Abstract
A tumor promoting role of macrophages has been described for a transgenic murine breast cancer model. In this model tumor-associated macrophages (TAMs) represent a major component of the leukocytic infiltrate and are associated with tumor progression. Shigella flexneri is a bacterial pathogen known to specificly induce apotosis in macrophages. To evaluate whether Shigella-induced removal of macrophages may be sufficient for achieving tumor regression we have developed an attenuated strain of S. flexneri (M90TΔaroA) and infected tumor bearing mice. Two mouse models were employed, xenotransplantation of a murine breast cancer cell line and spontanous breast cancer development in MMTV-HER2 transgenic mice. Quantitative analysis of bacterial tumor targeting demonstrated that attenuated, invasive Shigella flexneri primarily infected TAMs after systemic administration. A single i.v. injection of invasive M90TΔaroA resulted in caspase-1 dependent apoptosis of TAMs followed by a 74% reduction in tumors of transgenic MMTV-HER-2 mice 7 days post infection. TAM depletion was sustained and associated with complete tumor regression.
These data support TAMs as useful targets for antitumor therapy and highlight attenuated bacterial pathogens as potential tools.
Introduction
Ever since the American surgeon Coley described streptococcal infection as a potential cure of cancer [1], other bacteria have been explored and were shown to infiltrate, replicate and accumulate in tumors [2], [3]. For some extracellular bacteria, such as genetically modified obligate anaerobe Clostridium novyi, an anti-tumor effect was observed [4]. Other extracellular bacteria such as Escherichia coli accumulated in tumor tissue, induced some inflammatory responses, but failed to confer protection [5]. Facultative intracellular bacteria such as Salmonella have also been assessed for tumor therapy and their intratumoral accumulation was studied using different technologies, albeit beyond cellular resolution [6], [7], [8], [9]. In most syngenic experimental models the therapeutic effect was moderate, whereas in xenograft models a more pronounced effect was described [7], [9], [10], [11], [12]. It was speculated that induction of an inflammatory response was mediating the anti-tumor effect.
In contrast to extracellular bacteria, intracellular bacteria can deliver DNA into eukaryotic cells [13]. Therefore, intracellular bacteria could be employed to deliver toxins or prodrug converting enzymes directly into tumor cells. Yet, no quantitative information on the distribution of intracellular bacteria in different stromal versus tumor cells is available even though such data are key to the design of effective therapeutic regimens [14].
Tumors consist of a complex mixture of transformed cells and stroma cells [15]. In many tumors, tumor-associated macrophages (TAMs) represent a major component of the leukocytic infiltrate [16]. High macrophage numbers have been reported in breast, ovarian, prostate, pancreatic and cervical cancers and are associated with poor prognosis [16], [17]. Some authors have characterized TAMs as macrophages expressing protumoral functions, including promotion of tumor angiogenesis, metastasis, matrix remodelling and suppression of adaptive immunity [16], [18], [19], [20]. Similar results have recently been obtained for tumor associated neutrophils [21].
Removal of macrophages or neutrophils reduced the rate of tumor progression in murine tumor models [21], [22]. Evidence suggests that TAMs are tumor-educated macrophages that appear to have defective production of reactive oxygen and nitrogen intermediates and are impaired in phagocytic activity [16]. For normal macrophages it is known that they are a primary target of virulent Shigella flexneri [23]. Shigella flexneri infection triggers caspase-1 activation leading to apotosis and processing of IL-1 and IL-18 [23].
To analyse the distribution of shigellae after i.v. application in tumor models with high numbers of macrophages (Supporting Information S1: Fig. S1), we quantified the numbers of bacteria in the extracellular space or within tumor cells, distinguishing between the macrophages and non-macrophages. Here we show that TAMs were the primary target of invasive shigellae in a 4T1 tumor xenograft - and a transgenic MMTV-HER2 - breast cancer model. Metabolically attenuated, invasive Shigella flexneri were almost exclusively found intracellularly, whereas a non-invasive S. flexneri mutant predominantly located extracellularly. Invasive shigellae, but not non-invasive shigellae, were able to activate caspase-1 and induce apoptosis in TAMs in both breast cancer models. Shigellae induced apoptosis caused a substantial depletion of TAMs, which was correlated with a pronounced and long-lasting therapeutic effect in the 4T1 breast cancer model. Finally, apoptosis induction by Shigella was confirmed ex vivo with freshly isolated human TAMs.
Taken together the data suggests that invasive bacteria capable of inducing apoptosis in macrophages might represent a novel and promising therapeutic approach for a macrophage-targeted tumor therapy.
Materials and Methods
Ethics Statement
All animals experiments were carried out in accordance with protocols approved by the Regierung von Unterfranken, Germany.
Media, Chemicals and Other Reagents
Bacteria were grown on trypticase soy broth, trypticase soy agar (Becton Dickinson), LB broth, LB Agar (Sigma-Aldrich) or BHI (Becton Dickinson). Ampicillin-, chloramphenicol-, and kanamycin-resistant transformants were selected on agar containing the respective antibiotic at 100, 25, and 30 µg/ml. L-arabinose (Sigma-Aldrich) was used at 1 mM for gene expression induction. TSA containing 100 mg/l of Congo red dye was used to select Cr+ clones of S. flexneri. [24] Oligonucleotides (Eurofins MWG Operon), Enzymes (Fermentas) and Taq polymerase (Biotherm, Genecraft) were used according to manufacturers' instructions. Qiagen products were used to isolate plasmid DNA, gel-purify fragments, or purify PCR products.
Bacterial Strains, Growth Conditions
The S. flexneri serotype 5a strains used in this study are the wt M90T (streptomycin (Sm) resistant] and its noninvasive variant BS176 (lacking the virulence plasmid pWR100) [25], [26]. Strains containing pKD46 and pCP20 were incubated at 30°C unless otherwise noted.
