Abstract
In previous studies, acutely administered oral methylphenidate (MPD, 3 mg/kg) prior to testing improved performance on the radial arm maze in juvenile rats. In order to examine the mechanisms producing this improvement we administered MPD once prior to each test of anxiety, locomotor activity and attention. On postnatal day (PND) 22 on an elevated plus-maze, rats spent more time beyond the rails on the open arms and showed altered risk-assessment behaviors suggesting an anxiolytic-like effect of MPD. Grid crossings on the plus-maze indicated that MPD increased locomotor activity, as did activity recording on PND 23. In another group of juveniles, MPD improved performance in a multi-trial attention task in an age-dependent fashion. These data suggest that oral MPD has multifaceted effects on juvenile rats that together improve performance on cognitive tests such as the radial arm maze. In addition, our data support human studies finding multifaceted effects of MPD.
Keywords: Attention, Elevated plus-maze, Individual differences, Locomotor activity, Oral administration, Ritalin
1. Introduction
Methylphenidate (MPD, Ritalin) is prescribed to approximately 2.8 percent of children and adolescents aged 5 to 18 in the United States for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) (Safer, D. J. et al.1996; Robison, L. M. et al.1999). The neural mechanisms underlying the therapeutic efficacy of MPD remain poorly understood. Although human studies have demonstrated the effectiveness of MPD, clinical studies are difficult to control for the influences of factors such as diet, compliance, and environment. Therefore animal studies are useful to assess the biological effects of MPD under controlled conditions.
Few publications have characterized the effects of acutely administered MPD in juvenile animals using clinically relevant doses and routes of administration and testing during the active phase of the circadian cycle (Kuczenski, R. and Segal, D. S.2002) while chronic administration has been studied (e.g., Bethancourt, JA et al. 2009; Britton, GB. and Bethancourt, JA 2009; Dow-Edwards et al., 2008). The juvenile period in rats corresponds to childhood and early adolescence in humans when most patients take MPD. Dopamine (DA) and norepinephrine (NE) systems, the primary targets of MPD (Markowitz, J. S. et al.2006) are still developing during this period. In primate brain, DA varicosities in the prefrontal cortex (PFC) increase throughout childhood, plateau and then dramatically decline to adult levels in mid-puberty (Levitt, P.2003). The NE content in most regions of monkey brain increases at a slower rate until it reaches the adult level in mid-puberty (Brown, R. M. and Goldman, P. S.1977; Goldman-Rakic, P. S. and Brown, R. M.1982). The relative concentrations of DA and NE in brain regions such as prefrontal cortex also differ over the course of development (Brown, R. M. and Goldman, P. S.1977; Goldman-Rakic, P. S. and Brown, R. M.1982). Therefore, the response to MPD may be changing throughout the juvenile period and is expected to be different from that in the adult. The juvenile rat brain resembles the developing primate brain in many ways. For instance, basal DA synthesis in PFC increases considerably at postnatal day (PND) 30 and then declines until adulthood (Andersen, S. L. et al.1997); the NE levels reach the adult concentrations between 30-40 days (Loizou, L. A. and Salt, P.1970; Konkol, R. J. et al.1978; Morris, M. J. et al.1980). Thus we utilized juvenile rats (PND 22 to PND 40) to model the effects of MPD administration to children.
The developing DA and NE systems such as the locomotor pathways (see Koob, G. F. and Swerdlow, N. R.1988 for a review) and anxiety-related pathways (Schildkraut, J. J.1967; Asan, E.1998) respectively, subserve many complex behaviors. All behavioral measures are a compilation of multiple cognitive, motivational and experiential factors. Therefore MPD may contribute multifaceted effects to the outcome of behavioral studies.
A previous study showed enhanced performance in MPD-treated juvenile rats on the radial arm maze from PND 22 to 25 (Zhu, N. et al.2007). Olton and colleagues demonstrated the role of anxiety in performance on the radial arm maze by comparing the rat’s behaviors on elevated mazes with that on floor mazes (Olton, D. S. and Samuelson, R. J.1976; Olton, D. S. et al.1977). Locomotor activity, of course, was also required to perform the task. Thus we hypothesized that alterations of anxiety, locomotor activity and attention by MPD contributed to this enhanced performance. The present study therefore evaluated the acute effects of MPD administered with minimal stress at a clinically relevant dose on each of these aspects of behavior in juvenile male and female rats during the active phase of the circadian cycle. We expected to see decreased anxiety, somewhat increased locomotor activity and improved attention in the MPD-treated juvenile rats.
2. Experimental procedures
2.1. Subjects
Male and female Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) arrived in natural litters with the dams on PND 1. Animals were kept in a temperature-controlled room with lights off from 11:00 am to 11:00 pm. To be consistent with a previous study (Zhu, N. et al.2007), from PND 21 on rats were individually housed and food-deprived to 90% of body weight based on historical age-sex-matched free-fed controls. A split-litter design allotted 2 animals per litter in every sex-by-treatment group. All procedures were approved by the State University of New York Institutional Animal Care and Use Committee.
