Abstract
Background
The incidence of papillary thyroid cancer has been reported to be increasing during the past three decades, with a 65–126% increase between 1975 and 2004. The reason for the increase is currently unknown. This study examined the incidence pattern of papillary thyroid cancer in the United States, and evaluated the components of birth cohort (defined based on year of birth), time period, and age as determinants of the observed time trend of the disease.
Methods
Using the data from the National Cancer Institute's Surveillance, Epidemiology, and End Results program for 1973–2004, we conducted both univariate analysis and age–period–cohort modeling to evaluate birth cohort patterns and evaluate age, period, and cohort effects on incidence trends over time.
Results
The increasing incidence showed a clear birth cohort pattern for both men and women. The results from age–period–cohort modeling showed that, while period effect appeared to have had an impact on the observed incidence trends, birth cohort effect may also explain part of the increasing trend in papillary thyroid carcinoma during the study period, especially among women.
Conclusion
While a period effect that is likely due to advancements in diagnostic techniques and increased medical detection of small thyroid nodules may explain some of the observed increase in the incidence, we speculate that birth cohort–related changes in environmental exposures (such as increased exposure to diagnostic X-rays and polybrominated diphenyl ethers) have also contributed to the observed increase in papillary thyroid cancer during the past decades.
Introduction
Papillary thyroid cancer is the most common type of thyroid cancer, and the age-adjusted incidence rate for the disease has been reported to be increasing during the past decades (1–4). It is currently unknown whether the increase in papillary thyroid cancer is real or an artifact of improved diagnostic techniques or increased screening for small nodules.
Two studies have examined the time-trends of thyroid cancer incidence using the Surveillance, Epidemiology, and End Results (SEER) data (1,2); however, the studies have not examined the incidence pattern by birth cohort. Birth cohort examination could help to reveal birth cohort patterns; identify components of birth cohort, time period, and age as determinants of the observed incidence patterns; and generate hypotheses for eventually identifying the risk factors responsible for increasing incidence. Here, we report the results from a birth cohort analysis of papillary thyroid cancer using the National Cancer Institute's SEER data collected between 1973 and 2004.
Methods
Data source
The thyroid cancer incidence data for years 1973–2004 were obtained from the SEER program. The SEER 9 data included nine cancer registries in the United States (five states, Connecticut, Hawaii, Iowa, New Mexico, and Utah, and four metropolitan cities, Atlanta, Detroit, San Francisco, and Seattle), which accounts for approximately 12% of the U.S. population.
A total of 43,870 thyroid cancer cases were reported to these nine registries between January 1, 1973, and December 31, 2004. The SEER registries report patient demographic data such as age at diagnosis, race/ethnicity, and sex, as well as tumor information such as histological subtype, and tumor size (available between 1988 and 2003) for each patient found to have thyroid cancer. Thyroid cancer was classified according to histological subtype: papillary (International Classification of Diseases for Oncology, 3rd ed. [ICD-O-3] 8050, 8052, 8130, 8260, 8340–8344, 8450, and 8452), follicular (ICD-O-3 8290, 8330–8332, and 8335), medullary (ICD-O-3 8345, 8346, and 8510), or anaplastic (ICD-O-3 8021). Of all the thyroid cancer patients reported between 1973 and 2004 to these nine cancer registries, 34,490 (78.6%) had papillary thyroid cancer. The study was limited to papillary thyroid cancer incidence patterns where the vast majority of the increase in incidence was observed.
Data analysis
Sex-specific, age-adjusted incidence rates were calculated using SEER*Stat (6.3.6). The age-adjusted rates were adjusted to the 2000 U.S. standard population. The data were presented by calendar year and by cohort year of birth to examine the secular trends and the potential birth cohort patterns. The age–period–cohort (APC) analysis was conducted using a log-linear Poisson regression model, fitted to the age-specific incidence rates. This facilitates the assessment of the effects of period and birth cohort on the observed trends. Age, period, and cohort models were based on sixteen 5-year age intervals beginning at age 10, and six time period intervals (1973–1979, 1980–1984, 1985–1989, 1990–1994, 1995–1999, and 2000–2004). An age effect refers to disease rates that change with age independent of birth cohort and calendar time. Period effect refers to an artificial change in the disease rate due to a change in factors such as methods of diagnosis, access to the medical services, completeness of the reporting, and ascertaining systems. Cohort effect refers to the change in the risk factors of the disease that causes a real change in disease rate. Since it was unclear whether the period slope was actually increasing or decreasing or showing no overall trend during the study period, we set three βp parameter values (βp = 0, −0.02, or 0.02) to represent that there was no overall period slope (βp = 0), the period slope was decreasing (βp = −0.02), or the period slope was actually increasing (βp = 0.02) during the study period. The cohort effect can be evaluated under the different assumptions regarding the period effect. Because of the nonidentifiability problem, the linear APC relationship (cohort = year–age) does not allow for the evaluation of the independent effects of age, period, and cohort (5). As such, we estimated drifts, which represent the sum of the overall slopes for period and cohort, and thus the overall direction of the trends (5). Models were fit using SAS (9.1). The significance level was set at 0.05 for a two-sided test.
