Abstract
Background
Over 200 million people worldwide are affected by thyroid proliferative diseases, including cancer, adenoma, and goiter, annually. The incidences of thyroid malignancies are three to four times higher in women, suggesting the possible involvement of estrogen. Based on this observed sex bias, we hypothesize that estrogen modulates the growth and metastatic propensity of thyroid cancer cells.
Methods
In this study, two thyroid cell lines (Nthy-ori 3-1 and BCPAP) were evaluated for the presence of estrogen receptor (ER) by Western blot analysis and estrogen responsiveness by using a cell proliferation assay. In addition, the effect of estradiol (E2) on modulation of metastatic phenotype was determined by using in vitro adhesion, migration, and invasion assays.
Results
Thyroid cells expressed a functionally active ER-α and ER-β as evidenced by 50–150% enhancement of proliferation in the presence of E2. E2 also enhanced adhesion, migration, and invasion of thyroid cells in an in vitro experimental model system that, based on our results, is modulated by β-catenin.
Conclusion
Our data provide evidence that the higher incidence of thyroid cancer in women is potentially attributed to the presence of a functional ER that participates in cellular processes contributing to enhanced mitogenic, migratory, and invasive properties of thyroid cells. These findings will enable and foster the possible development of antiestrogenic therapy targeting invasion and migration, thus affecting metastatic propensity.
Introduction
Thyroid cancer (TCa) is the most common and prevalent of all endocrine malignancies accounting for more than 95% of all endocrine-related cancers (1,2). Thyroid disorders that include cancer and goiter affect more than 27 million people in the United States alone, with 38,000 new cases diagnosed every year (2,3). Thirty percent of thyroid disorders having palpable nodules are malignant (4). Thyroid carcinomas are classified as papillary, follicular, anaplastic, or medullary (5), with papillary thyroid carcinoma accounting for more than 70% of all cases (6,7). Well-differentiated papillary thyroid carcinoma can metastasize to the lymph nodes of the neck in 50% of the patients (8,9).
According to American Thyroid Association, the incidences of thyroid proliferative diseases (TPD) are four to five times more in women than in men. The risk of developing thyroid disorders in women is one in eight, which is comparable to that of sporadic breast cancer in women (10–12). Pregnancy and early menopause increases the risk of TPD with a decrease in the incidences of thyroid malignancies after menopause (13). Abortions and notably recurrent abortions, reproductive challenges, and infertility have all been associated with thyroid hormone abnormalities (13–16). The higher incidence of thyroid disorders in women and several lines of correlative evidence for thyroid disorders with estrogen in the etiology of TPD warrant an examination of its precise role in laboratory-based experimental models.
Estrogens consist of a group of three biochemically distinct hormones, estrone, estradiol (E2), and estriol, which are produced naturally by the body and are metabolized into estrogen metabolites such as 2-hydroxyestrone (2-OHE1) and 16-alphahydroxyestrone (16-OHE1) (17,18). These estrogen metabolites have stronger (16-OHE1) or weaker (2-OHE1) estrogenic ability, and their relative concentration in a female body can influence the risk of a woman for breast, uterine, and other cancers (17–19). Estrogen signaling is mediated primarily by two isoforms of the estrogen receptor (ER), ER-α and ER-β, which intersperse with the pro-survival mitogen-activated protein kinase and extracellular signal-regulated kinases signal transduction pathway, presumably leading to cell growth and proliferation (20–22).
Several epidemiological studies have tried to correlate incidences of thyroid malignancies with hormones and other reproductive factors, but the precise contribution of estrogen in TPD initiation and progression and in determining the risk of TPD in women is not yet known. Since E2-mediated genotypic and phenotypic changes are increasingly being implicated in a variety of hormonally induced cancers, treatment and preventive strategies using novel antiestrogens are becoming the mainstay of cancer prevention. In this study, we present data to suggest that E2 modulates thyroid cell growth, adhesion, migration, and invasion and that these phenotypic changes are associated with functional ER interspersing with growth-regulating signal transduction pathways. Our studies implicate the possible role of antiestrogens in prevention and/or therapy for TPD.
Materials and Methods
Cell culture
Cell lines used in this study—Nthy-ori 3-1 (human normal transformed thyroid cell line) and BCPAP (human papillary TCa cell line)—were cultured in Rosswell Park Memorial Institute (RPMI)-1640 (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals, Lawrenceville, GA), penicillin 10,000 IU/mL, streptomycin 10,000 μg/mL (Mediatech), and 2 mM L-glutamine (Mediatech). Nthy-ori 3-1 was kindly gifted by Dr. Norman L. Eberhardt (Mayo Clinic, Rochester, MN). BCPAP was purchased from DSMZ (Braunschh, Germany). MCF-7 (human breast cancer cell line) was purchased from American Type Culture Collection (Manassas, VA) and cultured in Dulbeco's Modified Eagles Medium (DMEM) supplemented with 10% FBS, penicillin, streptomycin, and L-glutamine.
Cell proliferation assay
Nthy-ori 3-1, BCPAP, and MCF-7 were harvested using 0.25% trypsin (Mediatech) and seeded at a density of 1 × 105 cells per well in six-well culture dishes and allowed to adhere overnight. After approximately 16–18 hours, cells were washed with phosphate-buffered saline (PBS) and starved for 24 hours. The starvation medium was phenol-red-free medium with 10% charcoal dextran-treated FBS (Sigma Chemical, St. Louis, MO) and 10,000 IU/mL penicillin, as opposed to the normal growth medium that contains 10% FBS. Subsequently, cells were treated with 10−8 M E2 (Sigma Chemical) or left untreated as control. After 24 hours, cells were harvested and stained using 0.4% trypan blue solution (Sigma Chemical). The number of viable (unstained) and dead (stained) cells was counted using a hemocytometer, and the stimulation of cell growth by E2 was calculated as the increase in viable cell count for cells treated with E2 relative to control cells.
