Abstract
Introduction and objectives
Prostatic inflammation could be a key component in prostate enlargement and benign prostatic hyperplasia (BPH) progression. Our aim was to characterize inflammatory cells infiltrate within BPH tissue and to correlate inflammation and clinical data.
Material and methods
Inflammation was profiled on three clinical outcome tissue microarrays (TMAs), including 282 patients treated by surgery for a complicated and/or symptomatic BPH. Inflammation score was defined by combining six cytological parameters and 5 markers on immunohistochemistry (IHC). Cytological parameters were lymphocytes, macrophages and polynuclears leukocytes infiltrates, and three glandular aspect modifications: glandular atrophy, glandular destruction and tissue fibrosis. IHC markers were CD3, CD4 and CD8 decorating T-lymphocytes, CD20 decorating B-lymphocytes, and CD163 decorating macrophages.
Results
The majority of patients had inflammatory cells infiltrating BPH tissues: 81% had T-lymphocytes markers (CD3), 52% had B-lymphocytes markers (CD20) and 82% had macrophages markers (CD163). IPSS score (21 vs 12; p=0,02) and prostate volume (77cc vs 62cc; p=0.002) were significantly higher in patients with high grade prostatic inflammation.
Conclusion
We characterized inflammatory cells infiltrate in a large cohort of surgically treated BPH specimens. The role of inflammation in BPH development was highlighted by the strong correlation between histological inflammation, IPSS and prostate volume. Prostate enlargement due to chronic inflammatory process may progressively conduce to BPH progression. Therefore, inflammation is a therapeutic target for BPH.
Keywords: Aged; Aged, 80 and over; Antigens, CD; metabolism; Antigens, CD20; metabolism; Antigens, CD3; metabolism; Antigens, Differentiation, Myelomonocytic; metabolism; B-Lymphocytes; immunology; Biological Markers; metabolism; Cohort Studies; Disease Progression; Humans; Immunohistochemistry; Macrophages; immunology; Male; Microarray Analysis; Middle Aged; Prostatic Hyperplasia; complications; pathology; Prostatitis; complications; metabolism; pathology; Receptors, Cell Surface; metabolism; Risk Assessment; T-Lymphocytes; immunology
Keywords: Benign prostatic hyperplasia, BPH, inflammation, CD3, CD4, CD8, CD20, CD163, lymphocyte
Introduction
Benign prostatic hyperplasia (BPH) assigns most of men after the age of fifty and represents the most common urologic disease among elderly males 1. BPH is histologically defined as an overgrowth of the epithelial and stromal cells from the transition zone and peri-urethral area. Incidence of histological BPH could be over 70% at 60 years old and over 90% at 70 years old 2. But histological BPH doesn’t systematically lead to clinical manifestations. BPH symptoms can range over a wide scale from minimal bother to urinary retention and renal failure. To date, we still have no precise knowledge of the cellular and molecular processes underlying the pathogenesis of BPH and leading to a symptomatic disease 3. Although the influence of androgens and estrogens has been demonstrated, hormonal factors alone may not fully explain BPH development.
There are some evidences that prostatic inflammation could be a key component in prostate enlargement and BPH progression. Two of the major clinical studies on BPH (MTOPS and Reduce study) recently demonstrated a link between histological prostatic inflammation and prostate enlargement or symptoms scores 4, 5. Numerous of the major key players in chronic inflammation have been studied in BPH: varieties of growth factors and cytokines have been shown to be involved both in the inflammatory process and in the epithelial/stromal prostatic cells interactions 6. These mediators are released in the prostatic gland by inflammatory cells that can be found on most of the surgery-derived BPH specimens 7.
Our aim was to characterize inflammatory cells infiltrate within BPH tissue and to correlate inflammation and clinical parameters in order to evaluate the BPH progression/complication risks in patients with prostatic inflammation.
