of the cell lines are closely related to each other and to those of undifferentiated carcinomas. Further, many cell lines have lost the expression of thyroid-specific genes and have altered karyotypes, while they exhibit activation of several oncogenes (BRAF, v-raf murine sarcoma viral oncogene homolog B1; RAS, rat sarcoma; and RET/PTC, rearranged in transformation/papillary thyroid carcinoma) and inactivation of tumor suppressor gene (TP53) which is known to be important for thyroid tumorigenesis.
Conclusions
A careful selection of thyroid cancer cell lines that reflect the major characteristics of a particular type of thyroid cancer being investigated could be used as a good model system to analyze the signaling pathways that may be important in thyroid carcinogenesis. Further, the review of literature also suggests that some of the limitations can be overcome by using multiple cell lines derived from the same type of tumor.
Introduction
Thyroid carcinoma is the most frequently occurring endocrine cancer and is one of the most rapidly increasing human cancers in the United States and other countries (1,2). A majority of patients with thyroid cancer who undergo appropriate treatment have an excellent outcome. However, in about 10% of patients with well-differentiated thyroid cancer (papillary thyroid cancer [PTC] and follicular thyroid cancer [FTC]), the tumor loses its ability to take up radioiodine, or becomes poorly differentiated or dedifferentiated, leading to recurrent disease and death. Further, patients with anaplastic thyroid cancer (ATC) have a very poor prognosis, with a mean survival time of less than 6 months from the time of diagnosis—an outcome that may not be significantly altered by current treatment regimens (3). Therefore, there is a compelling need for better understanding the thyroid tumorigenesis and for improving the treatment of these cases.
Molecular analysis has identified specific genetic alterations in different types of thyroid tumors that are associated with distinct differences in their phenotypes and biological properties. On the other hand, conventional techniques such as cytogenetic analysis of thyroid tumors have helped define the chromosomal translocations and their contribution to the observed abnormal activation in key receptor tyrosine kinases. Similarly, the assessment of ploidy has enabled more definitive determination of the stage of tumor progression. Molecular technologies such as microarrays, serial analysis of gene expression, and proteomics have shown promising results that may allow a more definitive diagnosis and proper molecular classification of thyroid cancers. In this regard, microarrays have been successfully used to identify prognostic markers that offer the possibility of tailoring better treatment regimens for thyroid cancer (4–9). Because of the heterogeneous nature of thyroid cancers it is imperative that appropriate experimental systems be used to delineate the underlying molecular mechanisms of tumorigenesis.
The traditional animal models serve to understand the underlying mechanisms of tumorigenesis and metastasis, whereas the widely used in vitro cell models aid in the dissection of molecular changes in key signaling pathways. The cellular models most frequently involve use of various thyroid tumor cell lines, although sometimes primary tumor cell cultures are used.
In this review, we have attempted to link some of the key findings made in the primary tumors to the corresponding cell lines. As cancer cell lines are routinely used in the identification of novel drug targets and to test the efficacy of a variety of treatments, we have summarized the uses, the limitations, and the existing problems associated with the available follicular cell-derived thyroid tumor cell lines (Table 1).
Table 1.
Thyroid Cancer Cell Lines and Their Reported Origins
| Cell line | Sex | Lesion | References |
|---|---|---|---|
| BCPAP | Female | PTC | Fabien et al. (20) |
| KTC-1 | Male | PTC | Kurebayashi et al. (62) |
| K1 | Male | PTC | Challeton et al. (63) |
| TPC1 | Female | PTC | Ishizaka et al. (64) |
| TT2609-CO2 | Male | FTC | Geldof et al. (65) |
| FTC133 | Male | FTC | Goretzki et al. (66) |
| ML1 | Female | FTC | Schönberger et al. (67) |
| WRO82-1 | Female | FTC | Estour et al. (21) |
| 8505C | Female | ATC | Ito et al. (68) |
| SW1736 | ATC | Xu et al. (34) | |
| Cal-62 | Female | ATC | Gioanni et al. (69) |
| T235 | Female | ATC | Rodrigues et al. (70) |
| T238 | Female | ATC | Rodrigues et al. (70) |
| Uhth-104 | Female | ATC | Lee et al. (33) |
| ACT-1 | ATC | Chung et al. (71) | |
| HTh74 | Female | ATC | Heldin et al. (72) |
| KAT18 | ATC | Ain et al. (73) | |
| TTA1 | ATC | Yano et al. (74) | |
| FRO81-2 | ATC | Nishihara et al. (75) | |
| HTh7 | ATC | Carlsson et al. (76) | |
| C643 | Male | ATC | Gustavsson et al. (77) |
| BHT101 | Female | ATC | Pályi et al. (78) |
| KTC-2 | Female | ATC | Kurebayashi et al. (79) |
PTC, papillary thyroid cancer; ATC, anaplastic thyroid cancer; FTC, follicular thyroid cancer.
Thyrotropin Signaling and Other Proliferative Pathways in Thyroid Gland
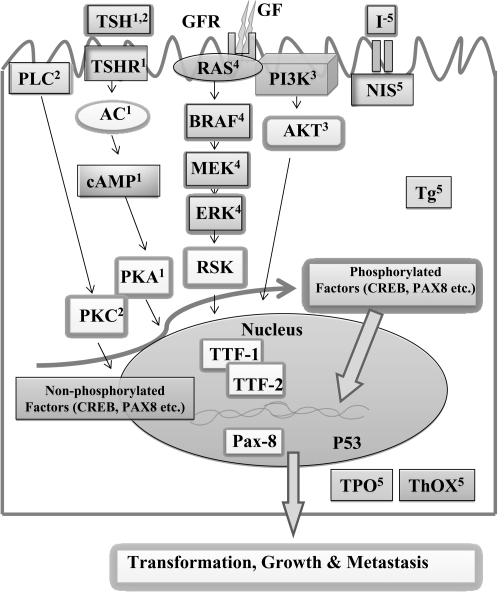
Figure 1 shows the established signaling network in the thyrocyte between thyrotropin (TSH)/protein kinase A and other proliferative pathways such as phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK). These signaling interactions certainly play critical roles in the onset and/or evolution of thyroid cancer. Therefore, studying molecular mechanisms that regulate these signaling pathways are important. Similarly, identification of various mutations that are often seen in thyroid cancers and understanding their effects on survival, proliferation, and apoptotic pathways are also of considerable interest.
FIG. 1.
Reported Mutations in Key Oncogenic Molecules in Thyroid Cancer Cell Lines
As shown in Figure 1, TSH is an important physiological regulator of growth and function in the thyroid gland. The TSH binds to its cognate heterotrimeric G protein-coupled receptor, TSH receptor (TSH-R), and causes dissociation of the G protein into α and βγ subunits. In turn, Gαs subunit stimulates the adenylate cyclase with subsequent increase in the levels of cyclic adenosine monophosphate (cAMP). The TSH can also stimulate the β isoform of phospholipase C, by coupling the receptor to members of the Gq/11 family of G proteins. The phospholipase C catalyzes the formation of the second messenger 1,2-diacylglycerol which activates protein kinase C. The participation of other factors such as insulin/insulin growth factor 1, basic fibroblast growth factor, or epidermal growth factor is required for TSH to display its full mitogenic activity through the activation of PI3K. Activation of PI3K can result in the activation of pyruvate dehydrogenase kinase, isozyme 1, leading to the phosphorylation of protein kinase B (Akt/PKB), which in turn can activate a number of growth-promoting genes. The Ras proteins (H-Ras, v-Ha-ras Harvey rat sarcoma viral oncogene homolog; N-Ras, neurobalstoma RAS viral (v-ras) oncogene homolog; and K-Ras, v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog) are membrane-bound guanine nucleotide-binding proteins and they transduce signals from cell membrane to nucleus, displaying a central role in the control of cell growth and differentiation. Ras, in its active guanosine triphosphate (GTP)-bound form, can interact with multiple downstream effectors including PI3K. The catalytic subunit (p110) of PI3K is recruited by Ras in a guanosine triphosphate (GTP)-dependent manner followed by the recruitment of the Raf family serine/threonine kinase which leads to the activation of a kinase cascade consisting of MAPK kinase (MEK-1 and MEK-2), which in turn activates p42 and p44 MAPK/extracellular signal-regulated kinases. Activated MAPKs translocate to the nucleus where they phosphorylate many substrates, including transcription factors (cAMP response element binding [CREB], thyroid transcription factors 1 and 2 [TTF-1 and TTF-2] and paired box 8 [PAX8]), resulting in gene induction. The function of the thyroid is to produce the quantity of thyroid hormone required to meet the demands of the peripheral tissues. This requires the daily uptake of sufficient iodide (I−) which is facilitated by the sodium-iodide symporter (NIS). Specific tyrosine residues of the thyroglobulin (Tg), a glycoprotein homodimer, are then iodinated at the apical margin of the thyroid cell to form diiodotyrosine and monotyrosine. This requires the formation of H2O2 by Duox1 and 2, also termed thyroid oxidase 1 (THOX1) and thyroid oxidase 2 (THOX2), and thyroid peroxidase (TPO), which catalyzes the oxidation of iodide and its transfer to tyrosine.