Western blot analysis
Cytoplasmic and nuclear protein extracts were prepared from untreated cells using NE-PER nuclear and cytoplasmic extraction reagent kit by Pierce (Rockford, IL). Manufacturer's protocol was followed to separate cytoplasmic and nuclear fractions. Whole cell lysates were prepared using 1 × 106 cells/100 μL of radioimmunoprecipitation assay buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.2% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 0.5% NP40, and 1 μM Pefabloc) and incubation on ice for 30 minutes with intermittent vortexing. The lysates were centrifuged at 14,000 rpm for 30 minutes at 4°C, and supernatants were collected. Cell lysates (15 μg protein) were subjected to 12% SDS–polyacrylamide gel electrophoresis under reducing conditions (presence of β-mercaptoethanol) as described earlier (23,24). Briefly, the proteins were transferred to Immobilon-P membranes at 220 mA for 2 hours, and membranes were blocked with 4% dried milk in Tris-Buffered Saline Tween-20 (TBST; 10 mM Tris-HCl, pH 7.5, 200 mM NaCl, 0.05% Tween-20) for at least 2–3 hours on a shaker at room temperature. Subsequently, the membrane was incubated overnight at 4°C with ER-α (Abcam, Cambridge, MA), ER-β (Santa Cruz Biotechnology, Santa Cruz, CA), α-catenin (Santa Cruz Biotechnology), β-catenin (Cell Signaling Technology, Danvers, MA), and actin (Santa Cruz Biotechnology) antibody (in TBS-T) on a shaker. Membranes were washed three times with TBS-T and incubated with respective secondary antibody for 2 hours at room temperature on a shaker. After four washes with TBS-T and one wash with TBS, membranes were developed by enhanced chemiluminescence (ECL; Pierce) and detected on X-ray film.
Coimmunoprecipitation studies
Cells were lysed using a modified lysis buffer (10 mM Tris-HCl [pH 7.4], 10 mM NaCl, 2 mM ethylenediaminetetraacetic acid, and 1% Triton X-100). ER-α and ER-β were immunoprecipitated from cell lysates (500 μg total protein) using 5 μg of either mouse anti-ER-α or rabbit anti-ER-β bound to 30 μL of GammaBind Plus Sepharose (Amersham Biosciences, Uppsala, Sweden) and incubated overnight at 4°C. Unbound proteins were removed by three washes with lysis buffer followed by three washes with PBS. Subsequently, the Protein G-Sepharose beads were resuspended in 50 μL of 1 × sample buffer, boiled for 10 minutes, and centrifuged. The supernatant was divided into three equal volume aliquots and loaded on a 12% SDS–polyacrylamide gel electrophoresis resolving gel. The proteins were transferred at 200 mA for 2 hours onto a polyvinylidene fluoride (PVDF) membrane and immunoblotted as previously described.
Cell adhesion assay
Nthy-ori 3-1, BCPAP, and MCF-7 cells were harvested as described and seeded at a density of 5 × 105 cells per well in six-well culture dishes. Cell culture medium was supplemented with 10−8 M E2 and/or 10−6 M fulvestrant or left untreated, and the cells were subsequently allowed to adhere for 2.5 hours. After indicated time points, the medium with nonadhered cells was discarded, and wells were gently washed twice with PBS to remove any loosely attached cells. Adherent cells were then scraped and counted using 0.4% trypan blue solution. Adherent cells were counted using a hemocytometer, and the stimulation of cell adhesion by E2 was expressed as percent increase in adherent cell count for cells treated with E2 relative to control cells.
Transwell migration assay
BD Biocoat Control Inserts (BD Biosciences, Bedford, MA) with 8-μm pore membrane filters were used for migration assay following the manufacturer's protocol. Briefly, cells were starved for 18 hours using the starvation medium (phenol-red-free medium supplemented with 10% charcoal stripped FBS and penicillin 10,000 IU/mL). After starvation, cells were harvested by trypsinization and cells (2.5 × 104 cells per well in 500 μL of 1% FBS) were loaded onto the upper chamber with or without 10−8 M E2 and 10−6 M fulvestrant, and 750 μL of the growth medium containing 5% FBS was loaded onto the bottom chamber. After 18 hours of incubation in a humidified tissue culture incubator at 37°C and 5% CO2, the nonmigrating cells were removed from the upper surface of the membrane by gently scrubbing using cotton tipped swab. Cells on the lower surface of the membrane were then fixed using methanol and then stained using 1% toluidine blue 1% borax stain followed by two washes with distilled water. Inserts were then allowed to air-dry and counted in 10 × field. Data are expressed as numbers of migrated cells per × 10 field micrograph for each sample well and normalized to cell counts obtained from the untreated control.
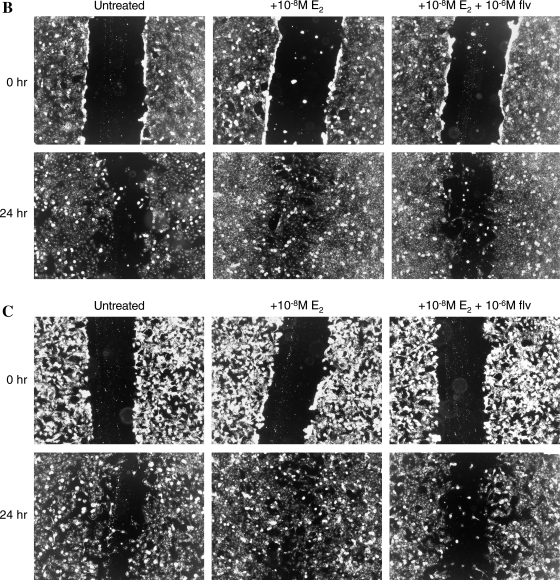
Scratch wound assay
Migratory ability of Nthy-ori 3-1 and BCPAP cells was also assessed by a scratch wound assay. About 5 × 105 cells were plated in a six-well plate and allowed to adhere and grow to semiconfluent cell monolayers. Subsequently, three vertical wounds were caused per well using a 2.5 μL sterile pipette tip followed by removal of any cellular debris and detached cells. The wounded cell monolayer was then incubated in fresh complete medium with or without E2 and fulvestrant. One horizontal line was made to allow observation of cells at the same point. The cells were inspected every 3 hours until the scratch cells fully migrated from one end of wound to other, and pictures were taken just above and below the horizontal mark using a light microscope at 5 × field.