Generation of Shigella aroA-Mutants
Linear DNA containing antibiotic resistance genes were prepared from the plasmids pKD3 according to the method described by Datsenko and Wanner [27]. Briefly, a PCR-product was generated by using primers AroA_up (GGGGTTTTTATTTCTGTTGTAGAGAGTTGAGTTCATGGAATCGTGTAGGCTGGAGCTGCTTC) and AroA_down (GGCCGTGCATTTGGGATCAAGAATCGTCACTGGTGTATCTGCATATGAATATCCTCCTTA) with 36 nucleotide extensions that are homologous to the AroA-gene. As a template, the pKD3 plasmid, which carries a chloramphenicol resistance gene flanked with FRT sites, was used. The PCR-product was transformed into electrocompetent Shigella flexneri BS176, carrying the recombinase encoding plasmid pKD46. In positive clones, the resistance cassette was eliminated using the flipase encoding helper plasmid pCP20 and finally the temperature sensitive helper plasmid was eliminated.
The resulting strain Shigella flexneri BS176ΔaroA (termed BS176ΔaroA) was transformed with the virulence plasmid pWR100 isolated from S. flexneri M90T and pCP20 as helper plasmid. After selection and elimination of the helper plasmid the mutant S. flexneri BS176ΔaroA pWR100 was obtained. The mutant carries the main feature of the virulent strain M90T and therefore was termed M90TΔaroA (M90TΔ).
Determination of CFU and Number of Infected Cells
CFUs were determined by plating serial dilutions in PBS containing 0.1% Triton-X (Roth) and plating on LB agar plates for shigellae strains. Colonies were counted after incubation overnight. For determination of the number of infected cells, serial dilutions were made in PBS, mixed with 5ml of 50°C heated SeaPlaque Agarose (Biozym Scientific) and dropped on LB agar plates. The number of bacterial colonies was counted after overnight incubation. Every colony marks an infected eukaryotic cell.
HeLa Cell Invasion Assays and Survival Assay
Invasion and survival in HeLa (American Type Culture Collection, ATCC; CCL-2) cells was assessed using a modified gentamicin protection assay as previously described [28]. Briefly, six-well plates were seeded with 2 ml of HeLa cell suspension adjusted to 2×105 cells/ml and incubated overnight to reach 90% confluency. Log-phase (OD600: 0.6–0.8) cultures of bacteria were added at an estimated MOI of 100 and plates were incubated at 37°C for 1 h. Subsequently plates were washed three times with PBS and then incubated with DMEM containing gentamicin (100 µg/ml) for 1 h at 37°C in 5% CO2. HeLa cells were lysed in a 0.1% Triton X-100 solution for 10 min at different time points and CFU was determined.
Cell to Cell Spreading Assay
To determine cell to cell spread with a growth attenuated strain a new assay was employed. HeLa cells (7×105) grown in 6-well tissue culture plates were infected at a MOI of 500 for 1 h and then washed twice with PBS. Infected cells were irradiated at 20 Gray. Subsequently, a monolayer of uninfected HeLa cells grown in 6 well plates was incubated with the irradiated Shigella-infected HeLa cells in a ratio of 70∶1 for 2 h, 8 h and 12 h. After 1 h incubation with 100 µg/ml gentamicin, the concentration of gentamicin was reduced to 10 µg/ml. The number of infected cells was determined as described above.
Analysis of Caspase-1, Caspase-3, IL-1β, IL-18 and PARP Processing by Western Blot
Up to 1×106 cells were washed twice with PBS and lysed in 120 µl of 2x Laemmli buffer at 100°C. 10–30 µl of lysates were separated by SDS-PAGE and transferred onto nitrocellulose membranes (Schleicher & Schuell). After 1 h blocking in 5% skimmed milk (Applichem), the membranes were incubated with the primary antibodies (anti-caspase-1/ICE, Sigma); anti-cleaved caspase-3 antibody (NEB), anti-IL1β antibody (BioVision), anti-IL18 antibody (BioVision) anti-cleaved PARP antibody (BD Pharmingen), anti-GAPDH antibody (Chemicon international) or anti-β-actin antibody (Sigma-Aldrich) diluted in 5% skimmed milk overnight at 4°C. After washing, the blot was incubated with peroxidase-conjugated secondary antibody (GE Healthcare). Detection was performed using the ECL kit (Amersham Biosciences) and exposure on X-ray film (Kodak).
Mice and Tumor Inoculation
Six- to eight-week-old female mice were injected subcutaneously with either 1×104 4T1 cytokeratine positive murine epithelial mammary cancer cells (ATCC, CRL-2539), 1×106 B78-D14 [29] melanoma cells or 1×106 P815-PSA [30] mastocytoma cells each resuspended in 50 µl PBS. Injections were done on both sides of the shaven abdomen.
Balb/c, C57/BL6, DBA-2 mice were obtained from Harlan Winkelmann GmbH, Germany. Transgenic MMTV-HER2/new FVB mice were obtained from Jackson Laboratory [31]. All animals were housed at the MSZ animal care facility and experiments were carried out in accordance with protocols approved by the Regierung von Unterfranken, Germany.
Histological and Immunohistochemical Analysis of Tumors
Tumors grown to approximately 1.5 cm in diameter were excised aseptically. The tumors were formalin-fixed, sectioned, and stained with hematoxylin and eosin (Sigma-Aldrich).
To identify macrophages at the tumor site, tissues were fixed in 4% buffered paraformaldehyde for one day, paraffin-embedded, and processed for sectioning. Sections were immunostained using the pan-macrophage anti-F4/80 rat monoclonal antibody (Acris Antibodies) and specific reactivity was detected using a peroxidase-based detection kit (Vector Laboratories). Inflammation by immune cells was detected using an anti-CD45 antibody (BD Pharmingen) and the peroxidase-based detection kit. For identification of epithelial cells an anti-Cytokeratin antibody (DAKO) and a biotinylated anti-IgG antibody (DAKO) were used.