Material and methods
BPH Samples
Inflammation was evaluated on three clinical outcome tissue microarrays (TMAs), including 282 patients treated by surgery for BPH. TMAs contained four cores per patient: 2 from predominantly glandular zone and 2 from stromal area. Prostate tissue was obtained after written consent from each patient and was approved by the local ethics committee. Mean age of patients was 70 (range 50–91). Surgical procedures were open prostatectomy in 106 patients and transurethral resection of the prostate (TURP) in 176 patients. In 22 cases, the procedure was a second TURP for persistent and bothersome BPH symptoms. The first TURP was performed at least one year earlier than the second one. Out of 282 patients, 250 had symptomatic BPH. Thirty two patients underwent BPH surgery for a diagnosis purpose: they had a suspicion of prostate cancer on the basis of prostate specific antigen (PSA) serum level, but they did not have LUTS requiring surgery. Eighty seven patients had a history of acute urinary retention. In 13 cases, prostate cancer was associated to BPH within the prostate. For them, prostate cancer was not represented on TMA. Mean preoperative PSA was 10.1 ng/ml (range 0.1–43). Prostate volume was calculated on transrectal ultrasonography. Mean prostate volume was 69 ml (range 20–220, median 60ml). Medical BPH treatment was considered when received by patients for at least 3 months before surgery. This treatment was 5 alpha reductase inhibitor (5-ARI) alone in 9 patients, alpha blocker therapy in 67 patients, phytotherapy in 10 patients, and 5-ARI/alpha blocker association in 10 patients. Eighty four patients had a history of negative prostate biopsies performed in the month prior surgery to eliminate a diagnosis of prostate cancer. Preoperative urine analysis and culture were sterile in all cases.
Immunohistochemistry (IHC)
Five markers were evaluated by IHC using antibodies (Ab) decorating T-lymphocytes, CD3 (rabbit polyclonal Ab A0452; antigen retrieval pH6; 1/300; Dako), CD4 (mouse monoclonal Ab clone 4B12; antigen retrieval EDTA pH8; 1/20; Novocastra-Menarini) and CD8 (mouse monoclonal Ab clone C8/144B; antigen retrieval pH9; 1/200; Dako), B-lymphocyes, CD20 (mouse monoclonal Ab clone L26; antigen retrieval pH6; 1/500; Dako), and macrophages, CD163 (mouse monoclonal Ab clone 10D6; antigen retrieval pH6; 1/800; Novocastra-Menarini). After antigen retrieval, the sections were processed on an automated instrument (Ventana nexes; Ventana Medical System, Paris, France) for immunostaining using an indirect biotin avidin system, the Ventana Basic 3,3′-diaminobenzidine detection kit (Ventana Medical System) according to the manufacturer’s instructions.
CD3, CD4, CD8 and CD20 antibodies are commonly used for the characterization of immune cells in tissue samples. The plasma membrane glycoprotein receptor CD163 is a member of the scavenger receptor cystein-rich family class B. It is involved in the innate immune response and expressed on most subpopulations of mature tissue macrophages 8.
Scoring of inflammation
Six cytological parameters were evaluated on TMAs including the type of immune cells (lymphocytes, macrophages and polynuclears leukocytes) and three modifications of glandular aspect: glandular atrophy, glandular destruction and tissue fibrosis. Modifications of glandular aspect were studied because they are known to be associated with chronic inflammatory process9.
Cytological and IHC parameters were separately quoted by two independent observers including one senior uropathologist (GR, YA). The presence or absence of macrophages, polynuclears leukocytes, atrophy, destruction, and fibrosis, was quoted using binary scores (0/1). The presence of lymphocytes and other IHC markers was quoted as absent, low, or high (0/1/2). When a difference was noticed between the two independent scores a third analysis was performed and was taken into account for the statistical analysis.
Statistical analysis
Individual patient’s inflammation score was obtained from assessing the maximal grade of all valid cores from the same patient. At least one valid core per patient was required.
Then, the 6 individual cytological grading (lymphocyte, macrophages, polynuclears leukocytes, atrophy, destruction, and fibrosis) were computed to make a “cytological score”. The five individual IHC grading (CD3, CD4, CD8, CD20, CD163) were computed to make an “IHC score”. Cytological and IHC scores were compared to clinical data using a non parametric correlation test, the Kendalls’tau-b test. Commercially available software was used for statistical analyses (SPSS inc., Chicago, II.).