Invasion assay
Invasion assay was carried out using BD Biocoat growth-factor-reduced Matrigel Invasion Chambers (BD Biosciences) with 8-μm pore membrane filters that were coated with Matrigel. The protocol was essentially the same as that of the migration assay except that growth-factor-reduced Matrigel invasion chambers were rehydrated for 2 hours using the serum-free RPMI medium at 37°C before loading cells onto inserts. Once rehydrated, 2.5 × 104 cells resuspended in the RPMI (500 μL) containing 1% FBS, and 10−8 M E2 and/or 10−6 M fulvestrant were carefully transferred onto the upper surface of filters in the chamber. Cells were allowed to invade for 18 hours, after which cells were stained and counted similarly as described for migration assay. Percent invasion was calculated by counting the number of cells invading through the growth-factor-reduced Matrigel invasion chambers in the experimental group (±E2 ± flv) relative to untreated controls. These calculations were based on the manufacturer's protocol (BD Biosciences).
Statistical calculation
Data presented are of three independent replicates, and the paired Student's t-test was used to assess statistical significance. A probability (p-value) ≤ 0.05 was used to reject the null hypothesis and considered statistically significant.
Results
Thyroid cells are estrogen responsive
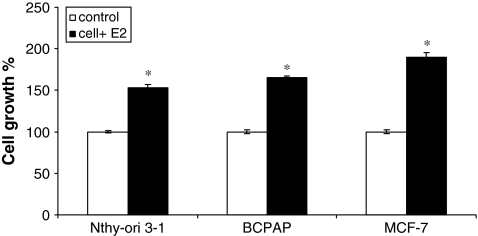
Estrogens induce proliferation in a wide variety of cancer cells. To determine whether thyroid cells are estrogen responsive or not, a papillary TCa cell line (BCPAP) and a normal thyroid cell line (Nthy-ori 3-1) were used. MCF-7, a classical estrogen-responsive breast cancer cell line, was used as a positive control. Figure 1 shows the effect of 17 β-E2 (the most abundant form of estrogen found in human body) on thyroid cells when cells were cultured under starvation conditions (phenol-red-free medium with 10% charcoal stripped serum) for 24 hours (white bars) and then stimulated with 10−8 M E2 (black bars). Cell viability was measured by the trypan blue exclusion dye method. We observed that removal of growth factors resulted in a quiescent state of thyroid cells, which was enhanced by addition of extraneous E2. The increase in cell growth 24 hours after E2 stimulation was 54% for Nthy-ori 3-1 and 65% for BCPAP, suggesting that thyroid cells are estrogen responsive and might be responsive to the growth regulatory cellular consequences of the E2–ER interaction.
FIG. 1.
Estrogen stimulates proliferation of thyroid cells. Effect of E2 on proliferation of Nthy-ori 3-1 and BCPAP was determined by the trypan blue dye exclusion assay. Cells were seeded at a density of 1 × 105 cells per well and allowed to adhere overnight. The starvation medium (10% charcoal-dextran-treated FBS) was then added for 24 hours, followed by stimulation with 10−8 M E2 for 24 hours. MCF-7, estrogen-responsive human breast cancer cells were used as a positive control. Cell growth is expressed as percent increase in viable cells + E2 (black bars) relative to untreated control (white bars). The asterisk denotes statistically significant increase (p < 0.05) in the experimental groups compared with controls. E2, estradiol; FBS, fetal bovine serum.
Thyroid cells express ER and ER is part of a multiprotein complex
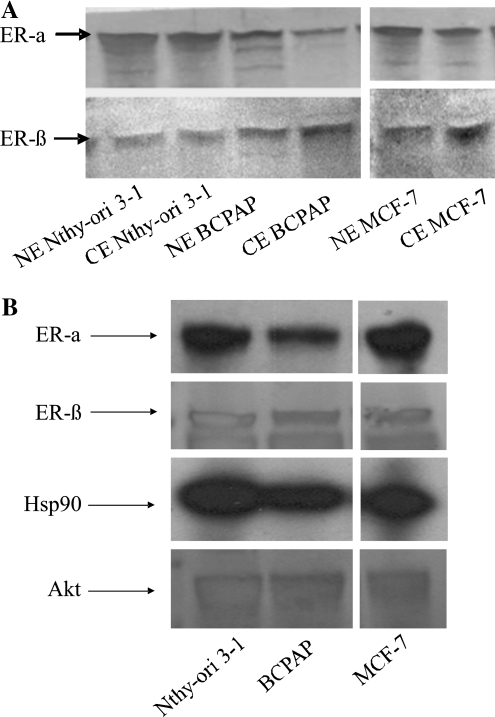
Thyroid cells are not known to act as traditional estrogen-responsive tissues such as breast. To determine the biochemical rationale for the estrogen responsiveness of these thyroid cells, Western blot analysis on both nuclear and cytoplasmic fractions of Nthy-ori 3-1 and BCPAP was performed. We observed that all the cell lines assayed expressed both nuclear and cytoplasmic form of ER-α and ER-β (Fig. 2A). MCF-7 was used as a positive control for detection of ER-α and ER-β. This suggests that these cells are presumably responsive to the E2–ER-mediated growth signaling pathway, and this is irrespective of the malignant phenotype as equivalent levels of ER was observed in both the cell lines examined.
