Abstract
Background
The zinc finger protein Sp2 (specificity protein 2) is a member of the glutamine-rich Sp family of transcription factors. Despite its close similarity to Sp1, Sp3 and Sp4, Sp2 does not bind to DNA or activate transcription when expressed in mammalian cell lines. The expression pattern and the biological relevance of Sp2 in the mouse are unknown.
Methodology/Principal Findings
Whole-mount in situ hybridization of mouse embryos between E7.5 and E9.5 revealed abundant expression in most embryonic and extra-embryonic tissues. In order to unravel the biological relevance of Sp2, we have targeted the Sp2 gene by a tri-loxP strategy. Constitutive Sp2null and conditional Sp2cko knockout alleles were obtained by crossings with appropriate Cre recombinase expressing mice. Constitutive disruption of the mouse Sp2 gene (Sp2null) resulted in severe growth retardation and lethality before E9.5. Mouse embryonic fibroblasts (MEFs) derived from Sp2null embryos at E9.5 failed to grow. Cre-mediated ablation of Sp2 in Sp2cko/cko MEFs obtained from E13.5 strongly impaired cell proliferation.
Conclusions/Significance
Our results demonstrate that Sp2 is essential for early mouse development and autonomous proliferation of MEFs in culture. Comparison of the Sp2 knockout phenotype with the phenotypes of Sp1, Sp3 and Sp4 knockout strains shows that, despite their structural similarity and evolutionary relationship, all four glutamine-rich members of the Sp family of transcription factors have distinct non-redundant functions in vivo.
Introduction
Specificity proteins (Sps) are transcription factors that control the expression of a variety of different genes including house keeping, tissue-specific, development-specific and cell-cycle-regulated genes (reviewed in [1]–[4]). Nine different Sp proteins, designated Sp1 to Sp9, have been identified in mammals [4]. They share a highly conserved zinc finger DNA-binding domain comprising three zinc fingers at the C-terminus (ZNF), the adjacent Buttonhead-box (Btd-box: CXCPXC), and the N-terminal Sp-box, a stretch of conserved amino acids (SPLALLAATCSK/RIG/E) of unknown function. The Sp subclass members Sp1, Sp2, Sp3 and Sp4 are further characterized by N-terminal glutamine-rich domains, whereas Sp5 to Sp9 contain proline, alanine or serine/threonine-rich domains [1], [4]. Sp1 and Sp3 are ubiquitously expressed, whereas Sp4 is most prominently found in neuronal tissues [1]–[3]. Sp1, Sp3 and Sp4 recognize the same promoter elements (GC- and GT-boxes) with similar specificity and affinity [5].
Gene targeting experiments revealed that individual Sp family members differ in their biological function, as they exhibit distinct phenotypes. Sp1null embryos die around embryonic day (E) 10.5 [6]. Sp3null mice develop until the end of pregnancy but die immediately after birth due to respiratory failure [7]. Sp3null embryos suffer from a variety of defects including impaired skeletal bone ossification, tooth development [7], [8], cardiac development [9], and placenta organisation [10]. Newborn Sp4-deficient mice do not show obvious abnormalities [11], [12]. However, two-thirds of the mice die within the first month after birth for unknown reasons. Surviving Sp4-deficient animals are growth-retarded [11], [12]. Furthermore, both the male and female Sp4 null mice have problems in reproduction; males do not breed and females are delayed in sexual maturation [11], [12]. Finally, Sp4 is required for specification of the cardiac conduction system [13], [14].
Sp2 is the least characterized member of the glutamine-rich subgroup of Sp factors. Originally, Sp2 was cloned by virtue of the similarity of its zinc finger domain with the zinc finger region of Sp1 [15]. Despite this similarity, Sp2 overexpressed in insect or mammalian cells binds poorly to DNA, and has little or no capacity to stimulate transcription from promoters that are activated by other Sp family members [16]. Consistently, endogenous Sp2 DNA-binding activity in nuclear extracts prepared from cells that express abundant amounts of Sp2 was not observed ([16], and our own unpublished results). Sp2 is associated with the nuclear matrix and localizes predominantly within subnuclear foci that are distinct from promyelocytic oncogenic domains [17]. However, the functional significance of this observation remains unclear.
Towards understanding the physiological relevance of Sp2, we targeted the Sp2 gene and generated Sp2 loss-of-function mutants. Sp2null embryos survive until E9.5 of gestation. They are severely retarded in growth and show a broad range of phenotypic abnormalities. Mouse embryonic fibroblasts (MEFs) derived from Sp2null embryos failed to grow indicating a cell-autonomous role of Sp2 in the control of cellular proliferation. This suggestion was corroborated by MEFs obtained from embryos carrying floxed Sp2 alleles (Sp2cko/cko). Cre-mediated ablation of Sp2 in Sp2cko/cko MEFs resulted in a strong decrease of proliferation. Collectively, we conclude that Sp2 is required for early mouse development and autonomous proliferation of MEFs in culture.
Results
Expression of Sp2 in the Mouse Embryo
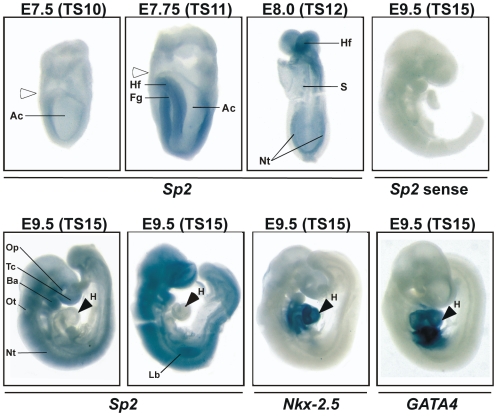
We analyzed expression of the zinc-finger transcription factor Sp2 at early embryonic stages by whole-mount in situ hybridization on E7.5 to E9.5 mouse embryos (Figure 1). At Theiler stage TS10 (E7.5) Sp2 is similarly expressed in embryonic as well as in extra-embryonic tissues. At TS11 and TS12 Sp2 expression is markedly increased in the embryonic tissues especially in the headfold. At a later stage (TS15) strong expression of Sp2 is observed throughout the embryo with exception of the heart. Control hybridizations with the heart-specific markers Nkx2-5 and GATA4 confirmed the accessibility of the heart for hybridization probes (Figure 1). Strongest expression at this stage occurs in the neural tissues, the neuroepithelium surrounding the optical and otic vesicles, the first branchial arch and the forelimb buds and the auditory pit (Figure 1). Taken together, our in situ hybridization experiments indicate abundant expression of Sp2 in most embryonic and extra-embryonic tissues during development.