Results
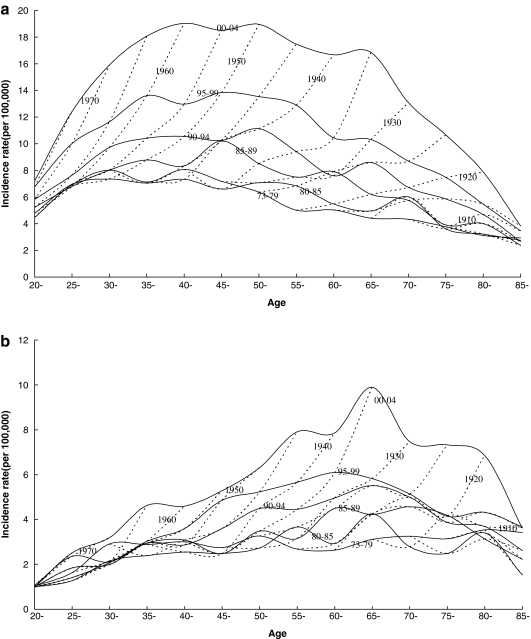
The cross-sectional age curves (solid lines) and the birth cohort age curves (dotted lines) for the incidence of papillary thyroid cancer are presented in Figure 1a (women) and Figure 1b (men). While the cross-sectional age curves showed a plateau between ages 40 and 60 among women, and peak at ages 65 and 69 among men, the birth cohort age curves, however, showed no such plateau or peak, but a continuing increase in incidence with increasing age among both men and women.
FIG. 1.
The Age-Adjusted Incidence Rates of Papillary Thyroid Cancer by Tumor Size and Sex in the United States (1988–2003)
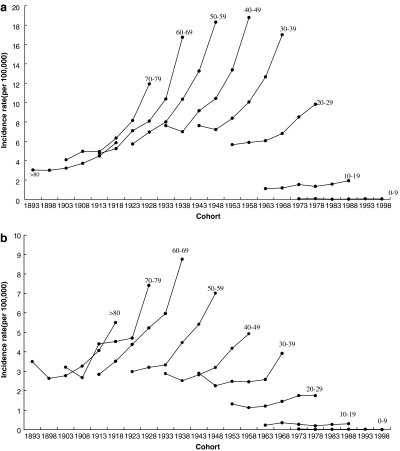
Figures 2a and 2b present the age-specific incidence rates of papillary cancer by year of birth. Figures 2a and 2b were constructed to show the effect of birth cohort on the age-specific rates. In both women (Fig. 2a) and men (Fig. 2b) at each age group greater than 20 years, the recent birth cohorts generally have much higher incidence rates than the earlier ones.
FIG. 2.
The Age-Adjusted Incidence Rates of Papillary Thyroid Cancer by Tumor Size and Sex in the United States (1988–2003)
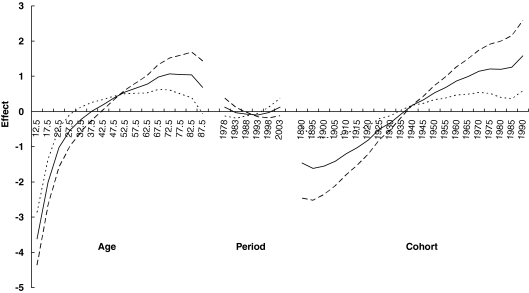
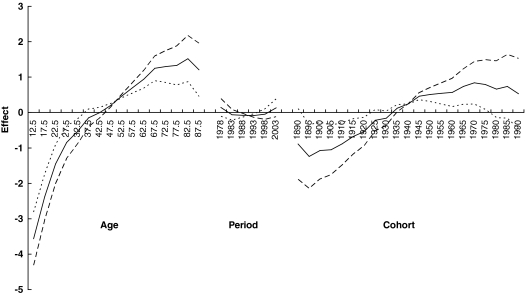
Figure 3 presents three sets of age, period, and cohort parameters for women using three different assumptions for the period slope (βp = 0, −0.02, or 0.02). The solid line is based on an assumption of no overall period slope. This assumption results in a steady increasing trend for the birth cohort effect shown by the corresponding solid cohort line. In fact, no matter what kind of assumption regarding period effect is made, the increasing birth cohort trend from papillary thyroid cancer persists in women. In men (Fig. 4), birth cohort slope also showed a continual increase until the 1970 birth cohort where the rates started to level off.