Molecular Genetics, TTF Expression, and Chromosomal Abnormalities in Thyroid Cancer and Related Cell Lines
Papillary thyroid cancer
Recently, both redundancy among thyroid cancer cell lines with different names and misidentification of cell lines as thyroid cancer cells that are not of thyroid origin have been reported (10–12). For example, PTC-derived BHP2-7, BHP10-3, BHP7-13, FB2, and TPC1 cell lines have been shown to be genetically identical to each other as well as K1 and K2 (10,11). Similarly, BHP5-16, BHP17-10, BHP14-9, and NPA87 cell lines have been found to be genetically identical to each other and to the MDA-MB-435S/M14 melanoma cell line (11). In contrast, BCPAP, TPC1, KTC-1, and K1 cell lines have been confirmed to be thyroid cancer cell lines that represent PTC (10,11).
The past decade has witnessed significant expansion in the understanding of the molecular basis of thyroid carcinogenesis. In particular, it has become apparent that PTCs frequently have genetic alterations leading to activation of the MAPK signaling pathway. These include rearranged in transformation (RET)/PTC rearrangement, and point mutations of the BRAF and RAS genes.
RET/PTC rearrangement is found on average in ∼20% of sporadic papillary carcinomas in adult patients. This rearrangement is even more common in tumors from patients with a history of radiation exposure (50–80%) and in papillary carcinomas from children and young adults (40–70%) (13). The RET/PTC rearrangement results from a fusion between the 3′-portion of the RET and the 5′-portion of various genes. The two most common rearrangements are RET/PTC1 and RET/PTC3. The RET/PTC1 has been described only in TPC1 and in its genetically identical BHP2-7, BHP10-3, BHP7-13, and FB2 cell lines (10,11,14,15), while BCPAP, K1, and KTC-1 cells have been shown to harbor the wild-type receptor (10,11,15).
Mutation of the BRAF gene is the most common known genetic alteration in papillary carcinomas and is found in ∼45% of these tumors. Virtually all mutations involve nucleotide 1799 that results in a valine-to-glutamate substitution at residue 600 (V600E, formerly V599E) (13). A V600E BRAF mutation has been reported in the BCPAP (homozygous as well as heterozygous) (10,11,14), KTC-1 (heterozygous) (15), and K1 (heterozygous) (10,11) cell lines. The V600E BRAF mutation in thyroid cancer cell lines has been shown to lead also to phosphorylation and activation of MAPK and its downstream effector, MEK (15).
The direct upstream activator of both BRAF and PI3K is a small G protein Ras. There are three types of Ras genes, namely H-Ras, N-Ras, and K-Ras. Point mutations involving several specific sites (codons 12, 13, and 61) of these three genes have been found in 10–20% of papillary carcinomas (13). Only a silent polymorphism of H-Ras (His27His) has been reported in TPC1 and K1 cell lines (10), whereas no mutations or polymorphisms have been described for N-Ras and K-Ras. These findings may limit the utility of these cell lines for studying Ras-mediated signaling pathways.
Besides the MAPK signaling pathway, the PI3K/Akt pathway plays an important role in the regulation of cell growth, proliferation, and survival (Fig. 1). Mutations typically involve various nucleotides in exons 20 and 9 of the PIK3CA gene that encodes the catalytic subunit of the lipid kinase. They are relatively common in follicular and anaplastic thyroid carcinomas but uncommon in PTCs (13). Only a missense mutation (Glu542Lys) in PIK3CA has been reported in K1 cells and in its genetically identical K2 cell line (10), thereby suggesting that further investigations are necessary to identify a suitable cell model to study the relevance of this signaling pathway in thyroid cancer.
The catenin gene (CTNNB1) that plays an essential role in Wnt signaling pathway is known to be unaffected in well-differentiated thyroid carcinomas—an observation that is in agreement with the findings in tumor specimens and PTC-derived cell lines (TPC1, BCPAP, and K1) (10).
Among the other genetic alterations involved in thyroid tumorigenesis, inactivating point mutations of the TP53 tumor suppressor gene, which encodes p53 protein, represent a late event and to date it has been identified in only few cases of PTC. A missense mutation in exon 7 (Asp259Tyr) in BCPAP cells and a silent polymorphism in exon 6 (Arg213Arg) in K1 cells have been reported (10).
Some of the above mutations can be orchestrated in the above cell models and the effects of a particular mutation in thyroid tumorigenesis can be investigated.
Expression analysis of differentiation markers such as TSH-R, TPO, PAX8, TTF-1 and TTF-2, and Tg is routinely used to ensure thyroid origin of cells. These markers also help in evaluating the differentiation status of thyroid cancers and cell lines (16–19) (Fig. 1 and Table 3).
Table 3.
The Observed Status of Various Thyroid Markers in Thyroid Cancer Cell Lines
| Cell line | Tumor | TSH-R | TPO | Tg | TTF-1 | PAX8 | NIS | THOX1 | THOX2 | TTF-2 |
|---|---|---|---|---|---|---|---|---|---|---|
| BCPAP | PTC | −(10,12)a | +(10,12) | −(10,12) | +(10,11,12) | +(10,11,12) | −(10,12) | −(10,12) | −(10,12) | −(12) |
| KTC-1 | PTC | −(62) | −(62) | +(62) | +(11,62) | +(11,62) | −(62) | |||
| K1 | PTC | −(10) | −(10) | −(10) | −(10,11) | +(10,11) | ||||
| TPC1 | PTC | −(10,12) | −(10,12) | −(10,12) | −(10,11)/+(12) | +(10,11,12) | −(12) | −(12) | −(12) | −(12) |
| TT2609-CO2 | FTC | +(11) | −(11) | |||||||
| FTC133 | FTC | −(12) | −(12) | −(12) | +(11,12) | +(11,12) | −(12) | −(12) | −(12) | −(12) |
| ML1 | FTC | +(11) | +(11) | |||||||
| WRO82-1 | FTC | −(12) | −(12) | −(12) | +(12)/−(11) | +(11,12) | −(12) | −(12) | −(12) | +(12) |
| 8505C | ATC | −(10) | −(10) | −(10) | +(10,11,12) | +(10)/−(11,12) | −(12) | −(12) | −(12) | −(12) |
| SW1736 | ATC | +(11b)(11c) | +(11) | |||||||
| Cal-62 | ATC | −(11) | +(11) | |||||||
| T235 | ATC | −(11) | +(11) | |||||||
| T238 | ATC | −(11) | +(11) | |||||||
| Uhth-104 | ATC | +(11) | +(11) | |||||||
| ACT-1 | ATC | +(11) | +(11) | |||||||
| HTh74 | ATC | −(10) | −(10) | −(10) | +(10,11b)/−(11c) | +(10)/−(11) | ||||
| KAT18 | ATC | −(11) | +(11b)/−(11c) | |||||||
| TTA1 | ATC | −(11) | −(11) | |||||||
| FRO81-2 | ATC | −(11) | −(11) | |||||||
| HTh7 | ATC | −(11) | −(11) | |||||||
| C643 | ATC | −(10) | −(10) | −(10) | +(10)/−(11) | +(10)/−(11) | ||||
| BHT101 | ATC | −(11) | −(11) | |||||||
| KTC-2 | ATC | −(11) | −(11) |
The numbers within parentheses are references.