FIG. 2.
(A) Thyroid cells express estrogen receptor (ER). Nuclear (NE) and cytoplasmic (CE) protein fractions (15 μg) were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and Western blot analysis, for ER-α (45 KDa) and ER-β (60 KDa), was performed. MCF-7, ER-positive cells were used as positive control. (B) ER is complexed with Akt and HSP90 in thyroid cells. Cell lysates were subjected to immunoprecipitation with antibodies to human ER-α and ER-β followed by Western blot analysis for ER-α, ER-β, HSP90, and Akt, respectively (top to bottom panel of immunoprecipitation and Western blot). MCF-7 (ER positive) was used as a positive control for detection of Hsp90 and Akt complex with ER.
By performing coimmunoprecipitation studies followed by Western blotting, ER was observed to be complexed with Hsp90 and Akt (Fig. 2B). When whole cell lysates were immunoprecipitated for ER-α or ER-β, we observed that along with ER, the proteins Akt and Hsp90 also get immunoprecipitated, suggesting that Akt, Hsp90, and ER are present as a multiprotein complex in these cells. The presence of this complex, similar to that of MCF-7, is an indication of existence of a functional ER assembly in thyroid cells. The detection of these complexes validated by estrogen responsiveness of thyroid cells as shown in Figure 1 is a testimony of a functional ER in thyroid cells.
Estrogen increases adhesion of thyroid cells
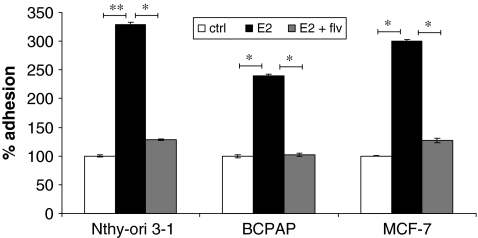
To metastasize to secondary sites, tumor cells have to adhere to the extracellular matrix (ECM) of secondary organs (25). We evaluated the effect of estrogen on adhesion of thyroid cells by performing an adhesion assay. About 5 × 105 cells (Nthy-ori 3-1, BCPAP, and MCF-7) were resuspended in a medium containing 10−8 M E2 and/or 10−6 M fulvestrant or left untreated and plated onto six-well culture dishes. After 2.5 hours adhered cells were counted and percent adhesion was calculated (Fig. 3). An increase in adhesion was observed when cells were treated with E2. The increase in adhesion with E2 was 137% for Nthy-ori 3-1 and 140% for BCPAP. Moreover, this increased ability of cells to adhere was inhibited by a classical ER antagonist, fulvestrant, suggesting that adhesion was an active phenotype possibly requiring functional ER activity.
FIG. 3.
Estrogen stimulates adhesion of thyroid cells. About 5 × 105 cells were plated in six-well culture dishes in the presence of ±10−8 M E2 ± fulvestrant. After 2.5 hours, the medium was removed, and wells were gently washed with phosphate-buffered saline to remove nonadherent cells. Viable adhered cells were removed by scraping and counted using trypan blue dye exclusion test. The groups are as follows: untreated (white bars), E2 treated (black bars), and E2 + fulvestrant treated (flv) (gray bars). MCF-7 was used as a positive control. E2-mediated increase in the number of adhesive cells expressed as % of control (100%). * denotes statistically significant differences (p < 0.05) between experimental and control groups. ** denotes statistically significant differences (p < 0.001) between experimental and control groups.
Estrogen increases cell migration
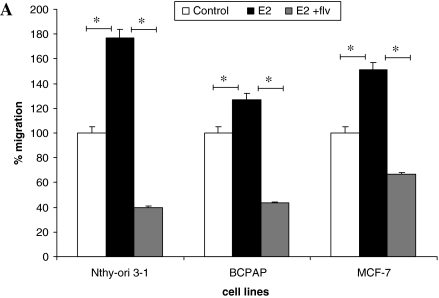
Tumor cells have an enhanced ability to migrate to neighboring tissue, and E2 has been observed to mobilize breast cancer cells, acting as a chemoattractant and stimulating them to migrate (26). Thyroid cells were starved and loaded on transwell migration chamber with E2 and/or fulvestrant and were allowed to migrate. We observed that the migratory ability of thyroid cells was enhanced with E2. This significant increase in migration (p < 0.05) was 76% for Nthy-ori 3-1 and 27% for BCPAP compared with control cells (Fig. 4A). This increase in cell migration was abrogated when fulvestrant was used, suggesting that the migratory ability of thyroid cells modulated by E2 requires ER, since this phenotype is ablated in the presence of the ER antagonist, fulvestrant.
FIG. 4.
Estrogen enhances the migration of thyroid cells. (A) About 2.5 × 104 cells were resuspended in 500 μL of Rosswell Park Memorial Institute (RPMI) with 1% FBS ± E2 and fulvestrant and seeded on upper chamber of BD Biocoat Control Inserts (8-μm pore membrane filters). Seven hundred and fifty microliters of Rosswell Park Memorial Institute (RPMI) containing 5% FBS was added to the bottom chamber as chemoattractant. After 18 hours, cells that migrated and adhered on the lower surface of the membrane were fixed, stained, and counted in 10 × field. The groups are as follows: untreated (white bars), E2 treated (black bars), and E2 + fulvestrant treated (gray bars). MCF-7 cells were used as a positive control for migration studies. Data are expressed as numbers of cells counted (migrated cells) per 10 × field micrograph for each sample well and normalized to the untreated control. The asterisk denotes statistically significant differences (p < 0.05) between experimental and control groups. (B) Scratch wound assay for Nthy-ori 3-1. (C) Scratch wound assay for BCPAP. About 5 × 105 cells were plated and were allowed to grow to semiconfluent cell monolayers when three vertical wounds were caused per well using a pipette tip, and the cells were allowed to migrate in the presence of E2 and/or fulvestrant. The cells were observed under 5 × field every 3 hours until the cells completely migrated from one end of scratch to other end.