Results
Seven cases on the TMA were not assessed because there was no valid core on TMAs. Inflammation was measured in the remaining 275 patients with a mean valid core per patient of 3.5.
Composition of the BPH inflammatory infiltrate
As shown on Figure 1, BPH inflammatory infiltrate was mostly constituted by T-lymphocytes (CD3 positive cells). T-lymphocytes were more frequently observed than other inflammatory cells: two third had CD8 expression and one third had CD4 expression.
Figure 1.
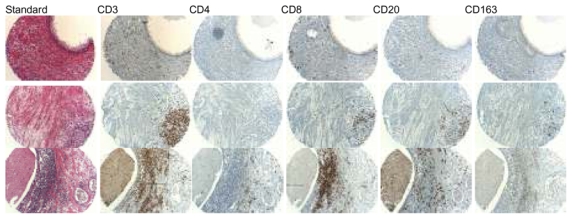
Standard coloration and immunostaining with CD3, CD4, CD8, CD20 and CD163 antibodies on different BPH samples from 3 surgery-derived BPH specimens: from low to high grade inflammation
Results of cytological and IHC scores are presented in tables 1 and 2. On cytological examination, 212 (77%) cases had a lymphocyte infiltrate. When focusing on IHC T-lymphocyte markers, CD8, CD3, and CD4 were expressed by 224 (81%), 218 (79%), and 108 (39%) patients, respectively. CD20, an IHC marker of B-lymphocytes was expressed by 142 (52%) patients. On cytology, 121 (44%) patients had a macrophage infiltrate, whereas CD 163, a macrophage IHC marker, was expressed by 226 (82%) patients. Polynuclear leukocyte infiltrate was observed in 15 (5%) patients. Finally, atrophy, destruction, and fibrosis were observed in 60 (22%), 21 (8%), 42 (15%) patients, respectively.
Table 1.
Description of the inflammatory infiltrate in 275 patients using cytological or IHC grading
| Cytological inflammation grading | IHC inflammation grading | ||||
|---|---|---|---|---|---|
| Grade | n | Grade | N | ||
| lymphocyte | 0 | 63 | CD3 | 0 | 59 |
| 1 | 128 | 1 | 117 | ||
| 2 | 84 | 2 | 101 | ||
| macrophage | 0 | 154 | CD4 | 0 | 169 |
| 1 | 121 | 1 | 96 | ||
| polynuclear | 0 | 260 | 2 | 12 | |
| 1 | 15 | CD8 | 0 | 54 | |
| atrophy | 0 | 215 | 1 | 135 | |
| 1 | 60 | 2 | 89 | ||
| destruction | 0 | 254 | CD20 | 0 | 133 |
| 1 | 21 | 1 | 85 | ||
| fibrosis | 0 | 233 | 2 | 57 | |
| 1 | 42 | CD163 | 0 | 51 | |
| 1 | 154 | ||||
| 2 | 72 | ||||
IHC: immunohistochemistry
Table 2.
Inflammation scores in 275 patients
| Cytological inflammation score | IHC inflammation score | ||
|---|---|---|---|
| Grade | n | Grade | n |
| 0 | 47 | 0 | 14 |
| 1 | 70 | 1 | 16 |
| 2 | 102 | 2 | 31 |
| 3 | 45 | 3 | 40 |
| 4 | 13 | 4 | 41 |
| 5 | 2 | 5 | 39 |
| 6 | 27 | ||
| 7 | 32 | ||
| 8 | 19 | ||
| 9 | 11 | ||
| 10 | 5 | ||
IHC: immunohistochemistry
Frequencies of IHC phenotypes are summarized in table 3. Four phenotypes were displayed by more than 10% of patients. All of them included CD8 expression.
Table 3.