Assessment of In Vivo Targeting and Apoptosis Induction
Shigella were harvested in mid-logarithmic phase, washed three times in PBS and diluted in PBS prior to injection. CFU was determined before by plating serial dilutions of infection aliquots. 4T1 tumor-bearing Balb/c mice 14 days post cell implantation or approx. 6 months old female MMTV-HER2 mice bearing spontaneous mammary carcinomas were injected with 1×106 CFU of bacteria into the tail vein. Bacterial titers were confirmed by plating serial dilutions. Mice were sacrificed at different time points and organs were removed. Organs were digested in 0.001% DNAse (Sigma-Aldrich) and 2 µg/ml dispase solution (Invitrogen) for 30 min at 37°C and homogenized with 70 µm and 40 µm cell strainers. Cell numbers of every cell fraction were counted using a Bürker counting chamber. For separation of cellular populations, parts of the cells were labelled with the pan-macrophage anti-F4/80 (anti-mouse IgG, Acris Antibodies; anti-human IgG, Santa Cruz) antibody for 20 minutes at 4°C. Subsequently, labelled cells were stained with an anti-IgG antibody conjugated to magnetic beads (Miltenyi-Biotec) for 10 minutes at 4°C. Cell separation was performed with the MACS Kit (Mitenyi-Biotec) according to the manufacturers protocol. Subsequently, every cell fraction was counted using a Bürker counting chamber. The CFU, the number of infected cells, caspase-1 processing and apoptosis induction was determined for every cell fraction. To determine the fraction of intra- and extracellular bacteria, CFU of total cells was determined in the presence or absence of 300 µg/ml gentamicin. For absolute values, detection limit is 10 for CFU determination and 1 for infected cells. For values relative to the cell numbers, detection limit is calculated for each experiment using the limit for absolute values divided by the highest number of cell counts for tumor cells, the macrophage or non-macrophage fraction. Detection limits for spleen cells (not shown) are usually 10 fold lower due to higher cell numbers.
FACS Analysis
The relative amount of macrophages in tumor tissues was determined by FACS. After blocking the Fcγ receptor (anti-CD16/32, BD Pharmingen), cells were stained with FITC-anti-mouse CD11b (Miltenyi-Biotec; 10 minutes at 4°C), phycoerythrin (PE)-anti-mouse Gr-1 (Miltenyi-Biotec; 10 minutes at 4°C) or PE-anti-mouse F4/80 (Acris Antibodies; 30 minutes at 4°C). FITC or PE coupled IgG2B antibody (RD Systems,) were used as isotype control. Total cell numbers were assessed in a FSC/SSC live gate. Flow cytometric analysis was performed on a FACSCalibur (BD Immunocytometry Systems) according to the manufacturer's instructions.
Efficacy Studies
Therapeutic efficacy of Shigella infection on tumor growth was explored in 28 six- to eight-week-old female Balb/c mice bearing 4T1 tumors. Tumor growth was determined every two days with a caliper. At day 14 post cell implantation, mean tumor volume had reached 170 mm3 and 24 mice were randomized into three groups. A total amount of 1×106 M90TΔaroA, BS176ΔaroA or 100 µl PBS were injected into the lateral tail vein of the tumor-bearing mice. Tumor size was monitored every second day. Tumor volume was calculated with the formula V = π/6*a*b2 (a>b). 31 days after tumor cell implantation the naϊve and the BS76ΔaroA group (tumor volume of approx. 4000 mm3) and two M90TΔaroA mice (macroscopic comparison of tumors, data not shown) were sacrificed. On day 62 post tumor cell implantation M90TΔaroA-infected mice were sacrified to determine CFU in tumor, liver, spleen and for FACS and histological analysis.
Statistical Analysis
Statistical analysis was performed using Graph Pad Prism 4 (GraphPad Software). Data were compared and analyzed for statistical significance by using a two-tailed Student's t-test whereas p<0.05 was considered as significant.
Results
Generation and In Vitro Characterization of Attenuated Shigella Strains
For infection, S. flexneri serotype 5a strains M90T and BS176 were used. BS176 is a variant of M90T lacking the virulence plasmid [32]. An auxotrophic and attenuated strain for animal studies was constructed, by chromosomal deletion of the aroA-gene locus. Isogenic virulent and avirulent strains were generated by attenuating Shigella flexneri strain BS176 to yield BS176ΔaroA followed by transfer of the virulence plasmid pWR100 [25]. The resulting mutant was called M90TΔaroA.
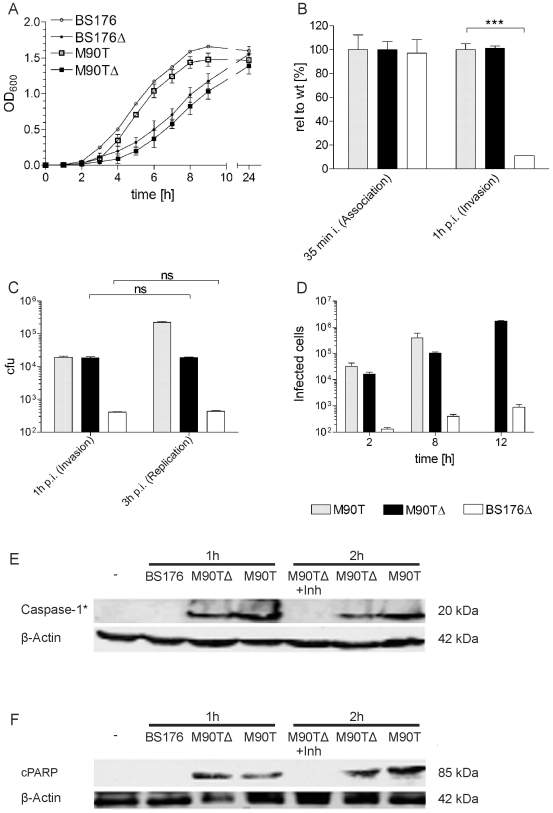
The strains were characterized with respect to extracellular and intracellular growth, adhesion, invasion and cell-to-cell spread in vitro (Fig. 1). The attenuated mutants had a reduced maximal growth rate in LB medium compared to the parental strains with functional aroA-genes (Fig. 1A). With respect to association and invasion, S. flexneri M90TΔaroA was indistinguishable from the M90T strain (Fig. 1B). Consistent with the behaviour of other aroA deleted mutants the intracellular replication was reduced (Fig. 1C). To determine the cell-to-cell spread, a spreading assay was applied, which is independent of intracellular replication (see material and methods section). S. flexneri M90TΔaroA was actively spreading as a 6- or 17-fold increase of infected cells was seen at 8 vs 12 hours post coincubation (Fig. 1D). In contrast the isogenic control BS176ΔaroA was only marginally active (Fig. 1D).