A validation of the migration assay discussed above was obtained by performing the scratch wound assay (27,28). Thyroid cells (Nthy-ori 3-1 and BCPAP) were grown in a monolayer in six-well plates followed by formation of three vertical scratches per well using a 2.5 μL pipette tip. Cells were then incubated with ± E2 and/or fulvestrant. One horizontal line was made to allow us to approximately observe cells at the same point. Photographic documentation was obtained above and below the horizontal mark. We found that the thyroid cells (Nthy-ori 3-1 and BCPAP) migrated around 80–85% (as observed visually) in 24 hours in the presence of E2, suggesting that the migratory ability of these cells is stimulated by E2 (Fig. 4B, C). On the other hand, the migratory ability of these cells was abrogated in the presence of fulvestrant, further suggesting that estrogen-mediated stimulation of migration of thyroid cells requires a functional ER and as such these cells behave as classical estrogen-responsive cells.
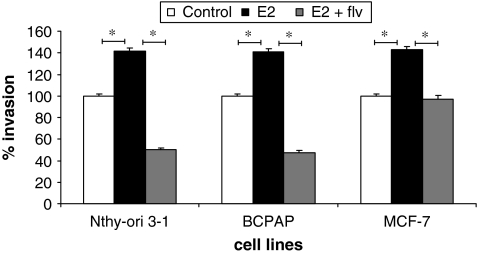
Thyroid cells gain invasiveness in response to estrogen
Formation of secondary metastatic foci by tumor cells requires invading the ECM of secondary organs. The invasive potential of Nthy-ori 3-1, BCPAP, and MCF-7 was assayed through the use of a transwell invasion chamber coated with biological matrix in vitro (Matrigel). We observed that when cells were treated with E2, cell invasion increased compared with control cells (untreated). This increase in invasion was approximately 40% for Nthy-ori 3-1 and BCPAP compared with control cells (normalized to 100% and p < 0.05). This increase in cell invasion was reversed when fulvestrant was used, suggesting that the estrogen plays an important role in increasing the invasive property of thyroid cells, whereas an ER antagonist can block the invasive properties (Fig. 5).
FIG. 5.
Estrogen enhances invasion of thyroid cells. Matrigel-coated invasion chambers (8-μm pore membrane filters) were used for invasion assay. About 2.5 × 104 cells per insert were resuspended in 500 μL medium containing 1% FBS ± 10−8 M E2 and/or 10−6 M fulvestrant were transferred onto the upper surface of filters in the chamber. Seven hundred and fifty microliters of growth medium containing 5% FBS was used as chemoattractant. Invasion was calculated based on the percent of cells invading through the growth-factor-reduced Matrigel invasion chambers relative to the cells migrating through control membrane after 18 hours when counted under 10 × field. The groups are as follows: untreated (white bars), E2 treated (black bars), and E2 + fulvestrant treated (gray bars). MCF-7 was used as a positive control for invasion. Data are represented as percent invasion (according to manufacturer's protocol), which is mean number of invaded cells per 10 × field micrograph for each sample well relative to the migration through the control membrane and normalized to the untreated control. * denotes statistically significant differences (p < 0.05) between experimental and control groups.
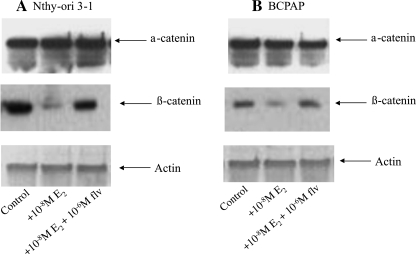
Estrodiol downregulates expression of β-catenin
E-cadherin–catenin complex is responsible for maintenance of cell adhesion and tissue integrity by forming a transmembrane complex with the actin cytoskeleton. Disruption of the integrity of this complex or decreased expression of one or all of its proteins has been shown to be correlative with progression of cancer proliferation and metastasis (29,30). To ascertain the molecular basis of increased in vitro metastatic phenotype of thyroid cells in the presence of E2, we analyzed protein expression of α-catenin and β-catenin by Western blot analysis in Nthy-ori 3-1 and BCPAP cells. The 24-hour treatment of thyroid cells with E2 caused a significant decrease in expression of β-catenin protein, with no change in expression of α-catenin (Fig. 6A, B). These observations are suggestive of a possible role of β-catenin in the migration and invasion of thyroid cells and can be a possible cellular marker of phenotypic change. The observed restoration of β-catenin levels in the presence of fulvestrant is again an indication of a possible interconnection of ER signaling and the β-catenin pathways.
FIG. 6.
E2 affects metastasis via downregulation of β-catenin. Nthy-ori 3-1 (A) and BCPAP (B) cells were treated with E2 ± fulvestrant. Whole cell protein (20 μg) was resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by Western blot analysis for α-catenin, β-catenin, and actin as a protein loading control.