Frequencies of inflammatory phenotypes expressed by 275 patients on IHC
| CD4 | CD8 | CD20 | CD163 | n (%) |
|---|---|---|---|---|
| + | + | + | + | 61 (22.2) |
| − | + | + | − | 57 (20.7) |
| − | + | − | − | 42 (15.3) |
| + | + | − | + | 28 (10.2) |
| − | − | − | + | 23 (8.4) |
| − | − | − | − | 14 (5.1) |
| − | + | − | − | 14 (5.1) |
| − | − | + | + | 9 (3.3) |
| − | + | + | + | 8 (2.9) |
| + | + | − | − | 7 (2.5) |
| + | + | + | − | 5 (1.8) |
| + | − | − | + | 3 (1.1) |
| + | − | − | − | 2 (0.7) |
| + | − | + | + | 2 (0.7) |
IHC: immunohistochemistry; (−): absence of expression; (+): presence of expression
Association between inflammation and clinical data
Clinical data was compared between patients with low and high grade inflammation (table 4). The median values of inflammation scores were used as thresholds to differentiate low from high grade inflammation patients. Prostate volume was significantly higher in the high grade inflammation group compared to the low one (77cc vs 62cc; p=0.002). Similarly, type of surgery was associated with inflammation: patients with high grade inflammation were more likely to be operated by open prostatectomy than those with low grade (62% vs 43%; p=0.001). As patients operated by open prostatectomy usually have higher prostate volume, the association between inflammation and type of surgery can be considered to be due to the one between inflammation and prostate volume.
Table 4.
Comparison of clinical parameters between patients with low and high grade inflammation
| All patients | Cytological score | IHC score | |||||
|---|---|---|---|---|---|---|---|
| n=275 | Low n=215 | High n=60 | p | Low n=142 | High n=133 | p | |
| Quantitative parameter (Student T-test): mean | |||||||
| Age (year) | 70 | 70 | 71 | 0.16 | 70 | 70 | 0.9 |
| PSA (ng/ml) | 10.1 | 9.9 | 11.4 | 0.6 | 9.8 | 10.6 | 0.7 |
| Prostate volume (cc) | 69 | 65 | 85 | 0.001 | 64 | 75 | 0.02 |
| IPSS | 14 | 13 | 15 | 0.8 | 12 | 21 | 0.02 |
| Qualitative parameters (Chi-square test): n (%) | |||||||
| Open Prostatectomy | 105 (38.2) | 73 (34.0) | 32 (53.3) | 0.010 | 42 (29.6) | 63 (47.4) | 0.003 |
| Second TURP | 22 (8.0) | 19 (8.8) | 3 (5.0) | 0.4 | 14 (9.9) | 8 (6.0) | 0.3 |
| No LUTS | 31 (11.3) | 21 (9.8) | 10 (16.7) | 0,2 | 16 (11.3) | 15 (11.3) | 1,0 |
| Associated PCa | 12 (4.4) | 7 (3.3) | 5 (8.3) | 0.1 | 7 (4.9) | 5 (3.8) | 0.8 |
| History of biopsies | 84 (30.5) | 66 (30.7) | 18 (30.0) | 1.0 | 34 (23.9) | 50 (37.6) | 0.018 |
| History of AUR | 87 (31.6) | 70 (32.6) | 17 (28.3) | 0.6 | 49 (34.5) | 38 (28.6) | 0.3 |
| Phytotherapy | 10 (3.6) | 8 (3.7) | 2 (3.3) | 1.0 | 7 (4.9) | 3 (2.3) | 0.3 |
| 5-ARI | 19 (6.9) | 13 (6.0) | 6 (10.0) | 0.3 | 12 (8.5) | 7 (5.3) | 0.3 |
| α-blocker therapy | 67 (24.4) | 54 (25.1) | 13 (21.7) | 0.7 | 32 (22.5) | 35 (26.3) | 0.5 |
IHC: immunohistochemistry; TURP: trans uretral resection of the prostate; PCa: prostate cancer; LUTS: lower urinary tract symptoms; AUR: acute urinary retention; 5-ARI: 5 alpha reductase inhibitor
Finally, history of previous prostate biopsy was more frequent (37.6% vs 23.9%; p=0.018) and IPSS score was higher (21 vs 12; p=0.02) in the high grade IHC inflammation group compared to the low one.
Discussion
Inflammatory cells infiltrate in BPH tissue
We studied inflammation cells infiltrate in a large cohort of BPH patients requiring surgical treatment. Using cytological analysis and IHC inflammation markers, we characterized the inflammation cells infiltrate.