Figure 1. In vitro characterization of aroA mutants.
(A) Determination of extracellular growth rate in LB medium. (B) Invasion assay (gentamicin protection assay). CFUs are shown relative to the wt M90T. (C) Intracellular replication rate in HeLa cells. (D) Cell to cell spread in HeLa cells assessed by L-Top agar assay. Western blot analysis for caspase-1 activation (caspase-1*) (E) and apoptosis induction was performed in J774A.1 Macrophages (PARP cleavage - cPARP) (F). Bars represent means +/- SD of three different experiments, *** P<0.001. BS176: Shigella flexneri BS176; BS176Δ: Shigella flexneri BS176ΔaroA; M90T: Shigella flexneri M90T; M90TΔ: Shigella flexneri M90TΔaroA.
The capacity of S. flexneri M90TΔaroA to induce both caspase-1 cleavage (Fig. 1E) and apoptosis (Fig. 1F) was confirmed in J774A.1 macrophages by western blot analysis (Supporting Information S1: Fig. S2). Apoptosis induction by S. flexneri M90TΔaroA was caspase-1 dependent, as the caspase-1 specific inhibitor YVAD-CHO fully blocked caspase-1 and PARP processing (Fig. 1E).
Infection of TAMs by Shigella In Vivo
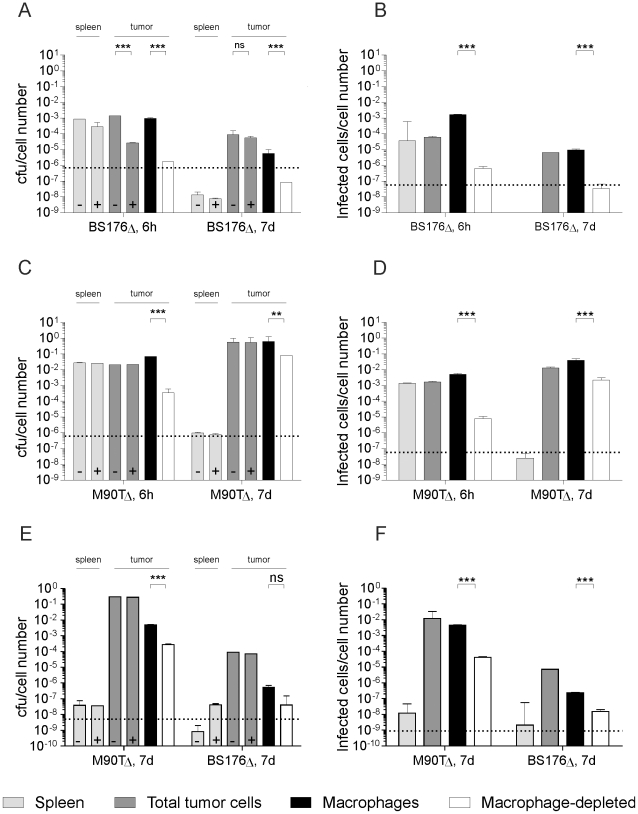
In the next set of experiments, 1×106 invasive S. flexneri M90TΔaroA or non-invasive BS176ΔaroA derivative were applied i.v. to 4T1 xenografted Balb/c or tumor-bearing transgenic MMTV-HER2 mice. After tumor removal, single cell suspensions were prepared and treated with or without gentamicin to determine the ratio of extra and intracellular bacteria. Parts of the tumor cell suspension were separated by MACS into a TAM fraction and TAM-depleted fraction using an F4/80 antibody (Supporting Information S1: Fig. S3,4,5). Supporting Information S1: Fig. S3,4 also depicts the phenotypic characterization of the TAMs. 83% of CD11b positive cells were F4/80 positive (Supporting Information S1: Fig. S3). The F4/80 positive population is DC-M negative, MHC class II low and Gr-1 negative (Supporting Information S1: Fig. S4). The number of infected cells was determined by plating in L-Top agar.
Similar to observations with other bacterial strains, both invasive and non-invasive Shigella accumulated and persisted in the tumor after i.v. infection, whereas they were rapidly eliminated in the spleen (Fig. 2A, C ). However, both strains showed marked differences with respect to their intra-tumoral distribution. In the 4T1 tumor model, gentamicin treatment revealed that 6 h after infection the invasive S. flexneri M90TΔaroA almost exclusively located intracellularly, whereas only 2% of the non-invasive derivative were located intracellularly (Fig. 2A ). Seven days after application invasive Shigella reached total CFU levels of up to 6×106 per tumor. Unexpectedly, the invasive strain was predominantly located in TAMs (>10 fold enrichment, Fig. 2C) even though M90TΔaroA readily infected epithelial 4T1 cells in culture (Supporting Information S1: Fig. S11). In case of the non-invasive BS176ΔaroA, only a small amount of bacteria were found that were restricted to macrophages 6 h p.i. ( Fig. 2A ), in line with previous reports [5]. Seven days after infection, non-invasive shigellae remained restricted to TAMs and were at the limit of detection (Fig. 2A). In contrast the ratio of invasive bacteria in TAMs vs non-TAMs was decreased at seven days p.i. presumably due to cell to cell spreading as observed in cell culture (Fig. 1E,F).
Figure 2. Shigella flexneri M90TΔaroA predominantly targeted TAMs in vivo.