Discussion
The incidence of differentiated TCa (papillary and follicular TCas) accounts for almost 90% of total TCas (3,5); because sizable numbers of patients suffer from secondary metastatic lesions, it is of importance to identify mediators of this phenomenon. With more than 27 million patients under treatment and an estimated 38,000 new patients this year, TCa is one of the fastest growing cancers in the United States (31) with more than 75% of patients being premenopausal women (2,13). This higher incidence of thyroid malignancies in premenopausal women suggests a possible role of estrogen. The higher levels of estrogen in women at onset of puberty, late menopause, or postmenopausal hormone replacement therapy have been repeatedly established as the cause for higher incidences of breast cancers in these women (32). The cancer-promoting properties of estrogen are mainly due to the binding of estrogen to its receptors, ER-α and ER-β, followed by binding to estrogen-responsive element resulting in transcription of several pro-survival pathways. This makes ER a key cellular and transcriptional regulator of proliferative mechanism of endocrine-related malignant disease (33,34). Although there is a noted sex bias in TCa, suggesting the possible involvement of estrogen, a correlation between estrogen and TCa has not been previously established.
A well-defined role of estrogen on cancer cells, such as breast cancer, is to induce proliferation (32,35,36). Several in vitro and in vivo studies have demonstrated that increased estrogen levels during puberty, menstrual cycles, and pregnancy not only regulate normal mammary gland growth and development but also can lead to enhanced risk of breast cancer in women (35–37). In contrast, few data exist that implicate the role of estrogen in thyroid cells. In one study, it was concluded that estrogen induces proliferation of rat thyroid follicular cells (38). More importantly, it was shown in a separate study that estrogen acts through mitogen-activated protein kinase pathway to induce growth of human thyroid tumor cells (39), but a correlation of estrogen and metastatic phenotype of thyroid cells is lacking. By using in vitro experimental models, we observed that thyroid cells Nthy-ori 3-1 and BCPAP are responsive to estrogen, as evidenced by their increased (50–150%) proliferation and expression of ERs, ER-α and ER-β, and that E2 affects its in vitro phenotypic characteristics.
Similar to MCF-7, a hormone-responsive breast cancer cell line, ER, was observed to be complexed with Hsp90 and Akt. The complex of Hsp90 and Akt with ER has major implications for nongenomic signaling. In the presence of ligand E2, Hsp90 dissociates, allowing ER to dimerize and induce gene expression. Akt, however, is now also free to manifest and participate in the signal transduction cascade. The presence of this complex in thyroid cells is a testimony of a functional ER assembly that can modulate cellular proliferation by both genomic and non genomic signaling when activated by the ligand E2.
Metastatic lesions are a complication arising in patients with TCa. Despite improved surgical and therapeutic processes, many TCa patients relapse, mainly due to secondary disseminated metastasis. Metastasis, a dynamic hallmark of cancer (40), consists of three essential events, adhesion, invasion, and migration, which have been observed to be modulated by such factors as estrogen in breast cancer (29,36,41). Thus, it is conceivable to hypothesize a role for estrogen in influencing the metastatic properties of TCa cells. Based on the fact that estrogen modulates adhesion, invasion, and migration in breast cancer, we wanted to determine whether the phenomenon exists in TCa. The most significant finding of our study and possibly of immense clinical significance is providing evidence for a link between estrogen and adhesion, invasion, and migration of thyroid cells. To metastasize throughout the body, cells must detach from the primary tumor and adhere to the ECM of a secondary organ. In our study, we observed that E2 increases the ability of thyroid cells to adhere dramatically (137–140%). This increase in adhesion was reverted in the presence of fulvestrant. When cells were allowed to adhere in the presence of E2 with fulvestrant, the adhesion was comparable to untreated cells.
In our study, the role of E2 on migration of TCa cells was investigated and was observed to increase the migration of cells (27–75%), which was abrogated in the presence of fulvestrant. To further test the migratory ability of these cells, a scratch wound assay was used as an experimental model for in vivo migration (28), though devoid of cell–cell interactions. E2 was able to induce the quick migration of thyroid cells in as early as 12 hours and fully completed by 24 hours. The ability of the thyroid cells to invade through Matrigel with E2 (38–40% increase) promoting the invasive ability of these cells is an intriguing finding of the present study.
One of the major findings of this study was the downregulation of tumor suppressive protein, β-catenin, in thyroid cells treated with E2. Downregulation of proteins associated with cadherin–catenin complex is correlated with increased cancer proliferation, adhesion, migration, and invasion in vitro and in vivo (29,30,42). Restitution of cadherin–catenin complex has been shown to suppress metastatic phenotype in several carcinomas, and our data using fulvestrant, where β-catenin levels are restored, may validate these studies. Nevertheless, a defective cadherin–catenin complex may not be the sole factor that determines the metastatic phenotype but may be a significant in vitro phenotypic marker for thyroid cells.
The in vitro adherence, invasiveness, and migration in response to E2 were found to be similar in normal transformed cells and cancer cells, presumably reflective of equivalent levels of ER in these cells. These observations are not paradoxical but are reflective of sequestered effects of E2–ER since antiestrogens completely abrogated our in vitro parameters of metastatic phenotype. In our earlier studies we have successfully demonstrated the abrogation of cellular and molecular effects of E2 by dietary antiestrogen 3,3′-diindolylmethane (24,43,44). Extrapolation of these observations to in vivo would be premature since in vivo metastases are multifactorially modulated and are a consequence of cell–cell interactions. Nevertheless, the primary role of E2 in modulating discrete events of metastasis initiation in thyroid cells is well demonstrated in our studies and that all of these cells have the propensity to be E2 modulated.
As a whole, our data suggest a strong link between estrogen and TCa cell proliferation and metastatic phenotype as evidenced by its effect on in vitro adhesion, migration, and invasion, but most significant was the ability of fulvestrant to inhibit any direct effect of estrogen on thyroid cells, thus allowing the possible development of an antiestrogenic therapy for thyroid malignancies that can target different stages of cancer progression such as cell proliferation, adhesion, invasion, and migration. In future studies, the cell-type specificities in modulating events leading to overt metastasis in in vivo models and the possible role of E2–ER and antiestrogens will be investigated. This will address a significant health problem in the United States and worldwide and lead to inhibition of the metastatic phenotype of TPD.
