Abstract
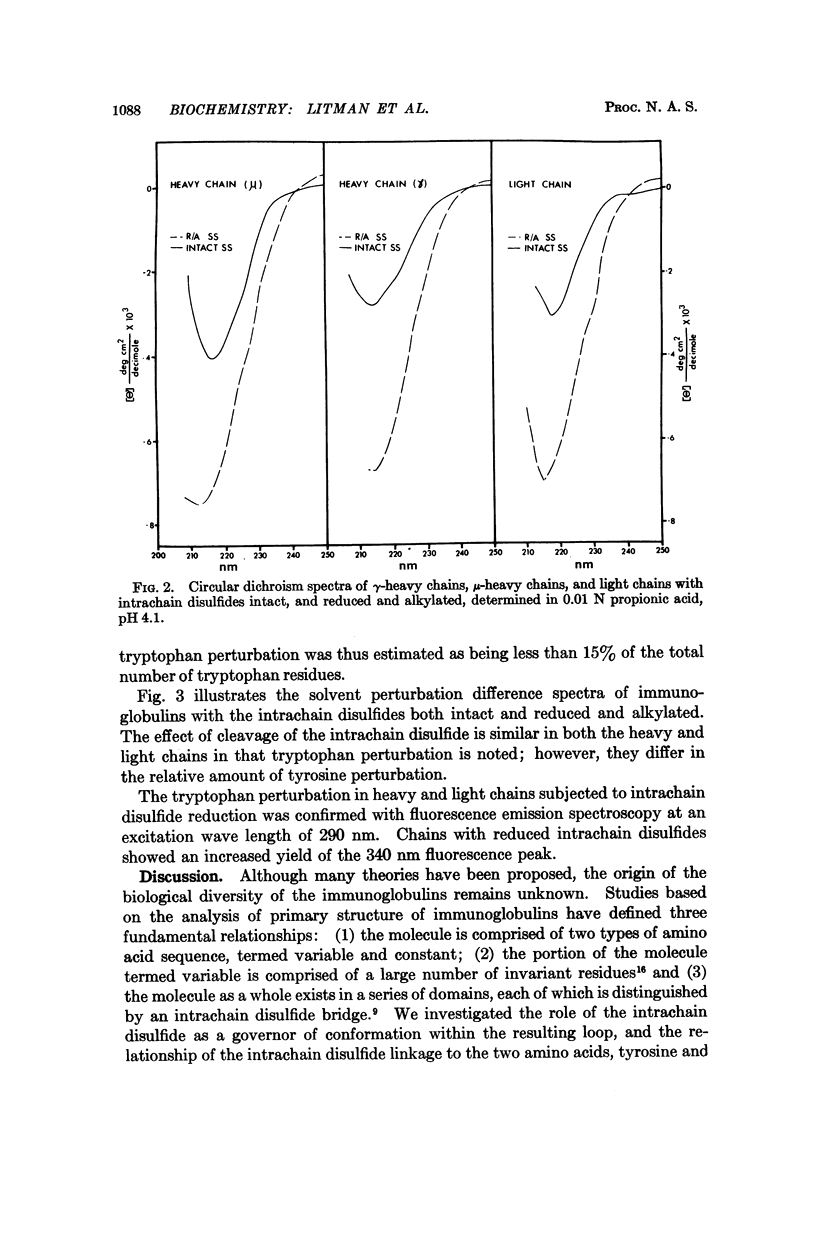
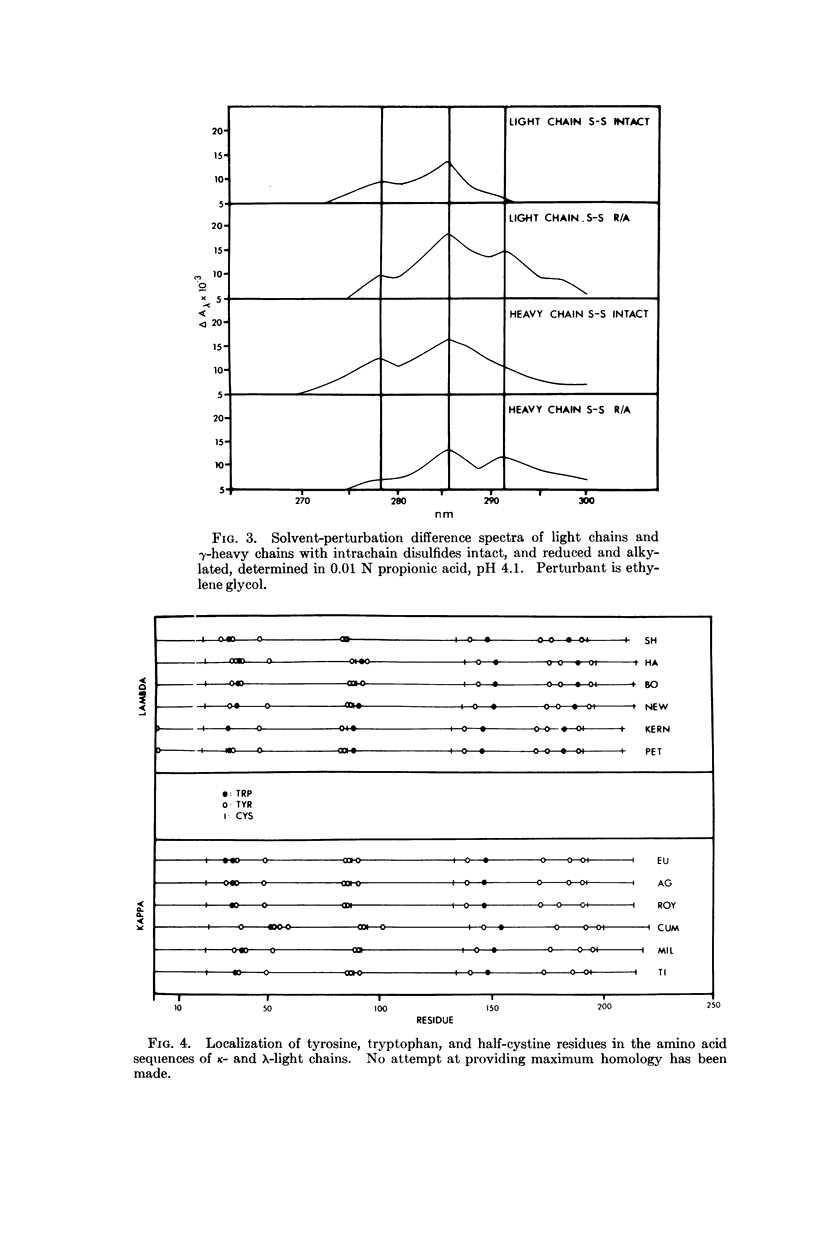
Biophysical studies of intact immunoglobulins, enzymatically derived immunoglobulin subunits, and chemically derived immunoglobulin chains are reported. All the forms studied lacked optical activity associated with the α-helix conformation. A dichroism band centered near 217 nm, which has been assigned to the β-sheet conformation, was present in all subunits that contained at least two covalently linked intrachain disulfide loop regions. This dichroism band could not be detected in Component II, the C-terminal 120 amino acids of the heavy chain. The reduction and alkylation of the intrachain disulfide linkages caused a large conformational change associated with short range interactions. The cleavage of the intrachain disulfide also produced a large change in the environment of two aromatic amino acids, tyrosine and tryptophan. These observations indicate some unique conformational relationships for immunoglobins which may be related to the functional demands placed on this class of macromolecule.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abaturov L. V., Nezlin R. S., Vengerova T. I., Varshavsky J. M. Conformational studies of immunoglobulin G and its subunits by the methods of hydrogen-deuterium exchange and infrared spectroscopy. Biochim Biophys Acta. 1969 Dec 23;194(2):386–396. doi: 10.1016/0005-2795(69)90099-3. [DOI] [PubMed] [Google Scholar]
- CRUMPTON M. J., WILKINSON J. M. AMINO ACID COMPOSITIONS OF HUMAN AND RABBIT GAMMA-GLOBULINS AND OF THE FRAGMENTS PRODUCED BY REDUCTION. Biochem J. 1963 Aug;88:228–234. doi: 10.1042/bj0880228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathou R. E., Kulczycki A., Jr, Haber E. Structural features of gamma-immunoglobulin, antibody, and their fragments. Circular dichroism studies. Biochemistry. 1968 Nov;7(11):3958–3964. doi: 10.1021/bi00851a024. [DOI] [PubMed] [Google Scholar]
- Coleman D. L., Blout E. R. The optical activity of the disulfide bond in L-cystine and some derivatives of L-cystine. J Am Chem Soc. 1968 Apr 24;90(9):2405–2416. doi: 10.1021/ja01011a034. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Pflumm M. N., Rutishauser U., Edelman G. M. Subgroups of amino acid sequences in the variable regions of immunoglobulin heavy chains. Proc Natl Acad Sci U S A. 1969 Nov;64(3):997–1003. doi: 10.1073/pnas.64.3.