Observation made in Fagin's lab, reported by Schweppe et al. (11).
Observation made in Haugen's lab, reported by Schweppe et al. (11).
TSH-R, thyrotropin receptor; TPO, thyroid peroxidase; Tg, thyroglobulin; TTF, thyroid transcription factor; PAX8, paired box 8; NIS, sodium-iodide symporter; THOX, thyroid oxidase.
Haugen and coworkers observed decreased levels of PAX8 and TTF-1 mRNA only in a subset of the papillary tumor tissue samples compared with their normal counterparts, indicating that these transcription factors may not be necessarily downregulated in all tumors (11). PAX8 mRNA levels in the PTC-derived cell lines are similar to those observed in the PTCs. In contrast, TTF-1 expression is lower in all of the cell lines compared with normal and PTC tissues, suggesting an accelerated dedifferentiation process underway in these cells in culture.
Maenhaut and coworkers have also determined the expression levels of other thyroid-specific genes such as ThOX2 (12). Although they failed to detect ThOX2 expression in the eight investigated cell lines, they found weak expression of a related gene ThOX1 in BCPAP cells. Among TTFs, PAX8 was found in FTC133, WRO82-1, BCPAP, and TPC1 cells, TTF-1 was expressed in all the cell lines and TTF-2 was faintly detected in WRO82-1, TPC1, and BCPAP cell lines. Their inability to detect TSH-R expression in all the cell lines differed from previous studies by other investigators (20–25). One can attribute the above discrepancies to different experimental conditions such as varying numbers of polymerase chain reaction cycles used and/or different durations of exposure of immunoblots (25). Alternatively, the differences could reflect emergence of novel strains of cell lines due to self-selection or mutation. Therefore, periodic and thorough evaluation of the cell lines for various thyroid markers is essential to confirm their origin and relationship to a particular type of thyroid cancer.
Thyroid tumors have been associated with specific chromosomal abnormalities (26). Therefore, accurate information on the karyotypes of cell lines derived from such tumors is of particular importance. Cytogenetic studies on primary cultures of PTC cells have shown that these tumors mainly have diploid karyotypes, and most cases exhibit the 10q11.2 rearrangement at the cytogenetic or molecular level (27). Among PTC-derived cell lines, to our knowledge, an extensive karyotype description has been reported only for the BCPAP cell line (28,29). Interestingly, the BCPAP cells do not express typical genetic features of papillary lesions, such as 10q11.2 rearrangement, and they exhibit a rather stable hypertriploid karyotype, indicating that this cell line has chromosomal anomalies that are different from those reported in PTCs. Interestingly, the BCPAP karyogram is reminiscent of that observed in primary follicular oncocytic thyroid tumors. Moreover, tumors grown in injected nude mice lack papillary architecture (20) and the appearance of the oncocytic features have been observed during cell line establishment in two studies (20,29). These and above described changes are consistent with a possible early in vitro selection for oncocytic features (28).
FTC
Recently, Haugen and coworkers described several unique FTC-derived cell lines such as TT2609-CO2, FTC133, ML1, and WRO82-1 (11). Mutations in the Ras genes as well as PAX8-PPARγ rearrangement were seen in 30–40% of conventional follicular carcinomas. In addition, mutations and other alterations of the genes coding key signaling molecules such as PI3K/Akt, particularly the PIK3CA gene that encodes the catalytic subunit of the lipid kinase, were also reported in 6–13% of follicular carcinomas (13).
In contrast, no Ras mutations, PAX8/PPARγ rearrangements, or PIK3CA mutations have been described so far in any of the FTC-derived cell lines. On the other hand, the V600E BRAF mutation, known to be restricted to papillary carcinoma and poorly differentiated or anaplastic carcinomas that originate from papillary carcinoma, has been reported in WRO82-1 cell line, but this finding is not universal (11). Finally, a point mutation in the tumor suppressor TP53 gene that has been reported in few cases of follicular carcinomas has also been found in the WRO82-1 cell line (Pro223Leu) (30). These features should be taken into consideration while using WRO82-1 cells for investigating the underlying mechanism of the development of thyroid follicular carcinoma.
Follicular carcinomas are well differentiated and express several thyroid markers. The FTC-derived cell lines such as FTC133 and WRO82-1 are known to express the thyroid-specific transcription factors PAX8 and TTF-1; in addition, the WRO82-1 cells also express TTF-2, albeit to a lesser extent, but fail to show the expression of other thyroid markers such as TSH-R, NIS, Tg, TPO, THOX1, and THOX2 (10,11,12).
Structural and numerical karyotype abnormalities have been described in follicular carcinomas. An extensive karyotype description has been reported only for the FTC133 and WRO82-1 cell lines (12,21,28). In particular, the FTC133 cells revealed significant gains in part or whole of chromosomes 1, 11, 6, 7, 8, 14, 15, 19, and 20, and losses in chromosomes 16, 21, and 22 (28). The hyperdiploid karyograms in the FTC133 and WRO82-1 cells (62–71 and 68–77 chromosomes, respectively) have also been reported (12). Divergence between present and previously reported karyotypes concerned numerical and structural alterations; in particular, the WRO82-1 chromosomal pattern (62–82 chromosomes) described by Klandorf and coworkers (21) has not yet been confirmed. This again suggests an in vitro evolution of changes in the cell line, a finding similar to that noted for the PTC-derived BCPAP cell line.
Oncocytic (Hurhtle cell) carcinoma of the thyroid is a variant of FTC. Clark's group established a well-differentiated thyroid cancer cell line from an oncocytic carcinoma designated XTC.UC1 (31). These cells retained their differentiated function in vivo as assessed by human Tg secretion and expression of thyroid differentiation markers such as PAX8, TSH-R, TTF-1, TPO, and Tg (10,31). Further, a silent polymorphism such as Gly610Gly in exon 15 of the BRAF gene, Gln787Gln in exon 20 of the EGFR gene and Val647Val in exon 15 of the RAF-1 gene has also been reported in the above cell line (10). Moreover, based on the ability to invade through reconstituted basement membrane in vitro and the potential to metastasize in vivo, this cell line may serve as a unique model for the investigation of mechanisms underlying the invasion and metastasis of a well-differentiated thyroid cancer.
Poorly differentiated and anaplastic thyroid cancer
Poorly differentiated carcinoma is a rare thyroid tumor that arises from follicular cells and is characterized by a partial loss of thyroid differentiation and less favorable prognosis in comparison with well-differentiated papillary or follicular carcinomas.
Anaplastic (undifferentiated) carcinoma represents the most undifferentiated type of thyroid tumors. Many cell lines derived from ATC are available. However, misidentification of some cell lines that are not of thyroid origin has been reported. In particular, Haugen and coworkers (11) showed that KAT5, KAT10, KAT4, KAT7, KAT50, KAK1, ARO81-1, and MRO87-1 cell lines that represent ATC are identical to each other, a finding confirmed by Maenhaut and coworkers (12), and to the HT-29 cell line, claimed to be derived from colon cancer. Moreover, DRO90-1 cell line has been found to be genetically identical to the A-375 cell line, claimed to be derived from melanoma, indicating that the identity of these cells is in doubt and thus their use for studies related to ATC may not be prudent. The unique ATC-derived cell lines currently available are listed in Table 1.