Determination of CFU/cell number (A, C, E) and infected cells/cell number (B, D, F) of separated tumor cells and spleen cells 6 h and 7d after i.v. infection with 1×106 bacteria of tumor-bearing 4T1 (A–D) and transgenic MMTV-HER2/neu mice (E, F). Corresponding raw data of CFU and infected cell counts are shown in Supporting Information S1: Fig. S6 and Supporting Information S1: Fig. S7. + indicates gentamicin treatment. Dashed lines indicate detection limits. All results shown are mean ± SD of values from three mice per experiment; ** P<0.01, *** P<0.001. BS176Δ: Shigella flexneri BS176ΔaroA; M90TΔ: Shigella flexneri M90TΔ aroA
The predominant localization in TAMs was not due to higher CFU counts per cell compared to non-macrophages, as the infectious center assay led to the same results (Fig. 2B, D). The selective targeting of invasive shigellae to TAMs was also observed in tumor bearing MMTV-HER2 transgenic mice (Fig. 2E, F).
Taken together, non-invasive BS176ΔaroA shigellae were mainly found extracellularly despite the presence of large numbers of macrophages in two different breast cancer models, whereas invasive M90TΔaroA shigellae almost exclusively targeted TAMs.
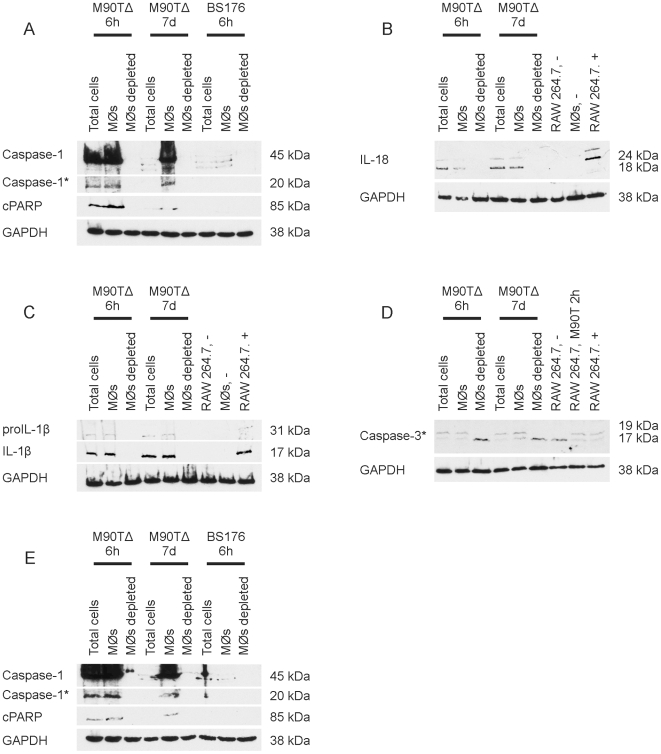
Apoptosis Induction in TAMs
To examine whether the apotosis induction observed in the established macrophage cell line J774A.1 in culture also occurred in TAMs in vivo, tumor bearing mice were infected. After infection with invasive M90TδaroA shigellae, caspase-1 activation (Fig. 3A ) was detectable in total cells and macrophage fractions of tumors taken 6 hours after infection. At seven days p.i. caspase-1 activation was exclusively seen in the macrophage fraction. Caspase-1 activation at either timepoint was invariantly associated with IL-18 and IL-1β processing (Fig. 3B,C ) in addition to processing of the effector caspase-3 and PARP cleavage (Fig. 3A,D), a hallmark of apoptosis. In contrast the non-invasive BS176ΔaroA did not induce apoptosis. Apoptosis induction was only observed in cellular fractions, where shigellae were located intracellularly and that contained TAMs. Similar data were obtained for transgenic MMTV-HER2 mice (Fig. 3E ). In both mouse models TUNEL staining in tumor tissue confirmed caspase-3 processing and PARP cleavage data (Supporting Information S1: Fig. S8).
Figure 3. Infection of tumor-bearing mice with Shigella flexneri M90TΔaroA, but not S. flexneri BS176ΔaroA induced consistent caspase-1 processing and apoptosis in TAMs in vivo.
Western Blot analysis for caspase-1 (A, E), IL-1β (C), IL-18 (B), caspase-3 (D) processing and PARP cleavage (A,E) in Balb/c (A–D) and transgenic MMTV-HER2 (E) mice. BS176Δ: Shigella flexneri BS176ΔaroA; M90TΔ: Shigella flexneri M90TΔaroA
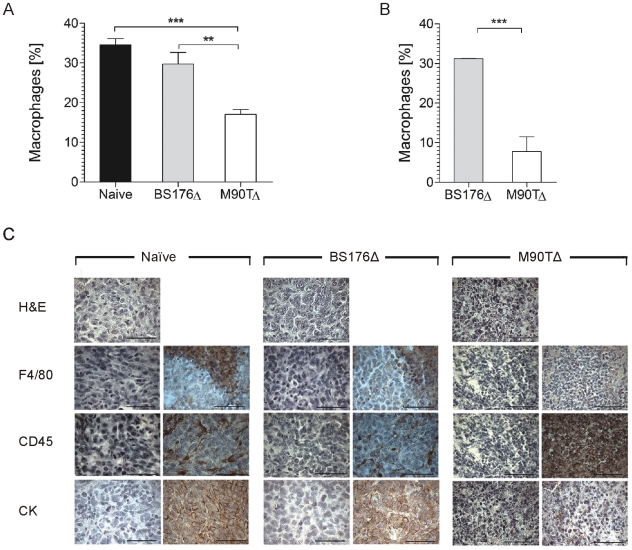
Next we investigated whether apoptosis induction in vivo was associated with depletion of macrophages. Figure 4 shows that M90TΔaroA, but not the avirulent shigellae reduced the relative content of TAMs in tumors from xenografted Balb/c mice by at least 50% seven days p.i. (Fig. 4A ). In transgenic mice a 74% reduction was achieved by M90TΔaroA but not control shigellae infection at the seven day timepoint (Fig. 4B). This result cannot be explained by differences in tumor sizes between animals (Supporting Information S1: Fig. S9) and therefore strongly suggests that infection with invasive bacteria induced the depletion of TAMs. In addition, the reduction of macrophages is not observed in spleens of infected animals suggesting that the reduction is confined to tumor tissues (data not shown).