Figure 1. Sp2 mRNA expression in mouse embryos.
Whole-mount in situ hybridization on embryos at the indicated developmental Theiler stages (E7.5-TS10, E7.75-TS11, E8.0-TS12 and E9.5-TS15) with the indicated probes (Sp2, Sp2 sense, Nkx-2.5 and GATA4). The two embryos at E9.5 (TS15) represent two independent in situ hybridization experiments with embryos obtained from different litters. Ac, amniotic cavity; Ba, first branchial arch; Hf, headfold; Fg, foregut; H, heart; Lb, limb bud; Nt, neuronal tissue; Op, optic pit; Ot, otic vesicle; S, somites; Tc, telencephalon. The white arrowheads denote the boundary between embryonic and extraembryonic tissue.
Targeting of the Mouse Sp2 Gene
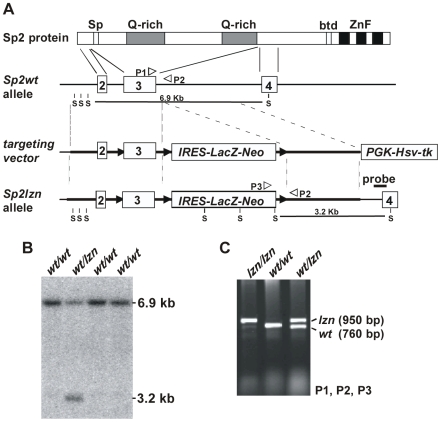
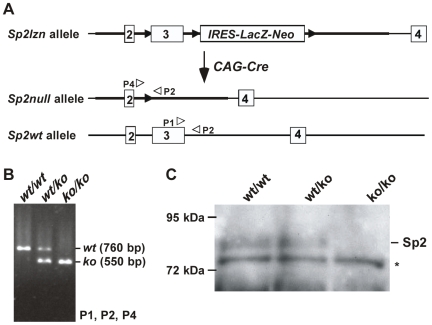
To investigate the role of Sp2 in development we generated Sp2 knockout mice by homologous recombination in embryonic stem cells. The open reading frame of the Sp2 gene encodes a polypeptide of 612 amino acids with a predicted molecular weight of 65 kDa. The entire amino acid sequence of Sp2 is encoded by seven exons. Concerned that the loss of Sp2 might produce early lethality, we employed a tri-loxP strategy to generate Sp2null mice and Sp2 floxed mice, which could be used for conditional knockout experiments. Similar to strategies that we used previously for targeting the Sp3 and Sp4 genes, we targeted exon 3, a large exon that encodes for most of the N-terminal part of Sp2 including the Sp-box and two glutamine-rich regions (Figure 2A). As Sp2 mRNA is expressed in mouse ES cells (data not shown) the targeting strategy allowed expression of a lacZ-neo fusion gene under the control of the endogenous Sp2 promoter (Figure 2A). Southern blot (Figure 2B) and PCR analyses (Figure 2C) revealed successful homologous recombination in 8 out of 80 ES cell clones analyzed.
Figure 2. Targeting strategy for the mouse Sp2 gene.
A) Schematic representation of the Sp2 protein structure, the wild type Sp2 allele, the targeting vector and the targeted Sp2lzn allele. The Sp-box (Sp), two glutamine-rich domains (Q-rich), the btd-box (btd) and the three zinc fingers (ZnF) of the Sp2 protein are indicated. Connecting lines with the corresponding murine Sp2 gene region indicate the derivation of N-terminal parts of the Sp2 protein. The Sp-box and two glutamine-rich regions are encoded by exon 3. In the targeted Sp2 locus exon 3 and the IRES-lacZ-neo cassette are floxed (black arrowheads). White arrowheads depict PCR primer locations (P1, P2 and P3). S, SacI sites. B) Southern blot analysis of transfected ES cells for the Sp2wt allele (6.9 kb) and the targeted Sp2lzn allele (3.2 kb) using SacI-restricted genomic DNA and the 387 bp probe indicated in Figure 2A. C) PCR analysis of E13.5 embryos for the Sp2lzn allele with the indicated primer combination (P1, P2 and P3).
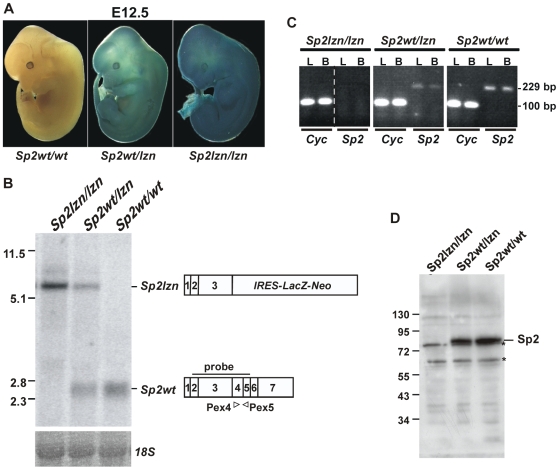
Mice heterozygous for the targeted Sp2 allele (Sp2wt/lzn) were obtained after injection of ES cells into blastocysts and subsequent crossing of the chimeric animals with wild type mice. Sp2wt/lzn mice are viable, reproduce normally and display no obvious abnormalities. Whole-mount LacZ staining on E12.5 embryos confirmed the notion that Sp2 is widely if not ubiquitously expressed during development (Figure 3A). As we expected that the insertion of the IRES-lacZ-neo cassette would result in impaired Sp2 expression, we crossed Sp2wt/lzn mice to homozygosity. Northern blot analyses revealed aberrant Sp2 mRNA expression (Figure 3B). The 7 kb transcript expressed from the Sp2lzn allele represents an RNA species containing exon 1 to 3 of the Sp2 gene fused to LacZ-neo sequences. RT-PCR analysis revealed that regions downstream of exon 3 are not expressed from the Sp2lzn allele (Figure 3C). The presence of an mRNA species containing Sp2 coding sequences was expected to result in expression of an N-terminal Sp2 fragment. Immunoblot analysis revealed the absence of the full-length Sp2 protein; a truncated Sp2 N-terminal fragment, however, was not detected (Figure 3D). Control experiments with recombinant Sp2 fragments showed that our Sp2 antiserum can detect the N-terminal part of Sp2 (data not shown). Likely, expression of the Sp2 N-terminal domain in Sp2lzn/lzn mice is below the detection limit.
Figure 3. Aberrant Sp2 mRNA expression in Sp2lzn/lzn mice.