ATC can arise de novo or from preexisting well-differentiated papillary or follicular carcinomas. Coexistence of areas of papillary or follicular carcinomas along with poorly differentiated or anaplastic carcinomas has been noted, thereby suggesting that these tumors may represent distinct steps in the progression from well-differentiated to undifferentiated thyroid carcinomas. Consistent with the above description, some molecular alterations, considered to be early events in thyroid carcinogenesis (i.e., mutations in Ras and BRAF), are found in tumors with different levels of dedifferentiation, whereas the late event mutations such as TP53 occur with increasing frequency in tumors that progressively lose thyroid differentiation.
Point mutations of the Ras genes have been reported in 18–27% of poorly differentiated carcinomas and in 50–60% of anaplastic carcinomas (13). Only a silent polymorphism (His27His) of H-Ras has been reported in 8505C and Hth74 cell lines (10). In contrast, the BRAF mutations occur in ∼15% of poorly differentiated carcinomas and can increase up to 20% in anaplastic carcinomas (13). In thyroid tumors, the V600E BRAF mutation is often seen in anaplastic carcinomas that arise from papillary carcinoma. A similar finding in cell lines established from anaplastic carcinomas and tumors containing areas of well-differentiated papillary carcinomas was also reported (32). Further, V600E BRAF mutation was found in the following cell lines: Uhth-104 (heterozygous) (33), 8505C (homozygous) (10,12,32), and SW1736 (heterozygous) (11,33,34). Wild-type BRAF is known to be present in Cal-62 (11,32), ACT-1 (11), HTh74 (10,11,33), KAT18 (11,34), TTA1 (11), HTh7 (11,33), C643 (10,11,33), and FRO81-2 (11). Although a V600 BRAF mutation was detected by Yamashita and coworkers (15) in the FRO81-2 cell line, the status of BRAF in T235, T238, BHT101, and KTC-2 cell lines remain unknown.
Point mutations in the PI3KCA gene (∼20%) and PTEN gene (∼15%) affecting PI3K/Akt pathway were found in ATCs (13); however, no PI3K mutations have been reported to date in any of the ATC-derived cell lines. Point mutations in exon 3 of CTNNB1 were reported in 25% of poorly differentiated and 66% of anaplastic carcinomas (13). However, only the occurrence of wild-type CTNBB1 has been described in C643, 8505C, and HTh74 cell lines (10).
Point mutations of TP53 were reported in 15–30% of poorly differentiated carcinomas and 60–80% of anaplastic carcinomas (13). However, in ATC-derived cell lines such as C643 and 8505C, only a missense mutation of TP53 (Arg248Gln) has been reported (10). Mutations of TP53 were found to be associated with the progressive loss of thyroid differentiated markers. Haugen and coworkers (11) reported undetectable levels of PAX8 and/or TTF-1 in ATC-derived 8505C, HTh74, and C643 cell lines in contrast to findings reported by Sobrinho-Simões and coworkers (10). It is possible that the above differences were due to either in vitro selection process that occurs during cell line propagation or the differences in experimental conditions. Further, Sobrinho-Simões and coworkers showed that the ATC cell lines did not express other thyroid differentiation markers such as TSH-R, TPO, and Tg (10).
Some of the chromosomal structural and ploidy abnormalities described for FTCs and PTCs were also detected in ATCs and ATC-derived cell lines. In particular, the 8505C cells showed hypotriploid set of chromosomes coupled with a structurally abnormal karyogram (12).
Epigenetic Alterations in Thyroid Cancer and Related Cell Lines
Epigenetic alterations are changes around a gene that alter gene expression without affecting the nucleotide sequence of the gene and they play a fundamental role in the regulation of gene expression. Two epigenetic mechanisms namely DNA methylation and histone modifications are commonly used by cells to regulate gene expression (35,36). Aberrant gene methylation plays an important role in human tumorigenesis, including thyroid tumorigenesis (37,38). Many tumor suppressor genes are aberrantly methylated in thyroid cancer, and some even in benign thyroid tumors, suggesting a role for this form of epigenetic event in early thyroid tumorigenesis. Methylation of some of the tumor suppressor genes tends to occur in certain types of thyroid cancer and is related to specific signaling pathways.
Methylation of phosphatase and tensin homolog PTEN (39) and ras association domain family member 1 RASSF1A (40,41) genes occurs mostly in FTC. Although activation of PTEN can downmodulate Akt phosphorylation, RASSF1A can lead to Ras activation which in turn can activate PI3K and lead to Akt phosphorylation. Thus PTEN and RASSF1A can regulate the PI3K/Akt signaling pathway, which plays an important role in FTC tumorigenesis.
Methylation of genes for tissue inhibitor of metalloproteinase-3, solute carrier family 5 member 8 (SLC5A8), and death-associated protein kinase occurs in PTC and is related to the MAPK pathway (42,43). Methylation of these genes is also associated with aggressive pathological characteristics of PTC; it is conceivable that silencing of these genes is an important mechanism mediating BRAF mutation-promoted progression and aggressiveness of thyroid cancers, particularly in the form of extrathyroidal invasion, metastasis, and recurrence of PTC.
Methylation of thyroid-specific genes, such as NIS (23,44), TSH-R (25), the genes for the putative thyroid follicular cell apical iodide transporters, solute carrier family 26 member 4 (SLC26A4 or pendrin) (45), and SLC5A8 (46,47), is also common in thyroid cancer and thyroid cell lines.
Interestingly, several thyroid-specific genes were shown to be silenced on activation of the BRAF/MAPK/MEK pathway, with induced expression of BRAF V600E in rat thyroid cell line PCCL3 (48,49). Suppression of MAPK pathway could restore the expression of these genes. In case of TSH-R gene, demethylation of the gene associated with its expression occurred with suppression of the BRAF/MAPK/MEK pathway (49). Although the tumorigenic role of these genes is not clear, the silencing of these thyroid-specific genes by methylation can cause failure of clinical radioiodine treatment of thyroid cancer. For example, aberrant methylation and associated silencing of NIS gene was demonstrated in FTC-derived WRO82-1 cell line, which could be reversed by treatment of cells with demethylating agents (23). Xing and coworkers reported similar findings for TSH-R gene in WRO82-1 (25).
Histone modification, including acetylation and methylation, is another important epigenetic event in gene regulation, but only fewer studies have been conducted to investigate their role in thyroid tumorigenesis. For example, a previous study tested the effect of the histone deacetylase (HDAC) inhibitor depsipeptide on expression of NIS and Tg and showed reexpression of these genes with increased iodine uptake and histone acetylation in FTC-derived FTC133 cell line and ATC-derived SW136 cell line (50). Another study demonstrated that other HDAC inhibitors increased NIS expression by enhancing the promoter activity of NIS through promoter acetylation in PTC-derived BCPAP and TPC1 cell lines and ATC-derived FRO81-2 cell line (51).
Detailed studies on epigenetic alterations in thyroid cancer and thyroid cell lines are required to uncover new molecular mechanisms in thyroid tumorigenesis that may provide novel therapeutic targets for thyroid cancer.
Conclusions
Cell lines derived from human tumors are widely used to study the mechanisms involved in cancer and they also serve as preclinical models to assess the efficacy of novel therapies. However, there are some limitations to the use of the cell lines because of reported cross-contaminations and misidentifications (10–12,52–60).
Based on the recent gene expression analysis carried out by Maenhaut's group, it is apparent that most of the thyroid cancer cell lines are not only closer to each other but are also related to the undifferentiated thyroid tumors regardless of the tumor type from which they were derived (12). The above findings suggest that the thyroid cancer cell lines currently in use may have evolved in culture and lost some of their original characteristics. At the same time, they show the expression of TTF-1, TTF-2, and PAX8 but not other thyroid-specific markers such as Tg or TPO, which are regulated by these factors (12) (Table 2). Although some of these cell lines have their origin in the thyroid, they appear to have lost the expression of most of the thyroid differentiation markers.