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi E., Jirgensons B. Circular dichroism studies on the acid denaturation of gamma-immunoglobulin G and its fragments. Biochemistry. 1970 Mar 3;9(5):1066–1073. doi: 10.1021/bi00807a003. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Gall W. E. The antibody problem. Annu Rev Biochem. 1969;38:415–466. doi: 10.1146/annurev.bi.38.070169.002215. [DOI] [PubMed] [Google Scholar]
- Frangione B., Milstein C., Franklin E. C. Intrachain disulphide bridges in immunoglobulin G heavy chains. The Fc fragment. Biochem J. 1968 Jan;106(1):15–21. doi: 10.1042/bj1060015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frommel D., Hong R. Studies on human immunoglobulin G Fc sub-fragments. Structural requirements for biological expression. Biochim Biophys Acta. 1970 Jan 20;200(1):113–124. doi: 10.1016/0005-2795(70)90049-8. [DOI] [PubMed] [Google Scholar]
- Glazer A. N. On the prevalence of "nonspecific" binding at the specific binding sites of globular proteins. Proc Natl Acad Sci U S A. 1970 Apr;65(4):1057–1063. doi: 10.1073/pnas.65.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskovits T. T., Sorensen M. Studies of the location of tyrosyl and tryptophyl residues in proteins. I. Solvent perturbation data of model compounds. Biochemistry. 1968 Jul;7(7):2523–2532. doi: 10.1021/bi00847a012. [DOI] [PubMed] [Google Scholar]
- Hilschmann N. Die chemische Struktur von zwei Bence-Jones-Proteinen (roy und Cum.) vom kappa-Typ. Hoppe Seylers Z Physiol Chem. 1967 Aug;348(8):1077–1080. [PubMed] [Google Scholar]
- Hilschmann N. Die vollständige Aminosäuresequenz des Bence-Jones-Proteins Cum. (kappa-Typ) Hoppe Seylers Z Physiol Chem. 1967 Dec;348(12):1718–1722. [PubMed] [Google Scholar]
- Kabat E. A. Comparison of invariant residues in the variable and constant regions of human K, human L, and mouse K Bence-Jones proteins. Proc Natl Acad Sci U S A. 1967 Jul;58(1):229–233. doi: 10.1073/pnas.58.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer B., Steinmetz-Kayne M., Hilschmann N. Die vollständige Aminosäuresequenz des Bence-Jones-proteins new (lambda-Typ) Subgruppen im variablen Teil bei Immunglobulin-L-Ketten vom lambda-Typ. Hoppe Seylers Z Physiol Chem. 1968 Jul;349(7):945–951. [PubMed] [Google Scholar]