Acknowledgments
The studies were supported by grants from National Cancer Institute 1R01CA131946-01A2 and clinical funding from New York Eye and Ear Infirmary, New York, New York.
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Hodgson NC. Button J. Solorzano CC. Thyroid cancer: is the incidence still increasing? Ann Surg Oncol. 2004;11:1093–1097. doi: 10.1245/ASO.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A. Siegel R. Ward E. Hao Y. Xu J. Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Vasko VV. Saji M. Molecular mechanisms involved in differentiated thyroid cancer invasion and metastasis. Curr Opin Oncol. 2007;19:11–17. doi: 10.1097/CCO.0b013e328011ab86. [DOI] [PubMed] [Google Scholar]
- 4.Mackenzie EJ. Mortimer RH. Thyroid nodules and thyroid cancer. Med J Aust. 2004;180:242–247. doi: 10.5694/j.1326-5377.2004.tb05894.x. [DOI] [PubMed] [Google Scholar]
- 5.Kondo T. Ezzat S. Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer. 2006;6:292–306. doi: 10.1038/nrc1836. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgibbons SC. Brams DM. Wei JP. The treatment of thyroid cancer. Am Surg. 2008;74:389–399. [PubMed] [Google Scholar]
- 7.Kitamura Y. Shimizu K. Ito K. Tanaka S. Emi M. Allelotyping of follicular thyroid carcinoma: frequent allelic losses in chromosome arms 7q, 11p, and 22q. J Clin Endocrinol Metab. 2001;86:4268–4272. doi: 10.1210/jcem.86.9.7853. [DOI] [PubMed] [Google Scholar]
- 8.Nikiforov YE. Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol. 2008;21(Suppl 2):S37–S43. doi: 10.1038/modpathol.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suster S. Thyroid tumors with a follicular growth pattern: problems in differential diagnosis. Arch Pathol Lab Med. 2006;130:984–988. doi: 10.5858/2006-130-984-TTWAFG. [DOI] [PubMed] [Google Scholar]
- 10.Chen AY. Jemal A. Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115:3801–3807. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 11.Cook MB. Dawsey SM. Freedman ND. Inskip PD. Wichner SM. Quraishi SM. Devesa SS. McGlynn KA. Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomarkers Prev. 2009;18:1174–1182. doi: 10.1158/1055-9965.EPI-08-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellevik AI. Asvold BO. Bjøro T. Romundstad PR. Nilsen TI. Vatten LJ. Thyroid function and cancer risk: a prospective population study. Cancer Epidemiol Biomarkers Prev. 2009;18:570–574. doi: 10.1158/1055-9965.EPI-08-0911. [DOI] [PubMed] [Google Scholar]
- 13.Levi F. Franceschi S. Gulie C. Negri E. La Vecchia C. Female thyroid cancer: the role of reproductive and hormonal factors in Switzerland. Oncology. 1993;50:309–315. doi: 10.1159/000227201. [DOI] [PubMed] [Google Scholar]
- 14.Lazarus JH. Obuobie K. Thyroid disorders—an update. Postgrad Med J. 2000;76:529–536. doi: 10.1136/pmj.76.899.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cline AM. Kutteh WH. Is there a role of autoimmunity in implantation failure after in-vitro fertilization? Curr Opin Obstet Gynecol. 2009;21:291–295. doi: 10.1097/gco.0b013e3283294879. [DOI] [PubMed] [Google Scholar]
- 16.Poppe K. Velkeniers B. Glinoer D; Medscape. The role of thyroid autoimmunity in fertility and pregnancy. Nat Clin Pract Endocrinol Metab. 2008;4:394–405. doi: 10.1038/ncpendmet0846. [DOI] [PubMed] [Google Scholar]
- 17.Lord RS. Bongiovanni B. Bralley JA. Estrogen metabolism and the diet-cancer connection: rationale for assessing the ratio of urinary hydroxylated estrogen metabolites. Altern Med Rev. 2002;7:112–129. [PubMed] [Google Scholar]
- 18.Vijayanathan V. Venkiteswaran S. Nair SK. Verma A. Thomas TJ. Zhu BT. Thomas T. Physiologic levels of 2-methoxyestradiol interfere with nongenomic signaling of 17 beta-estradiol in human breast cancer cells. Clin Cancer Res. 2006;12:2038–2048. doi: 10.1158/1078-0432.CCR-05-2172. [DOI] [PubMed] [Google Scholar]
- 19.Tiwari RK. Guo L. Bradlow HL. Telang NT. Osborne MP. Selective responsiveness of human breast cancer cells to indole-3-carbinol, a chemopreventive agent. J Natl Cancer Inst. 1994;86:126–131. doi: 10.1093/jnci/86.2.126. [DOI] [PubMed] [Google Scholar]
- 20.Wang TT. Milner MJ. Milner JA. Kim YS. Estrogen receptor alpha as a target for indole-3-carbinol. J Nutr Biochem. 2006;17:659–664. doi: 10.1016/j.jnutbio.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 21.He YY. Cai B. Yang YX. Liu XL. Wan XP. Estrogenic G protein-coupled receptor 30 signaling is involved in regulation of endometrial carcinoma by promoting proliferation, invasion potential, and interleukin-6 secretion via the MEK/ERK mitogen-activated protein kinase pathway. Cancer Sci. 2009;100:1051–1061. doi: 10.1111/j.1349-7006.2009.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis-Wambi JS. Jordan VC. Estrogen regulation of apoptosis: how can one hormone stimulate and inhibit? Breast Cancer Res. 2009;11:1–10. doi: 10.1186/bcr2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suriano R. Ghosh SK. Ashok BT. Mittelman A. Chen Y. Banerjee A. Tiwari RK. Differences in glycosylation patterns of heat shock protein, gp96: implications for prostate cancer prevention. Cancer Res. 2005;65:6466–6475. doi: 10.1158/0008-5472.CAN-04-4639. [DOI] [PubMed] [Google Scholar]