Table 2.
Reported Mutations in Key Oncogenic Molecules in Thyroid Cancer Cell Lines
| Cell line | Tumor | RET | HRAS | BRAF | PI3K | TP53 | CTNBB |
|---|---|---|---|---|---|---|---|
| BCPAP | PTC | WT (10,11,14,15)a | V600E (10,11,14) | Asp259Tyr (10) | WT (10) | ||
| KTC-1 | PTC | WT (10,11,15) | WT (15) | V600E (15) | |||
| K1 | PTC | WT (10,11,15) | His27His (10) | V600E/WT (10,11,33) | Glu542Lys (10) | Arg213Arg (10) | WT (10) |
| TPC1 | PTC | RET/PTC1 (10,11,14) | His27His (10) | WT (10,11,14,15) | WT (10) | ||
| TT2609-CO2 | FTC | ||||||
| FTC133 | FTC | ||||||
| ML1 | FTC | ||||||
| WRO82-1 | FTC | V600E/WT (11,15,33) | Pro23Leu (30) | ||||
| 8505C | ATC | WT (10) | His27His (10) | V600E (10,11,32) | Arg248Gln (80) | WT (10) | |
| SW1736 | ATC | WT (10,11,15) | V600E (11,33,34) | ||||
| Cal-62 | ATC | WT (11,32) | |||||
| T235 | ATC | ||||||
| T238 | ATC | ||||||
| Uhth-104 | ATC | V600E (33) | |||||
| ACT-1 | ATC | WT (11) | |||||
| HTh74 | ATC | WT (10,11,15) | His27His (10) | WT (11,33) | WT (10) | ||
| KAT18 | ATC | WT (10,11,15) | WT (10,34) | ||||
| TTA1 | ATC | WT (10) | |||||
| FRO81-2 | ATC | WT (10,11,15) | V600E/WT (11,15) | ||||
| HTh7 | ATC | WT (10,11,15) | WT (10,11,33) | ||||
| C643 | ATC | WT (10,11,15) | Gly13Arg (10) | WT (10,12,33) | Arg248Gln (80) | WT (10) | |
| BHT101 | ATC | ||||||
| KTC-2 | ATC |
The numbers within parentheses are references.
RET, rearranged in transformation; H-Ras, v-Ha-ras Harvey rat sarcoma viral oncogene homolog; BRAF, v-raf murine sarcoma viral oncogene homolog B1; PI3K, phosphatidylinositol 3-kinase; TP53, p53; CTNNB, catenin; WT, wild type.
Further, the thyroid tumor-derived cell lines retain the causal oncogenic event shown in Table 3 (e.g., BRAF activation for the BCPAP cell line and RET/PTC rearrangement for the TPC1 cells). Therefore, some of the cell lines represent an excellent starting point to analyze the signaling pathway that may be important in thyroid carcinogenesis.
In conclusion, the use of the thyroid cancer cell lines to investigate a particular type of thyroid carcinoma warrants prior careful and thorough validation to verify their origin and suitability as a model that reflects the major characteristics of the particular type of thyroid cancer being investigated. This sentiment is also echoed in a recent editorial by Dr. Ringel. (61). It is apparent from this review that it is prudent to use multiple cell lines derived from the same tumor type to overcome some of the limitations discussed in this review. Particular attention should be given to origin of cells that might reflect different genetic background and potential diversity acquired during their establishment in culture. Similar observations using these different cell lines are likely to enhance the reliability of observations and allow one to draw firmer conclusions about the involvement of a particular pathway in a particular cancer being investigated.
Acknowledgment
This work was supported by grant 5R01CA107506 from the National Institutes of Health.
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Davies L. Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;29:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Leenhardt L. Grosclaude P. Cherie-Challine L. Increased incidence of thyroid carcinoma in France: a true epidemic or thyroid nodule management effects? Report from the French Thyroid Cancer Committee. Thyroid. 2004;14:1056–1060. doi: 10.1089/thy.2004.14.1056. [DOI] [PubMed] [Google Scholar]
- 3.Pacini F. Cetani F. Miccoli P. Mancusi F. Ceccarelli C. Lippi F. Martino E. Pinchera A. Outcome of 309 patients with metastatic differentiated thyroid carcinoma treated with radioiodine. World J Surg. 1994;18:600–604. doi: 10.1007/BF00353775. [DOI] [PubMed] [Google Scholar]
- 4.Yano Y. Uematsu N. Yashiro T. Hara H. Ueno E. Miwa M. Tsujimoto G. Aiyoshi Y. Uchida K. Gene expression profiling identifies platelet-derived growth factor as a diagnostic molecular marker for papillary thyroid carcinoma. Clin Cancer Res. 2004;10:2035–2043. doi: 10.1158/1078-0432.ccr-0807-03. [DOI] [PubMed] [Google Scholar]
- 5.Kebebew E. Peng M. Reiff E. Duh QY. Clark OH. McMillan A. Diagnostic and prognostic value of cell-cycle regulatory genes in malignant thyroid neoplasms. World J Surg. 2006;30:767–774. doi: 10.1007/s00268-005-0308-2. [DOI] [PubMed] [Google Scholar]
- 6.Sunde M. McGrath KC. Young L. Matthews JM. Chua EL. Mackay JP. Death AK. TC-1 is a novel tumorigenic and natively disordered protein associated with thyroid cancer. Cancer Res. 2004;64:2766–2773. doi: 10.1158/0008-5472.can-03-2093. [DOI] [PubMed] [Google Scholar]
- 7.Wasenius VM. Hemmer S. Kettunen E. Knuutila S. Franssila K. Joensuu H. Hepatocyte growth factor receptor, matrix metalloproteinase-11, tissue inhibitor of metalloproteinase-1, and fibronectin are up-regulated in papillary thyroid carcinoma: a cDNA and tissue microarray study. Clin Cancer Res. 2003;9:68–75. [PubMed] [Google Scholar]
- 8.Zembutsu H. Ohnishi Y. Tsunoda T. Furukawa Y. Katagiri T. Ueyama Y. Tamaoki N. Nomura T. Kitahara O. Yanagawa R. Hirata K. Nakamura Y. Genome-wide cDNA microarray screening to correlate gene expression profiles with sensitivity of 85 human cancer xenografts to anticancer drugs. Cancer Res. 2002;62:518–527. [PubMed] [Google Scholar]
- 9.Nikolova DN. Zembutsu H. Sechanov T. Vidinov K. Kee LS. Ivanova R. Becheva E. Kocova M. Toncheva D. Nakamura Y. Genome-wide gene expression profiles of thyroid carcinoma: identification of molecular targets for treatment of thyroid carcinoma. Oncol Rep. 2008;20:105–121. [PubMed] [Google Scholar]
- 10.Meireles AM. Preto A. Rocha AS. Rebocho AP. Máximo V. Pereira-Castro I. Moreira S. Feijão T. Botelho T. Marques R. Trovisco V. Cirnes L. Alves C. Velho S. Soares P. Sobrinho-Simões M. Molecular and genotypic characterization of human thyroid follicular cell carcinoma-derived cell lines. Thyroid. 2007;17:707–715. doi: 10.1089/thy.2007.0097. [DOI] [PubMed] [Google Scholar]
- 11.Schweppe RE. Klopper JP. Korch C. Pugazhenthi U. Benezra M. Knauf JA. Fagin JA. Marlow L. Copland JA. Smallridge RC. Haugen BR. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab. 2008;93:4331–4341. doi: 10.1210/jc.2008-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Staveren WC. Solís DW. Delys L. Duprez L. Andry G. Franc B. Thomas G. Libert F. Dumont JE. Detours V. Maenhaut C. Human thyroid tumor cell lines derived from different tumor types present a common dedifferentiated phenotype. Cancer Res. 2007;67:8113–8120. doi: 10.1158/0008-5472.CAN-06-4026. [DOI] [PubMed] [Google Scholar]