- Milstein C., Clegg J. B., Jarvis J. M. Immunoglobulin lambda-chains. The complete amino acid sequence of a Bence-Jones protein. Biochem J. 1968 Dec;110(4):631–652. doi: 10.1042/bj1100631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISONOFF A., WISSLER F. C., LIPMAN L. N., WOERNLEY D. L. Separation of univalent fragments from the bivalent rabbit antibody molecule by reduction of disulfide bonds. Arch Biochem Biophys. 1960 Aug;89:230–244. doi: 10.1016/0003-9861(60)90049-7. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponstingl H., Hess M., Hilschmann N. Die vollständige Aminosäure-Sequenz des Bence-Jones-Proteins Kern Eine neue Untergruppe der Immunglobulin-L-Ketten vom lambda-typ. Hoppe Seylers Z Physiol Chem. 1968 Jun;349(6):867–871. [PubMed] [Google Scholar]
- Putnam F. W., Shinoda T., Titani K., Wikler M. Immunoglobulin structure: variation in amino acid sequence and length of human lambda light chains. Science. 1967 Sep 1;157(3792):1050–1053. doi: 10.1126/science.157.3792.1050. [DOI] [PubMed] [Google Scholar]
- Ross D. L., Jirgensons B. The far ultraviolet optical rotatory dispersion, circular dichroism, and absorption spectra of a myeloma immunoglobulin, immunoglobulin G. J Biol Chem. 1968 May 25;243(10):2829–2836. [PubMed] [Google Scholar]
- Sarkar P. K., Doty P. The optical rotatory properties of the beta-configuration in polypeptides and proteins. Proc Natl Acad Sci U S A. 1966 Apr;55(4):981–989. doi: 10.1073/pnas.55.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Thorpe N. O. On the location and structure of the active sites of antibody molecules. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1371–1378. doi: 10.1073/pnas.60.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M. W., Bennich H. Subfragments from the Fc fragment of human immunoglobulin G. Isolation and physicochemical charaterization. Biochem J. 1968 Mar;107(2):171–178. doi: 10.1042/bj1070171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINKLER M., DOTY P. Some observations on the configuration and precipitating activity of antibodies. Biochim Biophys Acta. 1961 Dec 23;54:448–454. doi: 10.1016/0006-3002(61)90084-1. [DOI] [PubMed] [Google Scholar]
- Wikler M., Titani K., Shinoda T., Putnam F. W. The complete amino acid sequence of a lambda type Bence-Jones protein. J Biol Chem. 1967 Apr 10;242(7):1668–1670. [PubMed] [Google Scholar]