FIG. 3.
The Age-Adjusted Incidence Rates of Papillary Thyroid Cancer by Tumor Size and Sex in the United States (1988–2003)
FIG. 4.
The Age-Adjusted Incidence Rates of Papillary Thyroid Cancer by Tumor Size and Sex in the United States (1988–2003)
The net drift for papillary thyroid cancer based on the periods since 1995 and cohorts since 1910 is 0.27/5-years for men (95% confidence interval: 0.20, 0.35), and 0.33/5-years for women (95% confidence interval: 0.29, 0.37). This translates into a net increase of 7.8% per year for women and 6.2% per year for men (data not shown).
Table 1 presents the results for the time trend of papillary thyroid cancer by tumor size for both men and women. It shows that the age-adjusted incidence rates of papillary cancer of the thyroid have been increasing for all tumor sizes among both men and women during the past decades. The female-to-male incidence rate ratio has ranged from 2.07 to 3.07 during the past three decades (data not shown).
Table 1.
The Age-Adjusted Incidence Rates of Papillary Thyroid Cancer by Tumor Size and Sex in the United States (1988–2003)
| |
Female (per 100,000) |
Male (per 100,000) |
||||||
|---|---|---|---|---|---|---|---|---|
| Year of diagnosis | 0.0–1.0 cm | 1.1–2.0 cm | 2.1–5.0 cm | >5.0 cm | 0.0–1.0 cm | 1.1–2.0 cm | 2.1–5.0 cm | >5.0 cm |
| 1988–1989 | 1.42 | 1.80 | 1.44 | 0.06 | 0.44 | 0.45 | 0.59 | 0.09 |
| 1990–1991 | 1.78 | 1.87 | 1.60 | 0.12 | 0.54 | 0.45 | 0.64 | 0.09 |
| 1992–1993 | 1.84 | 1.78 | 1.62 | 0.12 | 0.64 | 0.49 | 0.68 | 0.12 |
| 1994–1995 | 2.33 | 2.00 | 1.77 | 0.16 | 0.61 | 0.52 | 0.62 | 0.19 |
| 1996–1997 | 2.45 | 2.19 | 2.10 | 0.19 | 0.70 | 0.56 | 0.67 | 0.18 |
| 1998–1999 | 3.08 | 2.37 | 2.10 | 0.21 | 0.76 | 0.60 | 0.87 | 0.21 |
| 2000–2001 | 3.58 | 2.71 | 2.37 | 0.22 | 0.97 | 0.69 | 0.94 | 0.22 |
| 2002–2003 | 4.51 | 3.30 | 2.97 | 0.28 | 1.18 | 0.92 | 1.12 | 0.24 |
Discussion
Our results suggested that the period and birth cohort effects played an important role in the observed increasing incidence of papillary thyroid cancer in the United States during the past three decades. Birth cohort analyses and the APC modeling demonstrated a strong birth cohort effect on the observed incidence patterns. The results by tumor size also showed that the age-adjusted incidence rates of papillary cancer of the thyroid have been increasing for all tumor sizes among both men and women during the past decades. As stated previously, the time trend by tumor size is interesting because if increasing detection of small-sized tumors from screening is the only explanation for the observed increase of papillary thyroid cancer, we may find a corresponding decreasing or leveling off for larger-sized tumors. Thus, the observed increasing trends for all tumor sizes argue against the notion that the ultrasound and fine-needle aspiration facilitated overdiagnosis for small thyroid cancer as the only explanation for the observed increase in the incidence rate of papillary thyroid carcinoma. Change of histological classification for thyroid cancer also cannot be used as the explanation for the observed increase in the incidence of papillary carcinoma since the SEER data showed no corresponding decrease in the incidence of follicular thyroid carcinoma during the past decades.
Based on the SEER data, the mortality for thyroid cancer has not changed materially during the past two decades (0.47/100,000 and 0.40/100,000 in 1985–1989 and 0.47/100,000 and 0.46/100,000 in 2000–2004 for women and men, respectively). During the same time period, however, the incidence of papillary thyroid cancer experienced a rapid increase from 7.3/100,000 in 1985–1989 to 13.0/100,000 in 2000–2004 for women and from 3.0/100,000 in 1985–1989 to 4.6/100,000 in 2000–2004 for men. A relatively stable mortality against a background of rapid increase in incidence of papillary thyroid cancer has been argued by some as the evidence that the observed increase in the incidence of thyroid cancer in the United States is due to overdiagnosis from increased detection of small papillary carcinoma. However, if the increased detection of small papillary carcinoma was the only explanation for the observed increase of papillary thyroid cancer, we would anticipate a corresponding decrease in thyroid cancer mortality and a decrease in large-sized papillary thyroid cancers, but as observed in this study, there is no corresponding decrease in larger-sized tumors and also no decrease in thyroid cancer mortality (2) in the United States.