A) Whole-mount lacZ staining of E12.5 embryos with the indicated genotypes. B) Northern blot analysis of total RNA extracted from E18.5 embryos. RNA size markers are indicated on the left side. The 18S rRNA signal depicts the loading control. The wild type Sp2 mRNA and the aberrant Sp2-IRES-LacZ-Neo mRNA are drawn schematically along with the Northern probe and the primers Pex4 and Pex5 used for the PCR analysis shown in Figure 3C. The numbers 1 to 7 indicate the derivation of mRNA sequences from exons. C) Absence of RNA derived from exons 4 and 5 in Sp2lzn/lzn mice. RT-PCR was performed with RNA from liver (L) and brain (B) of Sp2lzn/lzn, Sp2wt/lzn and Sp2wt/wt embryos using the primers Pex4 and Pex5 indicated in Figure 3B. Amplification of cyclophilin (Cyc) mRNA sequences was used as a positive control. D) Western blot analysis of Sp2 using extracts from primary mouse embryonic fibroblasts derived from E13.5 embryos with the indicated genotypes. The asterisks denote cross-reacting proteins that serve as internal loading control.
Impaired Development of Targeted Sp2lzn/lzn Mice
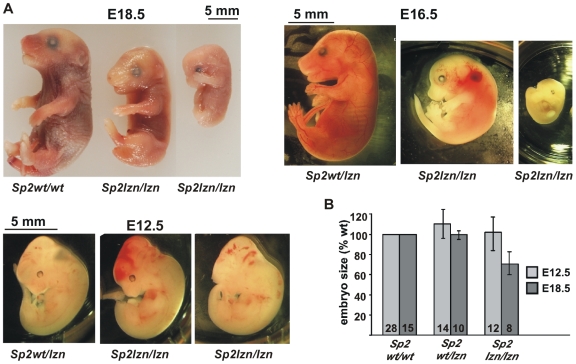
Genotyping of 91 10-day-old pups from heterozygous Sp2wt/lzn intercrosses revealed wild type and heterozygous animals at the expected 1∶2 ratio. Only one homozygous Sp2lzn/lzn mutant pup was found. This pub died before genotyping was finished and could not be examined. We then examined intercross embryos at E9.5 through E18.5 (Table 1). Overall, Sp2lzn/lzn embryos at E16.5 and E18.5 were smaller than their wild type and heterozygous littermates. They were generally developmentally retarded, albeit with considerable variation (Figure 4). Moreover, many of the Sp2lzn/lzn embryos were already dead at these developmental stages. In contrast, Sp2lzn/lzn embryos at E12.5 had a normal body size. However, they displayed hemorrhaging, predominantly in the head area (Figure 4). At E9.5 Sp2lzn/lzn embryos appeared apparently normal. Taken together the various developmental defects displayed by Sp2lzn/lzn mutant embryos indicate that Sp2 is required for normal embryonic development at late embryonic stages.
Table 1. Genotype distribution of Sp2wt/lzn intercrossings.
| Sp2wt/lzn x Sp2wt/lzn | Sp2wt/wt | Sp2wt/lzn | Sp2lzn/lzn | ||
| Born | n = 91 | (100%) | 26 (28.6%) | 64 (70.3%) | 1* (1.1%) |
| E18.5 | n = 54 | (100%) | 13 (24.1%) | 31 (57.4%) | 10 (18.5%) |
| E16.5 | n = 13 | (100%) | 3 (23.1) | 7 (53.8%) | 3 (23.1) |
| E13.5 | n = 72 | (100%) | 21 (29.2%) | 36 (50.0%) | 15 (20.8%) |
| E12.5 | n = 106 | (100%) | 27 (25.5%) | 50 (47.2%) | 29 (27.4%) |
| E9.5 | n = 51 | (100%) | 16 (31.4%) | 22 (43.1%) | 13 (25.5%) |
P, postnatal day; E, day of embryonic development; n, number of mice/embryos.
*died two weeks after birth.
Figure 4. Impaired development of Sp2lzn/lzn embryos.
A) Sp2lzn/lzn embryos at E18.5, E16.5 and E12.5 representing typical phenotypic variants. Sp2lzn/lzn embryos at E12.5 have a normal body size but display hemorrhaging predominantly at the head. B) Embryo size distribution at E12.5 and E18.5. The number of embryos analyzed is indicated at the bottom of the bars.
Early Embryonic Lethality of Sp2null Mice
Northern blot and PCR analyses of tissues form Sp2lzn/lzn embryos revealed aberrant mRNA expression from the targeted Sp2 locus. As expression of small amounts of N-terminal sequences of Sp2 could result in a hypomorphic phenotype we generated Sp2null mice by crossing heterozygous Sp2wt/lzn animals with CAG-Cre mice that express the Cre recombinase under control of the cytomegalovirus immediate early enhancer-chicken β-actin hybrid (CAG) promoter [18]. The resulting heterozygous Sp2wt/ko mice were healthy and fertile, and were intercrossed to generate Sp2null mice (Figure 5). Surprisingly, the phenotype of Sp2null embryos differs markedly from that of Sp2lzn/lzn embryos, strongly suggesting that the insertion of the LacZ-neo fusion gene into the third intron did not disrupt Sp2 functions completely.
Figure 5. Generation of Sp2null mice.
(A) Mice carrying an Sp2null allele were obtained by crossing heterozygous Sp2wt/lzn mice with CAG-Cre mice [18] thereby removing the selection marker (IRES-LacZ-Neo) and exon 3. Black arrowheads depict loxP sites, white arrowheads indicate PCR primer locations. B) PCR analysis of the Sp2 wild type (wt) and the knockout (ko) allele using primers P1, P2 and P4 indicated in panel A. C) Immunoblot analysis of Sp2 in extracts of primary mouse embryonic fibroblasts derived from E9.5 embryos with the indicated genotype. The asterisk denotes a cross-reacting protein.
No Sp2null embryos were found alive beyond E10.5 (Table 2). The few Sp2null embryos recovered at E12.5 to E13.5 were almost completely reabsorbed. Embryos at E9.5 and earlier stages displayed genotype distributions close to the expected Mendelian ratios (Table 2).