- 24.Garikapaty VP. Ashok BT. Tadi K. Mittelman A. Tiwari RK. 3,3′-Diindolylmethane downregulates pro-survival pathway in hormone independent prostate cancer. Biochem Biophys Res Commun. 2006;340:718–725. doi: 10.1016/j.bbrc.2005.12.059. [DOI] [PubMed] [Google Scholar]
- 25.Gupta GP. Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Suriano R. Chaudhuri D. Johnson RS. Lambers E. Ashok BT. Kishore R. Tiwari RK. 17β-estradiol mobilizes bone marrow–derived endothelial progenitor cells to tumors. Cancer Res. 2008;68:6038–6042. doi: 10.1158/0008-5472.CAN-08-1009. [DOI] [PubMed] [Google Scholar]
- 27.Meng Q. Qi M. Chen DZ. Yuan R. Goldberg ID. Rosen EM. Auborn K. Fan S. Suppression of breast cancer invasion and migration by indole-3-carbinol: associated with up-regulation of BRCA1 and E-cadherin/catenin complexes. J Mol Med. 2000;78:155–165. doi: 10.1007/s001090000088. [DOI] [PubMed] [Google Scholar]
- 28.Liang CC. Park AY. Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 29.Baranwal S. Alahari SK. Molecular mechanisms controlling E-cadherin expression in breast cancer. Biochem Biophys Res Commun. 2009;384:6–11. doi: 10.1016/j.bbrc.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng GZ. Zhang W. Wang LH. Regulation of cancer cell survival, migration, and invasion by Twist: AKT2 comes to interplay. Cancer Res. 2008;68:957–960. doi: 10.1158/0008-5472.CAN-07-5067. [DOI] [PubMed] [Google Scholar]
- 31.Scott AM. Thyroid cancer in adults. Radiol Technol. 2009;80:241–261. [PubMed] [Google Scholar]
- 32.Giretti MS. Fu XD. De Rosa G. Sarotto I. Baldacci C. Garibaldi S. Mannella P. Biglia N. Sismondi P. Genazzani AR. Simoncini T. Extra-nuclear signalling of estrogen receptor to breast cancer cytoskeletal remodelling, migration and invasion. PLoS One. 2008;3:1–6. doi: 10.1371/journal.pone.0002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Payne SJ. Bowen RL. Jones JL. Wells CA. Predictive markers in breast cancer—the present. Histopathology. 2008;52:82–90. doi: 10.1111/j.1365-2559.2007.02897.x. [DOI] [PubMed] [Google Scholar]
- 34.Rajoria S. Parmar P. Schantz S. Schaefer S. Chaudhuri D. Tiwari RK. Suriano R. Significance of estrogen receptor in thyroid cancer: molecular link of epidemiologic observations. Abstract presented at the Proceedings of the 99th Annual Meeting of the American Association for Cancer Research; San Diego, CA. Apr 12–16;2008 ; 2008. Abstract no. 4254. [Google Scholar]
- 35.Conner P. Lundström E. von Schoultz B. Breast cancer and hormonal therapy. Clin Obstet Gynecol. 2008;51:592–606. doi: 10.1097/GRF.0b013e318180b8ed. [DOI] [PubMed] [Google Scholar]
- 36.Malek D. Gust R. Kleuser B. 17-β-estradiol inhibits transforming-growth-factor-β-induced MCF-7 cell migration by Smad3-repression. Eur J Pharmacol. 2006;534:39–47. doi: 10.1016/j.ejphar.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 37.Colditz GA. Hormone replacement therapy increases the risk of breast cancer. Ann N Y Acad Sci. 1997;833:129–136. doi: 10.1111/j.1749-6632.1997.tb48598.x. [DOI] [PubMed] [Google Scholar]
- 38.Furlanetto TW. Nguyen LQ. Jameson JL. Estradiol increases proliferation and down-regulates the sodium/iodide symporter gene in FRTL-5 cells. Endocrinology. 1999;140:5705–5711. doi: 10.1210/endo.140.12.7197. [DOI] [PubMed] [Google Scholar]
- 39.Manole D. Schildknecht B. Gosnell B. Adams E. Derwahl M. Estrogen promotes growth of human thyroid tumor cells by different molecular mechanisms. J Clin Endocrinol Metab. 2001;86:1072–1077. doi: 10.1210/jcem.86.3.7283. [DOI] [PubMed] [Google Scholar]
- 40.Hanahan D. Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 41.Planas-Silva MD. Waltz PK. Estrogen promotes reversible epithelial-to-mesenchymal-like transition and collective motility in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 2007;104:11–21. doi: 10.1016/j.jsbmb.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 42.Kalluri R. Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tadi K. Chang Y. Ashok BT. Chen Y. Moscatello A. Schaefer SD. Schantz SP. Policastro AJ. Geliebter J. Tiwari RK. 3,3'-Diindolylmethane, a cruciferous vegetable derived synthetic anti-proliferative compound in thyroid disease. Biochem Biophys Res Commun. 2005;337:1019–1025. doi: 10.1016/j.bbrc.2005.09.143. [DOI] [PubMed] [Google Scholar]
- 44.Rajoria S. Wilson YL. Megwalu U. Schantz S. Schaefer S. Geliebter J. Suriano R. Tiwari RK. Role of estrogen in TPD: antiestrogens as novel therapeutic agents. Abstract present at the Proceedings of the 100th Annual Meeting of the American Association for Cancer Research; Denver, CO. Apr 18–22;2009 ; 2009. p. 361. Abstract no. 3907. [Google Scholar]