Figure 4. Infection with Shigella flexneri M90TΔaroA results in a decrease in TAMs number.
7d after IV infection of tumor bearing Balb/c (A) and MMTV-HER2 FVB mice (B) tumors were analysed for F4/80 positive cells by FACS to determine the relative amount of TAMs. Bars represent means +/− SD of four tumors analyzed per group, ** P<0.01, *** P<0.001. (C) Histology of tumor tissue from non-infected, BS176Δ- or M90TΔ- infected mice. In each case left panels represent antibody controls. Scale bars represent 50 µm. BS176Δ: Shigella flexneri BS176ΔaroA; M90TΔ: Shigella flexneri M90TΔaroA; CK: Cytokeratin.
Histological examination of non-infected (Fig. 4C left panels), BS176ΔaroA (Fig. 4C , middle panels) and M90TΔaroA (Fig. 4C right panels) infected mice by anti-F4/80 staining confirmed the substantial reduction of macrophages. Additionally the IHC experiments revealed massive infiltration of inflammatory cells (anti-CD45 staining) in 4T1-tumors upon infection with invasive but not non-invasive Shigella (Fig. 4C). Furthermore, cytokeratin staining of tumors derived from M90TΔaroA-infected mice, which identifies pockets of 4T1 tumor cells showed reduced content of cytokeratin positive cells. This result indicates that the depletion of macrophages is associated with a reduction of tumor cells as previously observed by other approaches targeting macrophages [19], [33], [34]. In the non-infected and BS176ΔaroA-infected tissue normal tumor structure was observed.
Tumor Regression by Depletion of TAMs
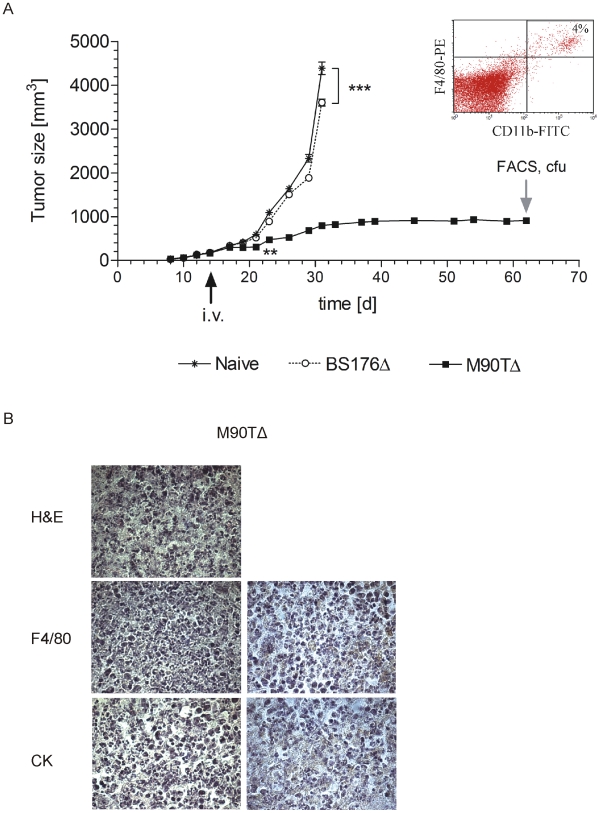
To investigate whether this substantial reduction in macrophage numbers and marked inflammation induced by S. flexneri M90TΔaroA is associated with a therapeutic effect, bacteria were applied to tumor-bearing Balb/c mice and tumor growth was assessed (Fig. 5A ). Infection with S. flexneri BS176ΔaroA resulted in a small, albeit significant reduction of tumor growth in comparison to the control animals. In contrast, a single i.v. infection with S. flexneri M90TΔaroA resulted in complete removal of tumor tissue (Fig. 5). 48 days p.i. the tumor showed very low macrophage (<4%) numbers and bacteria were not detectable (Fig. 5A ). Histological examinations of the tumor tissue 68 days after xenografting showed persistent reduction of macrophage numbers (anti-F4/80 staining) and complete depletion of 4T1 tumor cells (anti-cytokeratin staining) (Fig. 5B ).
Figure 5. M90TΔ, but not BS176Δ, completely blocked tumor growth after i.v. infection of 4T1 tumor-bearing mice.
(A) 14 days after tumor cell inoculation 1×106 bacteria were applied i.v. to 8 mice per group. Non-infected and BS176Δ infected mice were sacrificed 31 days after tumor cell inoculation. At day 62, M90TΔ mice were killed and CFU and macrophage numbers were determined. ** P<0.01, *** P<0.001. (B) Histology of tumor tissue from M90TΔ-infected mice. Left panels represent antibody controls. Scale bars represent 50 µm. BS176Δ; Shigella flexneri BS176ΔaroA; M90TΔ; Shigella flexneri M90TΔaroA;CK: Cytokeratin.
Discussion
In this work we have demonstrated that invasive S. flexneri preferentially target TAMs after i.v. infection in two different breast cancer models. The growth-attenuated virulent mutant M90TΔaroA, but not the non-invasive mutant BS176ΔaroA, was exclusively located intracellularly at all time points examined. Mutant M90TΔaroA, but not BS176ΔaroA, induced caspase-1 processing and apoptosis in TAMs leading to a long-lasting reduction of TAMs. The reduction of TAMs was accompanied by a complete tumor regression in a 4T1 breast cancer model and removal of transformed cells in the residual stroma. In contrast to bacterial tumor therapies that use extracellular bacteria, targeting TAMs with invasive shigellae was effective despite relatively low numbers of bacteria within the tumor. Finally, for a different gynaecological cancer type of human origin, human ovarian carcinoma, we could show ex vivo that infection of TAMs from ascites with S. flexneri M90TΔaroA induced caspase-1 activation and PARP cleavage (Supporting Information S1: Fig. S10).