Table 2. Genotype distribution of Sp2wt/ko intercrossings.
| Sp2wt/ko x Sp2wt/ko | Sp2wt/wt | Sp2wt/ko | Sp2null | ||
| P10 | n = 81 | (100%) | 34 (42%) | 47 (58%) | 0 (0%) |
| E17.5 | n = 7 | (100%) | 3 (42.9%) | 4 (57.1%) | 0 (0%) |
| E13.5 | n = 8 | (100%) | 0 (0.0%) | 8 (88.9%) | 1* (11.1%) |
| E12.5 | n = 23 | (100%) | 7 (26.9%) | 16 (61.5%) | 3* (11.5%) |
| E10.5 | n = 39 | (100%) | 18 (46.2%) | 20 (51.3%) | 1 (2.6%) |
| E9.5 | n = 303 | (100%) | 69 (22.8%) | 166 (54.8%) | 68+ (22.4%) |
| E8.5 | n = 34 | (100%) | 10 (29.4%) | 16 (47.1%) | 7 (20.6%) |
| E7.5 | n = 9 | (100%) | 1 (11.1%) | 6 (66.7%) | 2 (22.2%) |
P, postnatal day; E, day of embryonic development; n, number of mice/embryos.
*embryos were dead and reabsorbed.
7 out of 68 embryos were reabsorbed.
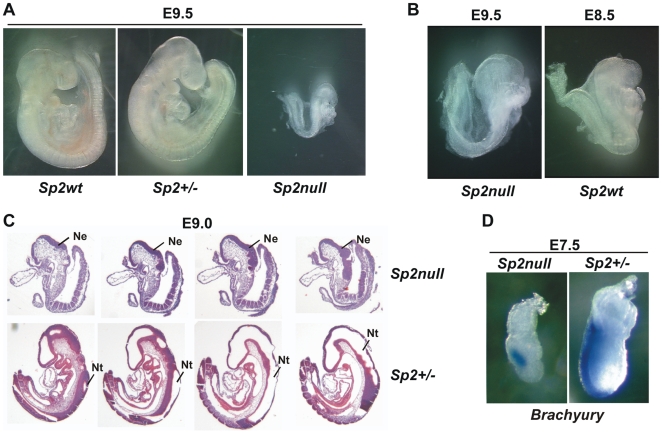
Sp2null embryos developed until the nine somite stage but failed to undergo body turning. At E9.5 Sp2null embryos were much smaller than their littermates (Figure 6A). However, heartbeat and superficial attachment of the allantois to the chorion were observed. Generally, the appearance of E9.5 Sp2null embryos was very similar to E8.5 wild type and heterozygous embryos (Figure 6B) indicating a general developmental delay. This conclusion is supported by serial longitudinal sections that revealed that E9 Sp2 knockout embryos have an open cranial neural tube (Figure 6C) typical for E8 wild type or Sp2 heterozygous embryos.
Figure 6. Phenotype of Sp2null embryos.
A) An Sp2null embryo at E9.5 in comparison to wild type (Sp2wt) and heterozygous (Sp2+/−) littermates. B) An Sp2null embryo at E9.5 in direct comparison to an E8.5 wild type embryo (Sp2wt). C) Serial longitudinal sections of heterozygous Sp2 (Sp2+/−) and Sp2null embryos from day E9.0. Sp2null embryos are smaller, have not turned and have an open cranial neural tube (Nt). Ne, neural epithelium. D) Whole-mount in situ hybridization on E7.5 embryos using the mesodermal marker Brachyury as a probe.
Growth and developmental retardation of Sp2-deficient embryos were already visible at E7.5 exemplified by the expression pattern of the mesodermal marker Brachyury. At this stage wild type and heterozygous Sp2 embryos displayed intense Brachyury expression. Expression of Brachyury in the smaller Sp2null littermates, however, was very weak (Figure 6D) resembling expression of Brachyury in the nascent primitive streak at the onset of gastrulation of E6.5 embryos [19], [20].
Generation of Conditional Sp2 Knockout Mice (Sp2cko/cko Mice)
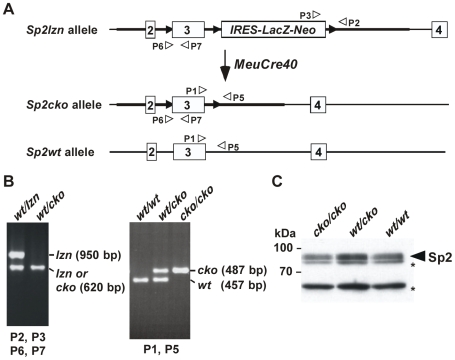
As inactivation of Sp2 is mid-gestational lethal we decided to generate mice with floxed exon 3 lacking the IRES-LacZ-neo cassette that would allow inactivating Sp2 in a stage-specific manner as well as in cell lines derived from these mice. Generation of such a conditional Sp2 knockout allele (Sp2cko allele) could be accomplished by selective removal of the IRES-lacZ-neo cassette upon partial Cre-loxP recombination (Figure 7). Injection of fertilized eggs with different concentrations of Cre RNA [21] resulted either in complete excision of exon 3 and the IRES-lacZ-neo cassette or excision of exon 3, but never in excision of only the IRES-lacZ-neo cassette. Therefore, we employed an in vivo approach.
Figure 7. Generation of conditional Sp2 mice (Sp2cko/cko mice).
A) Crossing of heterozygous Sp2wt/lzn mice with MeuCre40 mice resulted in generation of an Sp2cko allele in which the lacZ-Neo fusion gene is absent. B) PCR analysis of the Sp2 wild type (wt), Sp2-IRES-lacZ-Neo (lzn) and Sp2cko (cko) alleles with the indicated primers. C) Western blot analysis of Sp2 in extracts of Sp2cko/cko, Sp2cko/wt and Sp2wt/wt MEFs.
MeuCre40 (mosaic-early embryonic-ubiquitous Cre transgene), a transgenic mouse line that has been shown to generate partial mosaic Cre-loxP recombination patterns in the early embryo [22], were crossed with heterozygous Sp2wt/lzn mice. This resulted in mosaicism for recombination events at the Sp2lzn locus that was transmitted through the germline. After back crossing mosaic animals to wild type mice we found a single female, out of 130 progeny analysed, that carried an allele with the desired floxed exon 3 but lacking the IRES-lacZ-neo cassette (Sp2wt/cko) (Figure 7B), the Sp2null allele and Cre recombinase. Crossing of the single Sp2wt/cko female with wild type mice resulted also in male Sp2wt/cko animals.
Homozygous Sp2cko/cko mice in which both Sp2 alleles are floxed, were obtained by intercrossing of Sp2wt/cko mice. Consistent with wild type Sp2 protein expression from the floxed Sp2 locus (Figure 7C), Sp2cko/cko mice are phenotypically indistinguishable from wild type animals.