Most of surgery-derived BPH specimens are known to contain inflammatory infiltrates at pathological examination 7, 10. According to Theyer et al works, prostatic inflammatory infiltrate may be composed of a majority of T-lymphocytes (up to 70%) and of about 30% antigen-presenting cells (15% B-lymphocytes and 15% macrophages) 11. They also found that the CD4/CD8 ratio in BPH samples was reversed compared with normal prostate and stated that CD4-lymphocytes (memory T-helper cells) may represent up to 60% of T-cells infiltrate in BPH tissues. More recently Di Carlo et al described in detail the composition of the prostate-associated lymphoid tissue in normal prostate 12. They found that it was containing two main components: one with a majority of T-lymphocytes infiltrating the peri-glandular area, with up to 70% CD8 cells, and another one with lymphoid aggregates lying in the fibro-muscular stroma with B-lymphocyte follicules representing 50% of the hole aggregate surrounded by parafollicular T-lymphocytes with about twice as much CD4 than CD8 cells.
In our cohort, almost 80% of patients had a significant T-lymphocyte infiltrate with a predominance of CD8 expression. T-lymphocytes were more numerous than other inflammatory cells and were representing the major part of the inflammatory infiltrate observed in the prostatic gland. To build the TMA, four cores per patients were performed including 2 from predominantly glandular zone and 2 from stromal area. Thus, glandular and stromal areas are equally represented on the TMA which is not reflecting usual glandular/stromal ratio in the BPH transition zone. This might be an explanation for the unusual CD8/CD4 ratio in our study: CD8 cells are more frequently observed in the peri-glandular area and in the normal prostate tissue than in the lymphoid aggregates. When focusing on lymphoid aggregates observed on TMA cores, we also found a higher rate of CD4 cells (cf. figure 1, patient 3).
Smaller amounts of B-cells and macrophages were observed in respectively 52% and 82% of patients. Macrophages and B-lymphocytes are antigen-presenting cells that play a role in the first step of the inane inflammatory response. They may be stimulated directly by infectious agents or by prostatic epithelial cells themselves 6. Then, numerous cytokines and growth factors involved in the inflammatory response (IL-17, IFN-γ, TGF-β, FGF-2…) are secreted and stimulate growth of epithelial and stromal prostatic cells 13, 14. This immune response against possible infectious agents finally results in prostatic enlargement.
CD3 is expressed by any type of T-lymphocytes. Lymphocyte infiltrate quantification was almost similar using cytological examination method, and IHC method with CD3 marker (77% and 79%, respectively). In contrary, macrophage infiltrates was found much higher using an IHC method with CD 163 marker than with a cytological way (82% versus 44%).
Since they were present in 5% of patients only, polynuclear leukocytes might not play an important role in BPH development. Significant glandular changes such as atrophy, destruction and fibrosis were observed in a minority of cases. It is known that in the transition zone chronic cellular infiltrates are often associated with glandular atrophy. Once again, our TMA was build with equal number of glandular and stromal cores and we didn’t analyse the entire BPH transition zone. This is an explanation for the lower quality of the glandular architecture analysis in this study. Nonetheless, the statistical analysis highlighted an interesting link between glandular atrophy and T-lymphocyte infiltration of the prostatic gland (p<0.01). Therefore, we can suppose that chronic BPH inflammation might lead to significant glandular architecture changes.
BPH inflammation and clinical data
Prostate volume is known to have a real impact on BPH: baseline prostate size can be considered as a strong indicator of BPH progression, particularly for acute urinary retention and BPH-related surgery but also for long-term changes in symptoms, bother, quality of life, and flow rate 10. Two of the most important multicentric studies on BPH found a link between inflammation and prostate volume. MTOPS study demonstrated this link on the base of prostate biopsies 4. Authors were reporting 45% chronic inflammation and 3% acute inflammation in a large cohort of BPH patients (n=1197) treated with 5-ARI. They found an association between prostatic inflammation and higher prostate volume (41.1 vs 36.8cc; p= 0.0002), higher risk of urinary retention (5.6% vs 0%; p=0.003) or higher risk of symptomatic evolution (21% vs 13.2%; p=0.083). More recently these results were confirmed by the Reduce study 5. Authors were reporting 77.6% chronic inflammation on the base of prostate biopsies in a large cohort of patients (n=8824). Inflammation was associated with higher prostate volume (46.5 vs 43.4cc; p<0.0001), higher IPSS score (8.8 vs 8.2; p<0.0001) and higher iritative IPSS subscore (4.3 vs 4.1; p<0.0001).