- 13.Nikiforova MN. Nikiforov YE. Molecular genetics of thyroid cancer: implications for diagnosis, treatment and prognosis. Expert Rev Mol Diagn. 2008;8:83–95. doi: 10.1586/14737159.8.1.83. [DOI] [PubMed] [Google Scholar]
- 14.Finn S. Smyth P. O'Regan E. Cahill S. Toner M. Timon C. Flavin R. O'Leary J. Sheils O. Low-level genomic instability is a feature of papillary thyroid carcinoma: an array comparative genomic hybridization study of laser capture microdissected papillary thyroid carcinoma tumors and clonal cell lines. Arch Pathol Lab Med. 2007;131:65–73. doi: 10.5858/2007-131-65-LGIIAF. [DOI] [PubMed] [Google Scholar]
- 15.Namba H. Nakashima M. Hayashi T. Hayashida N. Maeda S. Rogounovitch TI. Ohtsuru A. Saenko VA. Kanematsu T. Yamashita S. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J Clin Endocrinol Metab. 2003;88:4393–4397. doi: 10.1210/jc.2003-030305. [DOI] [PubMed] [Google Scholar]
- 16.Kopp P. The TSH receptor and its role in thyroid disease. Cell Mol Life Sci. 2001;58:1301–1322. doi: 10.1007/PL00000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zannini M. Francis-Lang H. Plachov D. Di Lauro R. Pax-8, a paired domain-containing protein, binds to a sequence overlapping the recognition site of a homeodomain and activates transcription from two thyroid-specific promoters. Mol Cell Biol. 1992;12:4230–4241. doi: 10.1128/mcb.12.9.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brabant G. Maenhaut C. Kohrle J. Scheumann G. Dralle H. Hoang-Vu C. Hesch RD. von zur Muhlen A. Vassart G. Dumont JE. Human thyrotropin receptor gene: expression in thyroid tumors and correlation to markers of thyroid differentiation and dedifferentiation. Mol Cell Endocrinol. 1991;82:R7–R12. doi: 10.1016/0303-7207(91)90018-n. [DOI] [PubMed] [Google Scholar]
- 19.Carrasco N. Iodide transport in the thyroid gland. Biochim Biophys Acta. 1993;1154:65–82. doi: 10.1016/0304-4157(93)90017-i. [DOI] [PubMed] [Google Scholar]
- 20.Fabien N. Fusco A. Santoro M. Barbier Y. Dubois PM. Paulin C. Description of a human papillary thyroid-carcinoma cell-line—morphologic study and expression of tumoral markers. Cancer. 1994;73:2206–2212. doi: 10.1002/1097-0142(19940415)73:8<2206::aid-cncr2820730828>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 21.Estour B. Van Herle AJ. Juillard GJ. Totanes TL. Sparkes RS. Giuliano AE. Klandorf H. Characterization of a human follicular thyroid-carcinoma cell-line (UCLA-RO 82 W-1) Virchows Arch B Cell Pathol. 1989;57:167–174. doi: 10.1007/BF02899078. [DOI] [PubMed] [Google Scholar]
- 22.Schmutzler C. Hoang-Vu C. Rüger B. Köhrle J. Human thyroid carcinoma cell lines show different retinoic acid receptor repertoires and retinoid responses. Eur J Endocrinol. 2004;150:547–556. doi: 10.1530/eje.0.1500547. [DOI] [PubMed] [Google Scholar]
- 23.Venkataraman GM. Yatin M. Marcinek R. Ain KB. Restoration of iodide uptake in dedifferentiated thyroid carcinoma: relationship to human Na+/I− symporter gene methylation status. J Clin Endocrinol Metab. 1999;84:2449–2457. doi: 10.1210/jcem.84.7.5815. [DOI] [PubMed] [Google Scholar]
- 24.Chen ST. Shieh HY. Lin JD. Chang KS. Lin KH. Overexpression of thyroid hormone receptor beta1 is associated with thyrotropin receptor gene expression and proliferation in a human thyroid carcinoma cell line. J Endocrinol. 2000;165:379–389. doi: 10.1677/joe.0.1650379. [DOI] [PubMed] [Google Scholar]
- 25.Xing M. Usadel H. Cohen Y. Tokumaru Y. Guo Z. Westra WB. Tong BC. Tallini G. Udelsman R. Califano JA. Ladenson PW. Sidransky D. Methylation of the thyroid-stimulating hormone receptor gene in epithelial thyroid tumors: a marker of malignancy and a cause of gene silencing. Cancer Res. 2003;63:2316–2321. [PubMed] [Google Scholar]
- 26.Pierotti MA. Chromosomal rearrangements in thyroid carcinomas: a recombination or death dilemma. Cancer Lett. 2001;166:1–7. doi: 10.1016/s0304-3835(01)00439-6. [DOI] [PubMed] [Google Scholar]
- 27.Roque L. Nunes VM. Ribeiro C. Martins C. Soares J. Karyotypic characterization of papillary thyroid carcinomas. Cancer. 2001;92:2529–2538. doi: 10.1002/1097-0142(20011115)92:10<2529::aid-cncr1604>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 28.Corso C. Ulucan H. Parry EM. Parry JM. Comparative analysis of two thyroid tumor cell lines by fluorescence in situ hybridization and comparative genomic hybridization. Cancer Genet Cytogenet. 2002;137:108–118. doi: 10.1016/s0165-4608(02)00562-9. [DOI] [PubMed] [Google Scholar]
- 29.Dettori T. Frau DV. Garcia JL. Pierantoni G. Lee C. Hernandez JM. Fusco A. Morton CC. Vanni R. Comprehensive conventional and molecular cytogenetic characterization of BCPAP, a human papillary thyroid carcinoma-derived cell line. Cancer Genet Cytogenet. 2004;151:171–177. doi: 10.1016/j.cancergencyto.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 30.Fagin JA. Matsuo K. Karmakar A. Chen DL. Tang SH. Koeffler HP. High prevalence of mutations of the p53 gene in poorly differentiated human thyroid carcinomas. J Clin Invest. 1993;91:179–184. doi: 10.1172/JCI116168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zielke A. Tezelman S. Jossart GH. Wong M. Siperstein AE. Duh QY. Clark OH. Establishment of a highly differentiated thyroid cancer cell line of Hürthle cell origin. Thyroid. 1998;8:475–483. doi: 10.1089/thy.1998.8.475. [DOI] [PubMed] [Google Scholar]
- 32.Nikiforova MN. Kimura ET. Gandhi M. Biddinger PW. Knauf JA. Basolo F. Zhu Z. Giannini R. Salvatore G. Fusco A. Santoro M. Fagin JA. Nikiforov YE. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab. 2003;88:5399–5404. doi: 10.1210/jc.2003-030838. [DOI] [PubMed] [Google Scholar]
- 33.Lee JJ. Foukakis T. Hashemi J. Grimelius L. Heldin NE. Wallin G. Rudduck C. Lui WO. Höög A. Larsson C. Molecular cytogenetic profiles of novel and established human anaplastic thyroid carcinoma models. Thyroid. 2007;17:289–301. doi: 10.1089/thy.2006.0246. [DOI] [PubMed] [Google Scholar]
- 34.Xu X. Quiros RM. Gattuso P. Ain KB. Prinz RA. High prevalence of BRAF gene mutation in papillary thyroid carcinomas and thyroid tumor cell lines. Cancer Res. 2003;63:4561–4567. [PubMed] [Google Scholar]