Sp2 Is Essential for Proliferation of Mouse Embryonic Fibroblasts
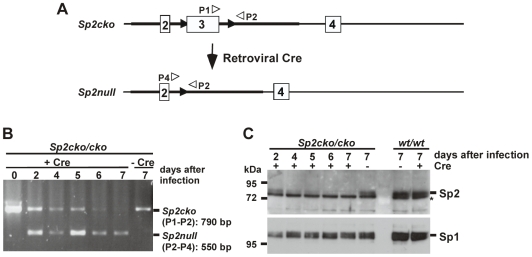
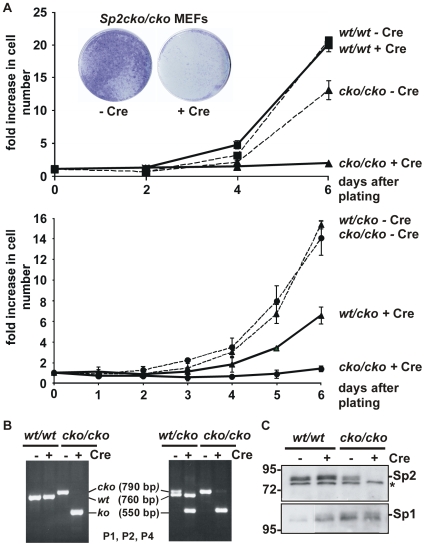
Mouse embryonic fibroblasts (MEFs) obtained from E9.5 Sp2null embryos became flat-shaped and did not divide, whereas wild type and heterozygous MEFs derived from siblings grew normally. The lack of proliferation of MEFs obtained from E9.5 Sp2null embryos could reflect the developmental retardation of these embryos or could be due to the lack of Sp2. To explore directly the potential role of Sp2 in cellular proliferation of MEFs we isolated MEFs carrying floxed Sp2 alleles (Sp2cko/cko) from E13.5 embryos. Sp2cko/cko MEFs and corresponding wild type and heterozygous MEFs (Sp2wt/cko) were immortalized by serial passages of primary MEFs until they pass their growth-crisis stage. Infection of Sp2cko/cko MEFs with a retrovirus expressing Cre recombinase resulted in a time-dependent deletion of the floxed Sp2 alleles and complete loss of the Sp2 protein at seven days post-infection (Figure 8).
Figure 8. Depletion of Sp2 in Sp2cko/cko MEFs upon retroviral Cre-infection.
A) Schematic presentation of the Sp2cko allele and the Sp2null allele obtained after retroviral Cre infection along with the positions of the primers used for genotyping. B) PCR analysis of Cre-infected Sp2cko/cko MEFs at various time points post-infection as indicated. C) Western blot analysis of Sp2 in Cre-infected Sp2cko/cko and Sp2wt/wt MEFs at various time points post-infection as indicated. The asterisk indicates a cross-reacting protein. To control for loading, the same blot was re-stained for Sp1.
Consistent with previous reports [23], [24] continuous Cre expression in wild type cells slightly decreased growth (Figure 9). However, Cre-mediated ablation of Sp2 in Sp2cko/cko MEFs resulted in strongly reduced proliferation relative to control retrovirus-infected cells (Figure 9). This finding together with the observation that MEFs derived from Sp2null embryos do not proliferate suggest a role of Sp2 in the control of cellular proliferation.
Figure 9. Sp2 is required for proliferation of MEFs.
A) MEFs with the indicated genotypes were infected with a Cre-expressing (+ Cre) or an empty control (- Cre) retrovirus, selected with puromycin for seven days and subsequently replated for determination of growth curves. The two growth curves were performed with two different Sp2cko/cko lines. Results are presented as fold increase in cell number relative to time 0. The insert in the upper panel shows a crystal violet staining of Sp2cko/cko MEFs infected with an empty retrovirus (- Cre) or a Cre-expressing retrovirus (+ Cre). B) PCR analyses of genomic DNA from Sp2wt/wt, Sp2wt/cko and Sp2cko/cko MEFs at day 0 of the growth curve determinations using the primer combination P1, P2, P4. C) Immunoblot analysis of Sp2 with MEF extracts of the indicated genotype.
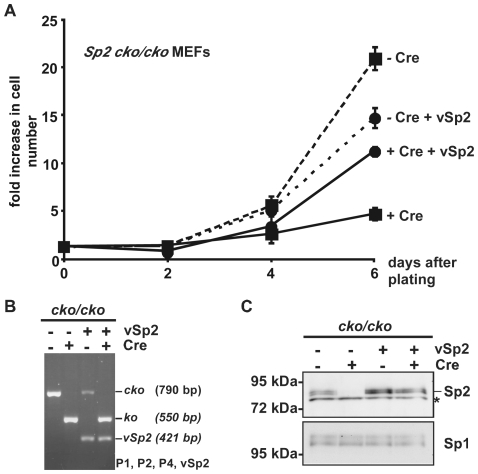
To further substantiate the conclusion that Sp2 is essential for proliferation of MEFs, we performed rescue experiments (Figure 10). First we infected Sp2cko/cko MEFs with a retroviral expression vector for Sp2 (pBABE-vSp2-puro), using infection with the corresponding empty vector (pBABE-puro) as a control. The resulting Sp2cko/cko-vSp2 MEFs expressed Sp2 ectopically in addition to endogenous Sp2. Cre-mediated ablation of endogenous Sp2 in Sp2cko/cko-pBABE-puro MEFs inhibited cell growth (compare -Cre and +Cre growth curves in Figure 10A). Retroviral Cre-infection of Sp2cko/cko-vSp2 led to a complete depletion of the floxed Sp2 alleles as well (Figure 10B). However, expression of ectopic Sp2 was unchanged (Figure 10C), and proliferation of these MEFs was affected much less severe (compare +Cre and +Cre+vSp2 growth curves in Figure 10A). These findings reinforce the conclusion that Sp2 plays an essential role in cell proliferation.
Figure 10. Partial rescue of proliferation by expression of exogenous Sp2.
A) Sp2cko/cko MEFs expressing exogenous Sp2 (vSp2) were infected with an empty control (- Cre) or a Cre-expressing retrovirus (+ Cre), selected for seven days with puromycin and subsequently replated for determination of the proliferation rates. Results are presented as fold increase in cell number relative to time 0. B) PCR analysis of Sp2cko/cko MEFs containing a retroviral expression vector for Sp2 (vSp2). The primer combination P1, P2, P4, vSp2 was used for amplification of the Sp2cko allele (P1–P2, 790 bp), the Sp2ko allele (P2–P4, 550 bp) and the Sp2 cDNA (P1-vSp2, 421 bp). C) Western blot analysis of Sp2 in control and Cre-infected Sp2cko/cko and Sp2cko/cko-vSp2 MEFs. The asterisk indicates a cross-reacting band. To control for loading, the same blot was re-stained for Sp1.