Experimental tumor therapy by infection with extracellular bacteria has a long history [3]. Recent observations with E.coli strains suggested that these bacteria were able to induce tumor-targeted inflammatory responses but were ineffective in killing tumor cells [5]. The non-invasive strain BS176ΔaroA showed a similar behavior, including a marginal, albeit significant, therapeutic efficacy. Bacterial tumor therapy is commonly thought to be based on the induction of an inflammatory response at the site of the tumor as a result of the accumulation of extracellular bacteria at this immunologically privileged site [4], [11], [35]. Whereas these bacteria remain extracellular at all times S. flexneri M90TΔaroA actively invades cells. This data confirm previous reports which have described phagocytosis defects in TAMs [16], [19].
In our experiments, the profound reduction of TAMs was associated with complete tumor regression [36]. The simplest interpretation of these data would be that TAMs are required to support tumor growth. However as we observed a massive infiltration of inflammatory cells simultaneous with bacterial infection and TAM depletion inflammation might also contribute to tumor regression. The extracellular BS176ΔaroA mutant did not induce caspase-1 processing and thus did neither induce IL-1β and IL-18 activation nor deplete TAMs. Therefore it is likely that for the induction of the inflammatory response by M90TΔaroA targeting of macrophages is also a necessary factor. However the low dose bacteria found under these conditions makes it difficult to come up with a firm conclusion in this respect. As neutrophils have been described to be potential mediators of inflammation induced tumor regression [21] it may be interesting in the future to determine whether M90TΔaroA can induce neutrophil infiltration in the presence or absence of TAMs.
Targeting TAMs as a strategy for bacterial tumor therapy may have the advantage that a stable cell population is attacked as opposed to the phenotypically unstable tumor cell population, that may quickly give rise to resistant cells. On the other hand macrophages found in tumors may potentially stimulate or inhibit tumor growth. It will therefore be important in the future to develop markers that differentiate between TAM populations and allow identification of tumors where TAM removal may be beneficial [37], [38]. Although at first sight Shigella would seem to be an unlikely candidate as a therapeutic agent based on its pathogenicity, the fact that attenuation was readily achieved and that a small number of bacteria at the tumor site was sufficient to induce a dramatic anti-tumor effect suggests that further investigation is warranted. At least in our experiments we did not observe signs of overt toxicity, albeit mice cannot be considered as a suitable model to assess toxicity of shigellae.
In summary, we suggest that targeting TAMs using attenuated S. flexneri is a promising option for future tumor therapy. Further studies are required with respect to the safety of the S. flexneri mutant and the efficacy of tumor targeting in humans. Furthermore, other intracellular bacteria like Salmonella enterica or Listeria monocytogenes might be suitable for a macrophage targeted bacterial tumor therapy.
Supporting Information
(15.15 MB DOC)
Acknowledgments
Plasmids were kindly provided by Christian Andersen, Würzburg. The authors wish to thank S. Roos and B. Oberwallner for technical assistance, M. Becker, R. Reuten, E. Zanucco and C. Korn, T. Dogan, S. Trüpschuch and H. Drexler for their support and helpful discussion.
Footnotes
Competing Interests: The authors declare that they have a competing financial interest. A patent application was filed by JF and Aeterna Zentaris: EP 08101045.6, US 61/024,225: “Attenuated bacteria capable of inducing apoptosis in macrophages, process of manufacturing and uses thereof”. The authors confirm that all authors adhere to all the PLoS ONE policies on sharing data and materials, despite filing the patent.
Funding: KG, CH and MH were supported by the International DFG Research Training Group 1141 Wuerzburg/Nice (GCWN.de) and the Franco-German University (ED-31-04;http://www.dfh-ufa.org/). This work was supported in part by Aeterna Zentaris (http://www.aeternazentaris.com/) and in part by the Bavarian Research Cooperations Abayfor (Foringen; http://www.bayfor.org/de/geschaeftsbereiche/forschungsverbuende/welt-des-lebens/foringen.html). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. JF is currently employed by Aeterna Zentaris and during this study he was the group leader in the unit and supervisor of Mrs. Galmbacher.
References
- 1.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin Orthop Relat Res. 1991:3–11. [PubMed] [Google Scholar]
- 2.Yu YA, Shabahang S, Timiryasova TM, Zhang Q, Beltz R, et al. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol. 2004;22:313–320. doi: 10.1038/nbt937. [DOI] [PubMed] [Google Scholar]
- 3.Van Mellaert L, Barbé S, Anné J. Clostridium spores as anti-tumour agents. Trends in Microbiology. 2006;14:190–196. doi: 10.1016/j.tim.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal N, Bettegowda C, Cheong I, Geschwind JF, Drake CG, et al. Bacteriolytic therapy can generate a potent immune response against experimental tumors. Proc Natl Acad Sci U S A. 2004;101:15172–15177. doi: 10.1073/pnas.0406242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weibel S, Stritzker J, Eck M, Goebel W, Szalay AA. Colonization of experimental murine breast tumours by Escherichia coli K-12 significantly alters the tumour microenvironment. Cell Microbiol. 2008 doi: 10.1111/j.1462-5822.2008.01122.x. [DOI] [PubMed] [Google Scholar]
- 6.Forbes NS, Munn LL, Fukumura D, Jain RK. Sparse initial entrapment of systemically injected Salmonella typhimurium leads to heterogeneous accumulation within tumors. Cancer Res. 2003;63:5188–5193. [PubMed] [Google Scholar]
- 7.Kasinskas RW, Forbes NS. Salmonella typhimurium Lacking Ribose Chemoreceptors Localize in Tumor Quiescence and Induce Apoptosis. Cancer Res. 2007;67:3201–3209. doi: 10.1158/0008-5472.CAN-06-2618. [DOI] [PubMed] [Google Scholar]