Thus, it is likely that increased detection is not the only explanation for the observed increase in thyroid cancer incidence, and that additional factor(s) may have been at least partly responsible for the observed long-term increase in papillary carcinoma of the thyroid and the strong cohort effects. Radiation exposure during childhood is the only established risk factor for thyroid cancer, and papillary carcinoma of the thyroid is the form that has been suggested to be related to radiation exposure (6). One would wonder whether there has been an increase in exposure to radiation among general population during the past several decades. It has been reported that the per capita use of X-rays in medical examination and dental care has increased significantly in the United States due to wider availability of services, new equipment, and increases in sophisticated diagnostic examinations since 1960s (7). While many medical or dental diagnostic examination or treatment procedures now may produce less exposure per film to ionizing radiation, the increased exposure opportunities from increasing use of medical or dental diagnostic or treatment procedures would increase the opportunity of population exposure to ionizing radiation. Epidemiological studies have linked medical and dental X-rays exposure to an increased risk of thyroid cancer (8–11). Thus, it is quite possible that increased exposure to diagnostic X-rays may explain part of the increase in the incidence of papillary thyroid cancer. In our opinion, the elucidation of the relationship between diagnostic X-rays and risk of thyroid cancer should be pursued, as it may provide important insights into etiology and potential opportunities for prevention interventions.
Thyroid cancer is one of few cancers that show a female dominance, with a fairly consistent female:male ratio of 3:1, suggesting that female hormones may play an important role in thyroid pathogenesis (12). This hormonal hypothesis points out the potential importance of endogenous hormones and endocrine disruptors on the development of papillary thyroid cancer. The recent emerging evidence support that polyhalogenated aromatic hydrocarbons (PHAHs), particularly polybrominated diphenyl ethers (PBDEs), may be associated with the risk of thyroid cancer, and thus, may be responsible for part of the observed increases in the incidence of papillary thyroid cancer, particularly in more recent cohorts (13). Both animal and human studies have shown that PBDEs and other PHAHs, such as polychlorinated biphenyls (PCBs), dichlorodiphenyldichloroethylene (DDE), and hexachlorobenzene (HCB), are potential thyroid carcinogens (14–17). The studies, however, did not provide information on thyroid cancer subtypes. In addition, human exposure to PBDEs and other PHAHs has been increasing during the past decades (18), which is parallel with the recent dramatic increase in thyroid cancer incidence.
In summary, in this descriptive epidemiological study, we found that the increased age-adjusted incidence rate of papillary thyroid carcinoma might be explained by the period and birth cohort effects. Our study was limited by the usual concerns related to analyses of registry data: nonstandardization of histopathologic diagnosis and incomplete data collection; however, our descriptive results are consistent with other population-based studies. Although advancements in diagnostic techniques and increased medical attention to small thyroid nodules may explain some of the observed increase in the incidence, increasing exposure to diagnostic X-rays and environmental hormone disruptors such as PBDEs and other PHAHs may also explain part of the observed increase. Population-based studies are urgently needed to clarify the role of environmental exposures on the risk of papillary thyroid cancer.
Acknowledgment
This research was supported by National Institutes of Health Fogarty Training Grant 1D43TW007864-01. This publication was made possible by Clinical and Translational Science Awards (CTSA) Grant UL1 RR024139 from the National Center for Research Resources, a component of the National Institutes of Health, and NIH Roadmap for Medical Research.
Disclaimer
Contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of National Center for Research Resources.