Discussion
Sp2 was originally identified by virtue of the similarity of its zinc finger region with the DNA-binding domain of Sp1 [15]. It is expressed in many different human and mouse cell lines [16] suggesting wide-spread expression in vivo. Our Sp2 expression analysis in mouse embryos supports this notion. Mouse Sp2 mRNA is detectable in embryonic stem cells and in all tissues during different stages of mouse embryogenesis, with exception of the heart. In Western blots, the mouse Sp2 protein appears as a double band. Whether this is due to posttranslational modifications or reflects splice variants is unknown at this stage.
Insertion of the IRES-lacZ-Neo cassette into the third intron of the Sp2 gene resulted in aberrant expression of an Sp2-lacZ-neo fusion RNA in Sp2lzn/lzn mice. Although no Sp2 protein, neither full-length nor a C-terminal truncated fragment was detectable, it is very likely that the glutamine-rich N-terminal part of Sp2 is expressed at a very low level. This would explain why the Sp2lzn/lzn embryos display a markedly less severe phenotype when compared to Sp2null embryos. Sp2lzn/lzn embryos alive are found beyond E12.5 whereas disruption of the Sp2 gene in Sp2null embryos causes severe developmental retardation and embryonic lethality before E9.5. The different phenotypes of Sp2lzn/lzn and Sp2null mice indicates that the glutamine-rich N-terminal part of Sp2 has a function independent of the C-terminal zinc finger domain that is believed to be responsible for DNA-binding [16].
No particular cell lineage or developmental process appears to be affected in Sp2null embryos. Rather, Sp2-deficiency causes a general cellular defect that precludes normal progression of development resulting in early embryonic lethality. This conclusion is consistent with the observation that MEFs derived from E9.5 Sp2null embryos did not grow in culture. Moreover, Sp2lzn/lzn MEFs derived from E13.5 embryos stopped growing in culture and repeatedly died after the first passage whereas MEFs from wild type and Sp2wt/lzn littermates grew normally. Finally, Cre-mediated ablation of Sp2 in Sp2cko/cko MEFs severely impaired proliferation that could be rescued by ectopic expression of exogenous Sp2. Altogether, these findings strongly suggest that Sp2 is essential for the autonomous growth of MEFs in culture. Further analyses will be necessary to unravel the cellular processes that are responsible for the growth arrest of Sp2-depleted MEFs and to identify key target genes of Sp2. Furthermore, the mice carrying floxed Sp2 alleles described here will allow for the analysis of the role of Sp2 in distinct cell types and in adult animals.
In conclusion, we have shown that Sp2 is required for normal progression of embryonic development, and that it is essential for survival of mouse embryos after E9.5. Sp2-deficient MEFs fail to grow in vitro, strongly indicating that the lack of Sp2 causes a cell-autonomous proliferation defect in these cells. Our data further strengthen the notion that, despite their structural similarities, Sp1, Sp2, Sp3 and Sp4 have non-redundant important functions during mouse development. Future research in our laboratories will be aimed at understanding the molecular basis for these distinct functions. Conditional knockout alleles, such as described here for the Sp2 gene, are essential tools to perform these studies.
Materials and Methods
Ethics Statement
Research involving mice have been conducted according to the German Animal Protection Law (Tierschutzgesetz). The application for the experiments was reviewed and approved by the responsible local authorities (Regierungspräsidium Giessen, reference number V 54 - 19 c 20/15 cMR20/27).
Whole-Mount β-Gal Staining and In Situ Hybridization
Whole-mount β-galactosidase staining of E13.5 embryos was performed according to Tewari et al. [25]. Whole-mount RNA in situ hybridization was performed essentially as described [26] using BM purple (Roche) as color substrate. For Sp2 mRNA detection, we used a 903 bp digoxygenin-labeled fragment of exon 3 (nucleotides 183 to 1085 of mouse Sp2 cDNA) cloned in pcDNA3. Appropriate plasmids to generate probes for Brachyury (T) and Nkx2-5 were obtained from Martin Eilers and Thomas Braun, respectively. The GATA4 probe is described in [9].
Generation of Sp2-Specific Antibodies and Immunoblot
A polyclonal antiserum against mouse Sp2 was generated by immunization of New Zealand White rabbits with recombinant full-length mouse Sp2 protein expressed in E. coli according to standard procedures. The antiserum was subsequently affinity-purified using immobilized recombinant Sp2. For immunoblots, whole cell extracts from MEFs were separated through 6% SDS-polyacrylamide gels and blotted to PVDF membranes. Primary antibodies were visualized with the Amersham ECL kit.
Generation of Sp2lzn Mutant Mice
A cosmid clone containing exons 2 and 3 as well as flanking intron sequences of the murine Sp2 gene was isolated from a mouse 129/ola library at the German Science Centre for Genome Research (RZPD). A targeting vector was designed to flank exon 3 of the Sp2 gene and the selection marker with loxP sites as follows. As starting vector, we used the pPNT plasmid containing the pgk-driven neomycin resistance gene (pgk-neo) and the herpes simplex thymidine kinase (hsv-tk) gene [27]. LoxP sites flanking the pgk-neo cassette were inserted as synthetic XbaI-SalI-LoxP-KpnI and NotI-XhoI-LoxP-SalI-[XhoI] oligonucleotides. A 1.8 kb fragment of intron 3 obtained by PCR was cloned into the EcoRI site located between pgk-neo and hsv-tk. A fragment containing exon 3 and flanking intron sequences was cloned as a 1.6 kb NotI-XhoI PCR fragment upstream of the floxed pgk-neo cassette. A third loxP site was then inserted as NotI-BamHI-LoxP-[NotI] oligonucleotide. Subsequently, a 3.2 kb Sp2 genomic fragment containing exon 2 and flanking intron sequences was inserted as NotI-BamHI PCR fragment. Finally, we replaced pgk-neo by an SA-IRES-lacZ-neo-SVpA cassette obtained as a 7.4 kb SalI fragment from pGT1.8Iresβgeo [28]. More detailed information concerning the generation of the targeting vector will be provided upon request. We verified all of the constructs by restriction analysis and sequencing. The functionality of the loxP sites in the final targeting vector was analyzed by appropriate restriction digests after transformation into the Cre-expressing E. coli strain 294-Cre [29].