Similarly, in our study, patients with high grade inflammation had higher IPSS score (21 vs 12) and higher prostate volume (77cc vs 62cc) than patients with low grade inflammation. Patients with high grade inflammation were also more likely to be operated by open prostatectomy (62% vs 43%). As patients operated by open prostatectomy had a higher average prostate volume than those operated by TURP (96cc vs 47cc; p<0.001 Student T-test), the association between inflammation and type of surgery was considered to be due to the one between inflammation and prostate volume.
Finally, patients with high grade inflammation had more frequently undergone transrectal ultrasound guided biopsies than those with low grade inflammation (37.6% vs 23.9%). Transrectal biopsies are known to be responsible for a bacteriological contamination of the prostatic gland. This bacteriological contamination is responsible for approximately 5% acute prostatitis but may also result in an important local immune response. Inflammatory cells may therefore remain detectable for a long time after transrectal biopsies of the prostatic gland.
It is quite surprising that half of patients operated for a diagnosis purpose had high grade prostatic inflammation while they had no LUTS (table 4). This is probably due to the fact that most of them had undergone at least 2 previous biopsy series before TURP which may have induced inflammation within the prostate.
Contrarily to MTOPS and Reduce studies, no association between BPH inflammation and urinary retention risk was found. This may be explained by the fact that the population we studied was different from the ones of other studies. Indeed, except 31 cases operated for a diagnosis purpose, patients included in our IHC analysis had symptomatic or complicated disease requiring surgical treatment. In MTOPS and reduce studies, patients had BPH symptoms not requiring surgical treatment.
Inflammation as a target for medical therapy
As prostatic inflammation is associated to prostate volume, it might be a therapeutic target in BPH. This was already investigated by others. Di Silverio et al studied the effects of 5-ARI and COX-2-inhibitor combination therapy. Patients taking the combination therapy had a significant increase in the apoptotic index compared to patients treated with 5-ARI alone 15. Unfortunately, this study was stopped because of cardio-vascular potential side effects of the combination therapy. In the MTOPS study, the use of 5-ARI was found to be more efficient in patients with prostatic inflammation 4. Vela Navarette et al studied the effects of serenoa repens phytotherapy on prostate inflammatory status16. They found a significant reduction in the number of lymphocytes B (58.2 vs 91.4; p=0.097) and other inflammatory markers (TNFα and IL-1β) after treatment. According to the authors, anti-inflammatory effect of serenoa repens phytotherapy could be mediated by the down-regulation of Leukotrienne B417.
In our study, no association was found with the type of medical therapy applied to the patient and severity of prostatic inflammation. As almost all patients included in our analysis required surgery, medical therapy they were receiving can be considered as inefficient. At this stage of the disease, inflammatory process may not be controlled by any medical therapy. Same treatment given to patients with a less severe disease not requiring surgery may modify inflammation infiltrate within BPH tissue.
Conclusion
Inflammatory cell infiltrate was evaluated in a large cohort of surgically treated BPH patients. CD8 T-lymphocytes and macrophages infiltrate were present in more than 80% of cases. The role of inflammation in BPH development was highlighted by the strong correlation between histological inflammation and IPSS or prostate volume. Prostate enlargement due to chronic inflammatory process may progressively conduce to BPH progression. Therefore, some drugs with anti-inflammatory effects could be used in the management of BPH.