- 35.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 36.Yoo CB. Jones PA. Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov. 2006;5:37–50. doi: 10.1038/nrd1930. [DOI] [PubMed] [Google Scholar]
- 37.Bird AP. The relationship of DNA methylation to cancer. Cancer Surv. 1996;8:87–101. [PubMed] [Google Scholar]
- 38.Baylin SB. Herman JG. Graff JR. Vertino PM. Issa JP. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res. 1998;72:141–196. [PubMed] [Google Scholar]
- 39.Alvarez-Nuñez F. Bussaglia E. Mauricio D. Ybarra J. Vilar M. Lerma E. de Leiva A. Matias-Guiu X; Thyroid Neoplasia Study Group. PTEN promoter methylation in sporadic thyroid carcinomas. Thyroid. 2006;16:17–23. doi: 10.1089/thy.2006.16.17. [DOI] [PubMed] [Google Scholar]
- 40.Schagdarsurengin U. Gimm O. Hoang-Vu C. Dralle H. Pfeifer GP. Dammann R. Frequent epigenetic silencing of the CpG island promoter of RASSF1A in thyroid carcinoma. Cancer Res. 2002;62:3698–3701. [PubMed] [Google Scholar]
- 41.Xing M. Cohen Y. Mambo E. Tallini G. Udelsman R. Ladenson PW. Sidransky D. Early occurrence of RASSF1A hypermethylation and its mutual exclusion with BRAF mutation in thyroid tumorigenesis. Cancer Res. 2004;4:1664–1668. doi: 10.1158/0008-5472.can-03-3242. [DOI] [PubMed] [Google Scholar]
- 42.Hoque MO. Rosenbaum E. Westra WH. Xing M. Ladenson P. Zeiger MA. Sidransky D. Umbricht CB. Quantitative assessment of promoter methylation profiles in thyroid neoplasms. J Clin Endocrinol Metab. 2005;90:4011–4018. doi: 10.1210/jc.2005-0313. [DOI] [PubMed] [Google Scholar]
- 43.Hu S. Liu D. Tufano RP. Carson KA. Rosenbaum E. Cohen Y. Holt EH. Kiseljak-Vassiliades K. Rhoden KJ. Tolaney S. Condouris S. Tallini G. Westra WH. Umbricht CB. Zeiger MA. Califano JA. Vasko V. Xing M. Association of aberrant methylation of tumor suppressor genes with tumor aggressiveness and BRAF mutation in papillary thyroid cancer. Int J Cancer. 2006;119:2322–2329. doi: 10.1002/ijc.22110. [DOI] [PubMed] [Google Scholar]
- 44.Neumann S. Schuchardt K. Reske A. Reske A. Emmrich P. Paschke R. Lack of correlation for sodium iodide symporter mRNA and protein expression and analysis of sodium iodide symporter promoter methylation in benign cold thyroid nodules. Thyroid. 2004;14:99–111. doi: 10.1089/105072504322880337. [DOI] [PubMed] [Google Scholar]
- 45.Xing M. Tokumaru Y. Wu G. Westra WB. Ladenson PW. Sidransky D. Hypermethylation of the Pendred syndrome gene SLC26A4 is an early event in thyroid tumorigenesis. Cancer Res. 2003;63:2312–2315. [PubMed] [Google Scholar]
- 46.Hu S. Liu D. Tufano RP. Carson KA. Rosenbaum E. Cohen Y. Holt EH. Kiseljak-Vassiliades K. Rhoden KJ. Tolaney S. Condouris S. Tallini G. Westra WH. Umbricht CB. Zeiger MA. Califano JA. Vasko V. Xing M. Association of aberrant methylation of tumor suppressor genes with tumor aggressiveness and BRAF mutation in papillary thyroid cancer. Int J Cancer. 2006;119:2322–2329. doi: 10.1002/ijc.22110. [DOI] [PubMed] [Google Scholar]
- 47.Porra V. Ferraro-Peyret C. Durand C. Selmi-Ruby S. Giroud H. Berger-Dutrieux N. Decaussin M. Peix JL. Bournaud C. Orgiazzi J. Borson-Chazot F. Dante R. Rousset B. Silencing of the tumor suppressor gene SLC5A8 is associated with BRAF mutations in classical papillary thyroid carcinomas. J Clin Endocrinol Metab. 2005;90:3028–3035. doi: 10.1210/jc.2004-1394. [DOI] [PubMed] [Google Scholar]
- 48.Riesco-Eizaguirre G. Gutiérrez-Martínez P. García-Cabezas MA. Nistal M. Santisteban P. The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I− targeting to the membrane. Endocr Relat Cancer. 2006;13:257–269. doi: 10.1677/erc.1.01119. [DOI] [PubMed] [Google Scholar]
- 49.Liu D. Hu S. Hou P. Jiang D. Condouris S. Xing M. Suppression of BRAF/MEK/MAP kinase pathway restores expression of iodide-metabolizing genes in thyroid cells expressing the V600E BRAF mutant. Clin Cancer Res. 2008;13:1341–1349. doi: 10.1158/1078-0432.CCR-06-1753. [DOI] [PubMed] [Google Scholar]
- 50.Kitazono M. Robey R. Zhan Z. Sarlis NJ. Skarulis MC. Aikou T. Bates S. Fojo T. Low concentrations of the histone deacetylase inhibitor, depsipeptide (FR901228), increase expression of the Na(+)/I(−) symporter and iodine accumulation in poorly differentiated thyroid carcinoma cells. J Clin Endocrinol Metab. 2001;86:3430–3435. doi: 10.1210/jcem.86.7.7621. [DOI] [PubMed] [Google Scholar]
- 51.Puppin C. D'Aurizio F. D'Elia AV. Cesaratto L. Tell G. Russo D. Filetti S. Ferretti E. Tosi E. Mattei T. Pianta A. Pellizzari L. Damante G. Effects of histone acetylation on sodium iodide symporter promoter and expression of thyroid-specific transcription factors. Endocrinology. 2005;146:3967–3974. doi: 10.1210/en.2005-0128. [DOI] [PubMed] [Google Scholar]
- 52.MacLeod RA. Dirks WG. Matsuo Y. Kaufmann M. Milch H. Drexler HG. Widespread intraspecies cross-contamination of human tumor cell lines arising at source. Int J Cancer. 1999;83:555–563. doi: 10.1002/(sici)1097-0215(19991112)83:4<555::aid-ijc19>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 53.Hughes P. Marshall D. Reid Y. Parkes H. Gelber C. The costs of using unauthenticated, over-passaged cell lines: how much more data do we need? Biotechniques. 2007;43:575–586. doi: 10.2144/000112598. [DOI] [PubMed] [Google Scholar]
- 54.Masters JR. Thomson JA. Daly-Burns B. Reid YA. Dirks WG. Packer P. Toji LH. Ohno T. Tanabe H. Arlett CF. Kelland LR. Harrison M. Virmani A. Ward TH. Ayres KL. Debenham PG. Short tandem repeat profiling provides an international reference standard for human cell lines. Proc Natl Acad Sci USA. 2001;98:8012–8017. doi: 10.1073/pnas.121616198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chatterjee R. Cell biology., When 60 lines don't add up. Science. 2007;315:929. doi: 10.1126/science.315.5814.929. [DOI] [PubMed] [Google Scholar]
- 56.Lacroix M. Persistent use of “false” cell lines. Int J Cancer. 2008;122:1–4. doi: 10.1002/ijc.23233. [DOI] [PubMed] [Google Scholar]
- 57.Chatterjee R. Cell biology. Cases of mistaken identity. Science. 2007;315:928–931. doi: 10.1126/science.315.5814.928. [DOI] [PubMed] [Google Scholar]
- 58.van Bokhoven A. Varella-Garcia M. Korch C. Johannes WU. Smith EE. Miller HL. Nordeen SK. Miller GJ. Lucia MS. Molecular characterization of human prostatecarcinoma cell lines. Prostate. 2003;57:205–225. doi: 10.1002/pros.10290. [DOI] [PubMed] [Google Scholar]