The targeting construct was introduced into C57BL/6×129/Ola F1 hybrid ES cells by electroporation and G418 resistant clones were screened for homologous recombination by Southern blotting using a 387 bp fragment overlapping intron 3 and exon 4 (Figure 2A). ES cells with a targeted Sp2 allele were injected into blastocysts. Blastocysts were transferred to pseudopregnant females and chimeric offspring were identified by PCR. Chimeric mice were crossed to heterozygosity resulting in Sp2wt/lzn mice.
Generation of Sp2null Mutant Mice
To generate an Sp2null allele, heterozygous Sp2wt/lzn mice were mated with CAG-Cre mice [18]. The resulting heterozygous Sp2wt/ko mice were intercrossed to generate Sp2null mice.
Generation of Conditional Sp2cko/cko Mice
Mosaic, early embryonic, ubiquitous Cre mice (MeuCre40) [22] were crossed with Sp2wt/lzn mice to remove the LacZ-Neo selection cassette from the Sp2 tri-loxP-allele. The F1 generation was screened for the presence of the Cre gene and partial recombination of the tri-loxP allele. Mice with partial and mosaic patterns, eg positive for Cre and the floxed exon 3 (primers P6–P7), were then mated with C57Bl/6 mice for segregation of the Cre-recombined alleles from MeuCre40 in the F2 generation.
Genotyping Embryos and MEFs by PCR
The following primers and amplicons were used for genotyping (see Figures 2, 5, 7 and 8 for orientation):
Primers:
P1: 5′-CCCTCTCAGAACTTTCAGATC-3′
P2: 5′-CTTAGGAGGGATCTAGACTAG-3′
P3: 5′- CATCGCCTTCTATCGCCTTCTTGA-3′
P4: 5′- ACCGAGAGCAAGTTCATGTC-3′
P5: 5′- GCTATTGCTCTTGTCTTTAGC-3′
P6: 5′-TATCCCTGCGGATCCATAACT-3′
P7: 5′-GGATACTTGCATTTGATCGGC-3′
vSp2: 5′-TCAGCCCACTGATAGTCAGG-3′
Cre 3′: 5′-CGATGCAACGAGTGATGAGGTTC-3′
Cre 5′: 5′-GCACGTTCACCGGCATCAAC-3′
Pex4: 5′-ATTCAGCTGCCATTCTCCGA-3′
Pex5: 5′-AGCCCACTGATAGTCAGGTT-3′
Amplicons:
Sp2 wild type allele: P1–P2: 760 bp; P1–P5: 457 bp
Lzn allele: P2–P3: 950 bp
Sp2null allele: P2–P4: 550 bp
Sp2cko allele: P1–P5: 487 bp
Lzn and Sp2cko alleles: P6–P7: 620 bp
pBABE-vSp2-puro: P1-vSp2: 421 bp
Sp2 cDNA: Pex4-Pex5: 229 bp
Northern Blot Analysis
Northern blot analysis was performed according to standard protocols with 20 µg of total RNA extracted from E18.5 embryos using a 32P-labeled 1.4 kb Sp2 cDNA probe encompassing exons 2 to 5 (Figure 3B).
Plasmids and Retroviral Infections
The pBABE-Cre-puro plasmid was previously described [30]. The retroviral pWLZ-Cre-neo plasmid was generated by cloning the Cre recombinase from pBABE-Cre-puro as a [XhoI]-EcoRI fragment into the [BamHI]-EcoRI-restricted pWLZ vector. The retroviral expression plasmid for Sp2 (pBABE-Sp2-puro) was obtained by cloning the full-length Sp2 cDNA into the BamHI site of pBABE-puro.
High-titre viruses were produced by transient transfection of retroviral constructs into the Phoenix-Eco packaging cell line using FuGENE HD transfection reagent (Roche) according to standard procedures. Retrovirally infected MEFs were selected with appropriate antibiotics using the following concentrations: 2 µg/mL of puromycin and 1 mg/mL of G418.
Determination of Growth Curves
MEFs were isolated from E9.5 and E13.5 embryos using standard methods. MEFs were immortalized by serial passages of primary MEFs. After retroviral infection MEFs were selected with appropriate antibiotics for seven days. Subsequently, 2.5×104 cells were plated on 6-cm dishes and duplicates were counted after 2, 4 and 6 days.
Histology
Embryos were dissected and a tail snip or the yolk sac was removed for genotyping. Embryos were fixed in Carnoy's solution (60% ethanol, 30% chloroform, 10% acetic acid) at 4°C overnight and embedded in paraffin according to standard procedures. Sections (8 µm) were stained with hematoxylin and eosin.
Acknowledgments
We thank Iris Rohner and Waltraud Sperling for excellent technical assistance. Martin Holzenberger generously provided MeuCre40 mice. The pBABE-Cre-puro plasmid was a gift from Kay-Uwe Wagner.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants of the Deutsche Forschungsgemeinschaft to GS (GRK767) and the Dutch Organisation for Scientific Research (Nederlandse Organisatie voor Wetenschappelijk Onderzoek, NWO) to SP (DN 82-294). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bouwman P, Philipsen S. Regulation of the activity of Sp1-related transcription factors. Mol Cell Endocrinol. 2002;195:27–38. doi: 10.1016/s0303-7207(02)00221-6. [DOI] [PubMed] [Google Scholar]
- 2.Philipsen S, Suske G. A tale of three fingers: the family of mammalian Sp/XKLF transcription factors. Nucleic Acids Res. 1999;27:2991–3000. doi: 10.1093/nar/27.15.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suske G. The Sp-family of transcription factors. Gene. 1999;238:291–300. doi: 10.1016/s0378-1119(99)00357-1. [DOI] [PubMed] [Google Scholar]
- 4.Suske G, Bruford E, Philipsen S. Mammalian SP/KLF transcription factors: bring in the family. Genomics. 2005;85:551–556. doi: 10.1016/j.ygeno.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Hagen G, Müller S, Beato M, Suske G. Cloning by recognition site screening of two novel GT box binding proteins: a family of Sp1 related genes. Nucleic Acids Res. 1992;20:5519–5525. doi: 10.1093/nar/20.21.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marin M, Karis A, Visser P, Grosveld F, Philipsen S. Transcription factor Sp1 is essential for early development but dispensable for cell growth and differentiation. Cell. 1997;89:619–628. doi: 10.1016/s0092-8674(00)80243-3. [DOI] [PubMed] [Google Scholar]
- 7.Bouwman P, Göllner H, Elsässer HP, Eckhoff G, Karis A, et al. Transcription factor Sp3 is essential for post-natal survival and late tooth development. EMBO J. 2000;19:655–661. doi: 10.1093/emboj/19.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Göllner H, Dani C, Phillips B, Philipsen S, Suske G. Impaired ossification in mice lacking the transcription factor Sp3. Mech Dev. 2001;106:77–83. doi: 10.1016/s0925-4773(01)00420-8. [DOI] [PubMed] [Google Scholar]