- 8.Zhao M, Yang M, Ma H, Li X, Tan X, et al. Targeted Therapy with a Salmonella Typhimurium Leucine-Arginine Auxotroph Cures Orthotopic Human Breast Tumors in Nude Mice. Cancer Res. 2006;66:7647–7652. doi: 10.1158/0008-5472.CAN-06-0716. [DOI] [PubMed] [Google Scholar]
- 9.Westphal K, Leschner S, Jablonska J, Loessner H, Weiss S. Containment of tumor-colonizing bacteria by host neutrophils. Cancer Res. 2008;68:2952–2960. doi: 10.1158/0008-5472.CAN-07-2984. [DOI] [PubMed] [Google Scholar]
- 10.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 11.Zhao M, Yang M, Li XM, Jiang P, Baranov E, et al. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci U S A. 2005;102:755–760. doi: 10.1073/pnas.0408422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vassaux G, Nitcheu J, Jezzard S, Lemoine NR. Bacterial gene therapy strategies. The Journal of Pathology. 2006;208:290–298. doi: 10.1002/path.1865. [DOI] [PubMed] [Google Scholar]
- 13.Pilgrim S, Stritzker J, Schoen C, Kolb-Maurer A, Geginat G, et al. Bactofection of mammalian cells by Listeria monocytogenes: improvement and mechanism of DNA delivery. Gene Ther. 2003;10:2036–2045. doi: 10.1038/sj.gt.3302105. [DOI] [PubMed] [Google Scholar]
- 14.Pawelek JM, Low KB, Bermudes D. Tumor-targeted Salmonella as a Novel Anticancer Vector. Cancer Res. 1997;57:4537–4544. [PubMed] [Google Scholar]
- 15.Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 16.Lewis CE, Pollard JW. Distinct Role of Macrophages in Different Tumor Microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 17.Kurahara H, Shinchi H, Mataki Y, Maemura K, Noma H, et al. Significance of M2-Polarized Tumor-Associated Macrophage in Pancreatic Cancer. J Surg Res. 2009 doi: 10.1016/j.jss.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 18.Low KB, Ittensohn M, Le T, Platt J, Sodi S, et al. Lipid A mutant Salmonella with suppressed virulence and TNFalpha induction retain tumor-targeting in vivo. Nat Biotechnol. 1999;17:37–41. doi: 10.1038/5205. [DOI] [PubMed] [Google Scholar]
- 19.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer and Metastasis Reviews. 2006;25:315–322. doi: 10.1007/s10555-006-9001-7. [DOI] [PubMed] [Google Scholar]
- 20.Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, et al. A Distinct Macrophage Population Mediates Metastatic Breast Cancer Cell Extravasation, Establishment and Growth. PLoS ONE. 2009;4:e6562. doi: 10.1371/journal.pone.0006562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng H-L, Trink B, Tzai T-S, Liu H-S, Chan S-H, et al. Overexpression of c-met as a Prognostic Indicator for Transitional Cell Carcinoma of the Urinary Bladder: A Comparison With p53 Nuclear Accumulation. J Clin Oncol. 2002;20:1544–1550. doi: 10.1200/JCO.2002.20.6.1544. [DOI] [PubMed] [Google Scholar]
- 23.Zychlinsky A, Kenny B, Menard R, Prevost MC, Holland IB, et al. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol Microbiol. 1994;11:619–627. doi: 10.1111/j.1365-2958.1994.tb00341.x. [DOI] [PubMed] [Google Scholar]
- 24.Maurelli AT, Blackmon B, Curtiss R., 3rd Temperature-dependent expression of virulence genes in Shigella species. Infect Immun. 1984;43:195–201. doi: 10.1128/iai.43.1.195-201.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sansonetti PJ, Kopecko DJ, Formal SB. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982;35:852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buchrieser C, Glaser P, Rusniok C, Nedjari H, D'Hauteville H, et al. The virulence plasmid pWR100 and the repertoire of proteins secreted by the type III secretion apparatus of Shigella flexneri. Mol Microbiol. 2000;38:760–771. doi: 10.1046/j.1365-2958.2000.02179.x. [DOI] [PubMed] [Google Scholar]
- 27.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elsinghorst EA. Measurement of invasion by gentamicin resistance. Methods Enzymol. 1994;236:405–420. doi: 10.1016/0076-6879(94)36030-8. [DOI] [PubMed] [Google Scholar]
- 29.Rymsa B, Becker HD, Lauchart W, de Groot H. Hypoxia/reoxygenation injury in liver: Kupffer cells are much more vulnerable to reoxygenation than to hypoxia. Res Commun Chem Pathol Pharmacol. 1990;68:263–266. [PubMed] [Google Scholar]
- 30.Fensterle J, Bergmann B, Yone, CLRP, Hotz C, Meyer SR, et al. Cancer immunotherapy based on recombinant Salmonella enterica serovar Typhimurium aroA strains secreting prostatespecific antigen and cholera toxin subunit B. Cancer Gene Therapy. 2008;15:85–93. doi: 10.1038/sj.cgt.7701109. [DOI] [PubMed] [Google Scholar]
- 31.Volpers C, Thirion C, Biermann V, Hussmann S, Kewes H, et al. Antibody-mediated targeting of an adenovirus vector modified to contain a synthetic immunoglobulin g-binding domain in the capsid. J Virol. 2003;77:2093–2104. doi: 10.1128/JVI.77.3.2093-2104.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sansonetti PJ, Ryter A, Clerc P, Maurelli AT, Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986;51:461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin EY, Gouon-Evans V, Nguyen AV, Pollard JW. The Macrophage Growth Factor CSF-1 in Mammary Gland Development and Tumor Progression. Journal of Mammary Gland Biology and Neoplasia. 2002;7:147–162. doi: 10.1023/a:1020399802795. [DOI] [PubMed] [Google Scholar]
- 34.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-Stimulating Factor 1 Promotes Progression of Mammary Tumors to Malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoiseth SK, Stocker BA. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 36.Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, et al. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95:272–281. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis C, Murdoch C. Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. Am J Pathol. 2005;167:627–635. doi: 10.1016/S0002-9440(10)62038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loessner H, Endmann A, Leschner S, Bauer H, Zelmer A, et al. Improving live attenuated bacterial carriers for vaccination and therapy. Int J Med Microbiol. 2008;298:21–26. doi: 10.1016/j.ijmm.2007.07.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(15.15 MB DOC)