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Albores-Saavedra J. Henson DE. Glazer E. Schwartz AM. Changing patterns in the incidence and survival of thyroid cancer with follicular phenotype—papillary, follicular, and anaplastic: a morphological and epidemiological study. Endocr Pathol. 2007;18:1–7. doi: 10.1007/s12022-007-0002-z. [DOI] [PubMed] [Google Scholar]
- 2.Davies L. Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds RM. Weir J. Stockton DL. Brewster DH. Sandeep TC. Strachan MW. Changing trends in incidence and mortality of thyroid cancer in Scotland. Clin Endocrinol (Oxf) 2005;62:156–162. doi: 10.1111/j.1365-2265.2004.02187.x. [DOI] [PubMed] [Google Scholar]
- 4.Zheng T. Holford TR. Chen Y. Ma JZ. Flannery J. Liu W. Time trend and age-period-cohort effect on incidence of thyroid cancer in Connecticut, 1935–1992. Int J Cancer. 1996;67:504–509. doi: 10.1002/(SICI)1097-0215(19960807)67:4<504::AID-IJC7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 5.Holford TR. Monitoring the Health of Populations Statistical Principles and Methods for Public Health Surveillance. Oxford University Press; New York: 2003. [Google Scholar]
- 6.Hall P. Holm LE. Radiation-associated thyroid cancer—facts and fiction. Acta Oncol. 1998;37:325–330. doi: 10.1080/028418698430539. [DOI] [PubMed] [Google Scholar]
- 7.Interagency Working Group on Medical Radiation. Federal Guidance Report No. 9: Radiation Guidance For Diagnostic X Rays. U.S. Environmental Protection Agency; Washington, DC: 1976. [Google Scholar]
- 8.Hallquist A. Hardell L. Degerman A. Wingren G. Boquist L. Medical diagnostic and therapeutic ionizing radiation and the risk for thyroid cancer: a case-control study. Eur J Cancer Prev. 1994;3:259–367. doi: 10.1097/00008469-199403030-00004. [DOI] [PubMed] [Google Scholar]
- 9.Hallquist A. Nasman A. Medical diagnostic X-ray radiation—an evaluation from medical records and dentist cards in a case-control study of thyroid cancer in the northern medical region of Sweden. Eur J Cancer Prev. 2001;10:147–152. doi: 10.1097/00008469-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Inskip PD. Ekbom A. Galanti MR. Grimelius L. Boice JD. Medical diagnostic x rays and thyroid cancer. J Natl Cancer Inst. 1995;87:1613–1621. doi: 10.1093/jnci/87.21.1613. [DOI] [PubMed] [Google Scholar]
- 11.Wingren G. Hatschek T. Axelson O. Determinants of papillary cancer of the thyroid. Am J Epidemiol. 1993;138:482–491. doi: 10.1093/oxfordjournals.aje.a116882. [DOI] [PubMed] [Google Scholar]
- 12.Franceschi S. Boyle P. Maisonneuve P. La Vecchia C. Burt AD. Kerr DJ. MacFarlane GJ. The epidemiology of thyroid carcinoma. Crit Rev Oncog. 1993;4:25–52. [PubMed] [Google Scholar]
- 13.Zhang Y. Guo GL. Han X. Zhu C. Kilfoy BA. Zhu Y. Boyle P. Zheng T. Do polybrominated diphenyl ethers (PBDEs) increase the risk of thyroid cancer? Biosci Hypotheses. 2008;1:195–199. doi: 10.1016/j.bihy.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NTP toxicology and carcinogenesis studies of decabromodiphenyl oxide (CAS No. 1163–19-5) in F344/N rats and B6C3F1 mice (feed studies) Natl Toxicol Program Tech Rep Ser. 1986;309:1–242. [PubMed] [Google Scholar]
- 15.Cabral JR. Shubik P. Mollner T. Raitano F. Carcinogenic activity of hexacholorobenzene in hamsters. Nature. 1977;269:510–511. doi: 10.1038/269510a0. [DOI] [PubMed] [Google Scholar]
- 16.Grimalt JO. Sunyer J. Moreno V. Amaral OC. Sala M. Rosell A. Anto JM. Albaiges J. Risk excess of soft-tissue sarcoma and thyroid cancer in a community exposed to airborne organochlorinated compound mixtures with a high hexachlorobenzene content. Int J Cancer. 1994;56:200–203. doi: 10.1002/ijc.2910560209. [DOI] [PubMed] [Google Scholar]
- 17.Mayes BA. McConnell EE. Neal BH. Brunner MJ. Hamilton SB. Peters AC. Ryan MJ. Toft JD. Singer AW. Brown JF., Jr Menton RG. Moore JA., Jr Comparative carcinogenicity in Sprague-Dawley rats of the polychlorinated biphenyl mixtures Aroclors 1016, 1242, 1254, and 1260. Toxicol Sci. 1998;41:62–76. doi: 10.1093/toxsci/41.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hale RC. La Guardia MJ. Harvey EP. Mainor TM. Duff WH. Gaylor MO. Polybrominated diphenyl ether flame retardants in Virginia freshwater fishes (USA) Environ Sci Technol. 2001;35:4585–4591. doi: 10.1021/es010845q. [DOI] [PubMed] [Google Scholar]