- 9.Van Loo PF, Mahtab EA, Wisse LJ, Hou J, Grosveld F, et al. Transcription factor Sp3 knockout mice display serious cardiac malformations. Mol Cell Biol. 2007;27:8571–8582. doi: 10.1128/MCB.01350-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krüger I, Vollmer M, Simmons DG, Elsässer HP, Philipsen S, et al. Sp1/Sp3 compound heterozygous mice are not viable: impaired erythropoiesis and severe placental defects. Dev Dyn. 2007;236:2235–2244. doi: 10.1002/dvdy.21222. [DOI] [PubMed] [Google Scholar]
- 11.Supp DM, Witte DP, Branford WW, Smith EP, Potter SS. Sp4, a member of the Sp1-family of zinc finger transcription factors, is required for normal murine growth, viability, and male fertility. Dev Biol. 1996;176:284–299. doi: 10.1006/dbio.1996.0134. [DOI] [PubMed] [Google Scholar]
- 12.Göllner H, Bouwman P, Mangold M, Karis A, Braun H, et al. Complex phenotype of mice homozygous for a null mutation in the Sp4 transcription factor gene. Genes Cells. 2001;6:689–697. doi: 10.1046/j.1365-2443.2001.00455.x. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen-Tran VT, Kubalak SW, Minamisawa S, Fiset C, Wollert KC, et al. A novel genetic pathway for sudden cardiac death via defects in the transition between ventricular and conduction system cell lineages. Cell. 2000;102:671–682. doi: 10.1016/s0092-8674(00)00089-1. [DOI] [PubMed] [Google Scholar]
- 14.St Amand TR, Lu JT, Zamora M, Gu Y, Stricker J, et al. Distinct roles of HF-1b/Sp4 in ventricular and neural crest cells lineages affect cardiac conduction system development. Dev Biol. 2006;291:208–217. doi: 10.1016/j.ydbio.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Kingsley C, Winoto A. Cloning of GT box-binding proteins: a novel Sp1 multigene family regulating T-cell receptor gene expression. Mol Cell Biol. 1992;12:4251–4261. doi: 10.1128/mcb.12.10.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moorefield KS, Fry SJ, Horowitz JM. Sp2 DNA binding activity and trans-activation are negatively regulated in mammalian cells. J Biol Chem. 2004;279:13911–13924. doi: 10.1074/jbc.M313589200. [DOI] [PubMed] [Google Scholar]
- 17.Moorefield KS, Yin H, Nichols TD, Cathcart C, Simmons SO, et al. Sp2 localizes to subnuclear foci associated with the nuclear matrix. Mol Biol Cell. 2006;17:1711–1722. doi: 10.1091/mbc.E05-11-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakai K, Miyazaki J. A transgenic mouse line that retains Cre recombinase activity in mature oocytes irrespective of the cre transgene transmission. Biochem Biophys Res Commun. 1997;237:318–324. doi: 10.1006/bbrc.1997.7111. [DOI] [PubMed] [Google Scholar]
- 19.Rivera-Perez JA, Magnuson T. Primitive streak formation in mice is preceded by localized activation of Brachyury and Wnt3. Dev Biol. 2005;288:363–371. doi: 10.1016/j.ydbio.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Wilkinson DG, Bhatt S, Herrmann BG. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature. 1990;343:657–659. doi: 10.1038/343657a0. [DOI] [PubMed] [Google Scholar]
- 21.de Wit T, Drabek D, Grosveld F. Microinjection of cre recombinase RNA induces site-specific recombination of a transgene in mouse oocytes. Nucleic Acids Res. 1998;26:676–678. doi: 10.1093/nar/26.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leneuve P, Colnot S, Hamard G, Francis F, Niwa-Kawakita M, et al. Cre-mediated germline mosaicism: a new transgenic mouse for the selective removal of residual markers from tri-lox conditional alleles. Nucleic Acids Res. 2003;31:e21. doi: 10.1093/nar/gng021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loonstra A, Vooijs M, Beverloo HB, Allak BA, van Drunen E, et al. Growth inhibition and DNA damage induced by Cre recombinase in mammalian cells. Proc Natl Acad Sci U S A. 2001;98:9209–9214. doi: 10.1073/pnas.161269798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silver DP, Livingston DM. Self-excising retroviral vectors encoding the Cre recombinase overcome Cre-mediated cellular toxicity. Mol Cell. 2001;8:233–243. doi: 10.1016/s1097-2765(01)00295-7. [DOI] [PubMed] [Google Scholar]
- 25.Tewari R, Gillemans N, Harper A, Wijgerde M, Zafarana G, et al. The human beta-globin locus control region confers an early embryonic erythroid-specific expression pattern to a basic promoter driving the bacterial lacZ gene. Development. 1996;122:3991–3999. doi: 10.1242/dev.122.12.3991. [DOI] [PubMed] [Google Scholar]
- 26.Belo JA, Bouwmeester T, Leyns L, Kertesz N, Gallo M, et al. Cerberus-like is a secreted factor with neutralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech Dev. 1997;68:45–57. doi: 10.1016/s0925-4773(97)00125-1. [DOI] [PubMed] [Google Scholar]
- 27.Tybulewicz VLJ, Crawford CE, Jackson PK, Bronson RT, Mulligan RC. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-able proto-oncogene. Cell. 1991;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 28.Mountford P, Zevnik B, Duwel A, Nichols J, Li M, et al. Dicistronic targeting constructs: reporters and modifiers of mammalian gene expression. Proc Natl Acad Sci USA. 1994;91:4303–4307. doi: 10.1073/pnas.91.10.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchholz F, Angrand PO, Stewart AF. A simple assay to determine the functionality of Cre or FLP recombination targets in genomic manipulation constructs. Nucleic Acids Res. 1996;24:3118–3119. doi: 10.1093/nar/24.15.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krempler A, Henry MD, Triplett AA, Wagner KU. Targeted deletion of the Tsg101 gene results in cell cycle arrest at G1/S and p53-independent cell death. J Biol Chem. 2002;277:43216–43223. doi: 10.1074/jbc.M207662200. [DOI] [PMC free article] [PubMed] [Google Scholar]