- 59.van Bokhoven A. Varella-Garcia M. Korch C. Miller GJ. TSU-Pr1 and JCA-1 cells are derivatives of T24 bladder carcinoma cells and are not of prostatic origin. Cancer Res. 2001;61:6340–6344. [PubMed] [Google Scholar]
- 60.van Bokhoven A. Varella-Garcia M. Korch C. Hessels D. Miller GJ. Widely used prostate carcinoma cell lines share common origins. Prostate. 2001;47:36–51. doi: 10.1002/pros.1045. [DOI] [PubMed] [Google Scholar]
- 61.Ringel MD. “Thyroid cancer” cell line misidentification: a time for proactive change. J Clin Endocrinol Metab. 2008;93:4226–4227. doi: 10.1210/jc.2008-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kurebayashi J. Tanaka K. Otsuki T. Moriya T. Kunisue H. Uno M. Sonoo H. All-trans-retinoic acid modulates expression levels of thyroglobulin and cytokines in a new human poorly differentiated papillary thyroid carcinoma cell line, KTC-1. J Clin Endocrinol Metab. 2000;85:2889–2896. doi: 10.1210/jcem.85.8.6732. [DOI] [PubMed] [Google Scholar]
- 63.Challeton C. Branea F. Schlumberger M. Gaillard N. de Vathaire F. Badie C. Antonini P. Parmentier C. Characterization and radiosensitivity at high or low dose rate of four cell lines derived from human thyroid tumors. Int J Radiat Oncol Biol Phys. 1997;37:163–169. doi: 10.1016/s0360-3016(96)00449-x. [DOI] [PubMed] [Google Scholar]
- 64.Ishizaka Y. Itoh F. Tahira T. Ikeda I. Ogura T. Sugimura T. Nagao M. Presence of aberrant transcripts of ret proto-oncogene in a human papillary thyroid carcinoma cell line. Jpn J Cancer Res. 1989;80:1149–1152. doi: 10.1111/j.1349-7006.1989.tb01645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Geldof AA. Versteegh LRT. van Mourik JC. Rooimans MA. Arwert F. Hermsen MA. Schadee-Eestermans IL. van Dongen GA. van der Valk P. van der Clement EHP. Lips P. Teule GJ. Clonally related but phenotypically divergent human cancer cell lines derived from a single follicular thyroid cancer recurrence (TT2609) Thyroid. 2001;11:909–917. doi: 10.1089/105072501753210966. [DOI] [PubMed] [Google Scholar]
- 66.Goretzki PE. Frilling A. Simon D. Rastegar M. Ohmann C. Growth regulation of human thyrocytes by thyrotropin, cyclic adenosine monophosphate, epidermal growth factor, insulin-like growth factor. In: Goretzki PE, editor; Röher HD, editor. Frontiers of Hormone Research. Karger; Basel: 1989. pp. 56–80. [Google Scholar]
- 67.Schönberger J. Bauer J. Spruss T. Weber G. Chahoud I. Eilles C. Grimm D. Establishment and characterization of the follicular thyroid carcinoma cell line ML-1. J Mol Med. 2000;78:102–110. doi: 10.1007/s001090000085. [DOI] [PubMed] [Google Scholar]
- 68.Ito T. Seyama T. Iwamoto KS. Hayashi T. Mizuno T. Tsuyama N. Dohi K. Nakamura N. Akiyama M. In vitro irradiation is able to cause RET oncogene rearrangement. Cancer Res. 1993;53:2940–2943. [PubMed] [Google Scholar]
- 69.Gioanni J. Zanghellini E. Mazeau C. Zhang D. Courdi A. Farges M. Lambert JC. Duplay H. Schneider M. Characterization of a human cell line from an anaplastic carcinoma of the thyroid gland. Bull Cancer. 1991;78:1053–1062. [PubMed] [Google Scholar]
- 70.Rodrigues RF. Roque L. Krug T. Leite V. Poorly differentiated and anaplastic thyroid carcinomas: chromosomal and oligo-array profile of five new cell lines. Br J Cancer. 2007;96:1237–1245. doi: 10.1038/sj.bjc.6603578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chung SH. Onoda N. Ishikawa T. Ogisawa K. Takenaka C. Yano Y. Hato F. Hirakawa K. Peroxisome proliferator-activated receptor gamma activation induces cell cycle arrest via the p53-independent pathway in human anaplastic thyroid cancer cells. Jpn J Cancer Res. 2002;93:1358–1365. doi: 10.1111/j.1349-7006.2002.tb01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heldin NE. Cvejić D. Smeds S. Westermark B. Coexpression of functionally active receptors for thyrotropin and platelet-derived growth factor in human thyroid carcinoma cells. Endocrinology. 1991;129:2187–2193. doi: 10.1210/endo-129-4-2187. [DOI] [PubMed] [Google Scholar]
- 73.Ain KB. Taylor KD. Tofiq S. Venkataraman G. Somatostatin receptor subtype expression in human thyroid and thyroid carcinoma cell lines. J Clin Endocrinol Metab. 1997;82:1857–1862. doi: 10.1210/jcem.82.6.4013. [DOI] [PubMed] [Google Scholar]
- 74.Yano Y. Kamma H. Matsumoto H. Fujiwara M. Bando H. Hara H. Yashiro T. Ueno E. Ito K. Uchida K. Growth suppression of thyroid cancer cells by adenylcyclase activator. Oncol Rep. 2007;18:441–445. [PubMed] [Google Scholar]
- 75.Nishihara E. Nagayama Y. Mawatari F. Tanaka K. Namba H. Niwa M. Yamashita S. Retrovirus-mediated herpes simplex virus thymidine kinase gene transduction renders human thyroid carcinoma cell lines sensitive to ganciclovir and radiation in vitro and in vivo. Endocrinology. 1997;138:4577–4583. doi: 10.1210/endo.138.11.5509. [DOI] [PubMed] [Google Scholar]
- 76.Carlsson J. Nilsson K. Westermark B. Pontén J. Sundström C. Larsson E. Bergh J. Påhlman S. Busch C. Collins VP. Formation and growth of multicellular spheroids of human origin. Int J Cancer. 1983;31:523–533. doi: 10.1002/ijc.2910310502. [DOI] [PubMed] [Google Scholar]
- 77.Gustavsson B. Hermansson A. Andersson AC. Grimelius L. Bergh J. Westermark B. Heldin NE. Decreased growth rate and tumour formation of human anaplastic thyroid carcinoma cells transfected with a human thyrotropin receptor cDNA in NMRI nude mice treated with propylthiouracil. Mol Cell Endocrinol. 1996;121:143–151. doi: 10.1016/0303-7207(96)03859-2. [DOI] [PubMed] [Google Scholar]
- 78.Pályi I. Péter I. Daubner D. Vincze B. Lõrincz I. Establishment, characterization and drug sensitivity of a new anaplastic thyroid carcinoma cell line (BHT-101) Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;63:263–269. doi: 10.1007/BF02899271. [DOI] [PubMed] [Google Scholar]
- 79.Kurebayashi J. Otsuki T. Tanaka K. Yamamoto Y. Moriya T. Sonoo H. Medroxyprogesterone acetate decreases secretion of interleukin-6 and parathyroid hormone-related protein in a new anaplastic thyroid cancer cell line, KTC-2. Thyroid. 2003;13:249–258. doi: 10.1089/105072503321582042. [DOI] [PubMed] [Google Scholar]
- 80.Wyllie FS. Haughton MF. Blaydes JP. Schlumberger M. Wynford-Thomas D. Evasion of p53-mediated growth control occurs by three alternative mechanisms in transformed thyroid epithelial cells. Oncogene. 1995;10:49–59. [PubMed] [Google Scholar]