Acknowledgments
This study was granted by Pierre Fabre Medicament
References
- 1.Garraway WM, Collins GN, Lee RJ. High prevalence of benign prostatic hypertrophy in the community. Lancet. 1991;338:469. doi: 10.1016/0140-6736(91)90543-x. [DOI] [PubMed] [Google Scholar]
- 2.Carter HB, Coffey DS. The prostate: an increasing medical problem. Prostate. 1990;16:39. doi: 10.1002/pros.2990160105. [DOI] [PubMed] [Google Scholar]
- 3.Lee KL, Peehl DM. Molecular and cellular pathogenesis of benign prostatic hyperplasia. J Urol. 2004;172:1784. doi: 10.1097/01.ju.0000133655.71782.14. [DOI] [PubMed] [Google Scholar]
- 4.Roehrborn CG, Kaplan SA, Noble WD, et al. The impact of acute or chronic inflammation in baseline biopsy on the risk of clinical progression of BPE: Results from the MTOPS study. AUA Meeting; 2005. [Google Scholar]
- 5.Nickel JC, Roehrborn CG, O’Leary MP, et al. The Relationship between Prostate Inflammation and Lower Urinary Tract Symptoms: Examination of Baseline Data from the REDUCE Trial. Eur Urol. 2008;54:1379. doi: 10.1016/j.eururo.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007;51:1202. doi: 10.1016/j.eururo.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Di Silverio F, Gentile V, De Matteis A, et al. Distribution of inflammation, pre-malignant lesions, incidental carcinoma in histologically confirmed benign prostatic hyperplasia: a retrospective analysis. Eur Urol. 2003;43:164. doi: 10.1016/s0302-2838(02)00548-1. [DOI] [PubMed] [Google Scholar]
- 8.Fabriek BO, Dijkstra CD, van den Berg TK. The macrophage scavenger receptor CD163. Immunobiology. 2005;210:153. doi: 10.1016/j.imbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Borowsky AD, Dingley KH, Ubick E, et al. Inflammation and atrophy precede prostatic neoplasia in a PhIP-induced rat model. Neoplasia. 2006;8:708. doi: 10.1593/neo.06373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nickel JC. Inflammation and benign prostatic hyperplasia. Urol Clin North Am. 2008;35:109. doi: 10.1016/j.ucl.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theyer G, Kramer G, Assmann I, et al. Phenotypic characterization of infiltrating leukocytes in benign prostatic hyperplasia. Lab Invest. 1992;66:96. [PubMed] [Google Scholar]
- 12.Di Carlo E, Magnasco S, D’Antuono T, et al. The prostate-associated lymphoid tissue (PALT) is linked to the expression of homing chemokines CXCL13 and CCL21. Prostate. 2007;67:1070. doi: 10.1002/pros.20604. [DOI] [PubMed] [Google Scholar]
- 13.Kramer G, Steiner GE, Handisurya A, et al. Increased expression of lymphocyte-derived cytokines in benign hyperplastic prostate tissue, identification of the producing cell types, and effect of differentially expressed cytokines on stromal cell proliferation. Prostate. 2002;52:43. doi: 10.1002/pros.10084. [DOI] [PubMed] [Google Scholar]
- 14.Steiner GE, Stix U, Handisurya A, et al. Cytokine expression pattern in benign prostatic hyperplasia infiltrating T cells and impact of lymphocytic infiltration on cytokine mRNA profile in prostatic tissue. Lab Invest. 2003;83:1131. doi: 10.1097/01.lab.0000081388.40145.65. [DOI] [PubMed] [Google Scholar]
- 15.Di Silverio F, Bosman C, Salvatori M, et al. Combination therapy with rofecoxib and finasteride in the treatment of men with lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia (BPH) Eur Urol. 2005;47:72. doi: 10.1016/j.eururo.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Vela Navarrete R, Garcia Cardoso J, Barat A, et al. Effects of the lipido-sterolic extract of Serenoa repens (Permixon) on infiltrating cells and inflammatory markers in prostatic tissues from BPH patients. Eur Urol. 2002;1:62. [Google Scholar]
- 17.Paubert-Braquet M, Mencia Huerta JM, Cousse H, et al. Effect of the lipidic lipidosterolic extract of Serenoa repens (Permixon) on the ionophore A23187-stimulated production of leukotriene B4 (LTB4) from human polymorphonuclear neutrophils. Prostaglandins Leukot Essent Fatty Acids. 1997;57:299. doi: 10.1016/s0952-3278(97)90548-2. [DOI] [PubMed] [Google Scholar]