2.2. Apparatus
2.2.1. Elevated Plus-Maze
The black Plexiglas maze was elevated 58 cm above the floor. All 4 arms were 51 cm L×11 cm W. Each arm had 3 white-painted grid lines. 42 cm high walls enclosed two opposing arms (enclosed arms). The other two arms (open arms) had only short rails (23 cm L×6 cm H) adjacent to the center start box.
2.2.2. Locomotor Activity Monitors
Digiscan Activity Monitor (model VMRXYZ16; Accuscan Instruments, Columbus, OH) had 48 infrared sensors spaced 2.5 cm apart with 16 sensors along each side to measure horizontal activity. Additional 16 sensors were 10 cm from the floor of the box to measure vertical activity. A transparent Plexiglas box with removable lid (42 L×42 W×30 H cm) confined the animal in the center of the monitor. Each monitor was within a white laminate sound-attenuating chamber (60 L×60 W×37 H cm inside) containing two 6-W red-light bulbs and a fan (model 30 CFM, Dayton).
2.2.3. Attention Task
This appetitive task was carried out in the home cage of every rat. Wood boxes with removable lids were utilized to conceal food reward (Honey Nut Cheerios®, General Mills, MN). The boxes were 9 L × 6 W × 4 H cm outside and 7.7 L × 4 W × 3.2 H cm inside. Two boxes were plain wood while 4 other boxes were covered with black-rubber, sandpaper, green-paper and white rubber-mesh, respectively. All boxes contained a thin layer of shavings mixed with bits of crumbled Cheerios® to mask olfactory cues. The equipment included three plain wood lids, a black-rubber-covered lid and a sandpaper-covered lid. Every lid was 11 L × 7.7 W cm. All lids were made heavy with weights (~20 g), except one plain wood lid for pre-training (~9 g).
2.3. Drug Administration
2.3.1. Selection of Dose
Therapeutic doses of MPD in treatment of ADHD typically produce peak plasma drug levels in the range of 8 to 40 ng/ml (reviewed in Swanson, J. M. and Volkow, N. D.2002). In rats, oral MPD at 2.0 mg/kg and 3.5 mg/kg produce plasma levels of 36 ng/ml and 62 ng/ml, respectively (Aoyama, T. et al.1990). A previous study in rat has shown that 3.0 mg/kg of MPD via gavage did improve performance on radial arm maze task but did not elevate locomotor activity at the end of the dosing period (Dow-Edwards, D.L. et al., 2008). Therefore, we chose 3.0 mg/kg of oral MPD for the current study. MPD at this dose should yield peak plasma levels approximately between 30 and 60 ng/ml, corresponding to the upper end of the clinical range.
2.3.2. Dosing Procedure
MPD HCl (Research Triangle Institute, Research Triangle Park, NC) was dissolved in sterile water to a concentration of 4 mg/ml. The solution was administered at a volume of 0.75 ml/kg body weight on a portion of a small cracker (Pathmark Oyster Cracker) to produce a dose of 3 mg/kg of MPD. Rats were weighed daily and doses were adjusted daily. Rats weighing below 100 g and above 100 g received an eighth and a quarter of a cracker, respectively. All rats consumed the entire dose within 1 min on each day of testing.
2.4. Behavioral Testing
2.4.1. Elevated Plus-Maze
On PND 22, rats received a single dose of MPD or sterile water on a cracker. 30 min after dosing, each rat was placed in the start box in the center of the maze, facing the open arm away from the experimenter. After 5 seconds, the start box was removed and a session began. Each rat was video-recorded for 10 min. Tapes were analyzed with ethological software (Observer 5.0, Noldus, Netherlands) for time spent in the open arms (Open Arm Time), entries to the open arms and time spent beyond the rails (Time Beyond Rails). Stretch-attended postures and head dips were recorded as measures of risk-assessment behaviors, which are more sensitive measures of anxiety on the elevated plus maze than Open Arm Time (Rodgers, R. J. and Cole, J. C.1993; Rodgers, R. J. et al.1999). A stretch-attended posture was a posture where the rat stretched forward to its full body length without moving the hind limbs and then returned to its original position. A head dip occurred when the rat stretched its head and shoulders over the edge of the maze and looked down to the floor. The enclosed arms, the center platform and the railed areas of the open arms were classified “protected areas”. The segments beyond the rails were considered “unprotected areas”. Stretch-attended postures and head dips were considered “protected” when displayed with the hind body in the protected areas, and “unprotected” when displayed with the hind body in the unprotected areas. Grid crossing, rearing and grooming were also assessed from the videotape.
2.4.2. Locomotor Activity Recording
On PND 23, rats tested on the elevated plus maze the previous day were individually placed into Digiscan Activity Monitors. After 15-min recording of basal activity, a cracker treated with MPD or sterile water was given to the rat. Rats received the same drug/vehicle treatment as on the previous day. Recording resumed for another 40 min once the entire cracker was consumed (within 1 min). Total distance traveled in cm was recorded in 5-min time blocks. Time spent in the margin area (Margin Time), also called thigmotaxis/wall-hugging (Treit, D. and Fundytus, M.1989), and time spent in center area (Center Time) were recorded in seconds as measures of anxiety.
2.4.3. Attention task
Drug-naïve subjects were tested in a simple attention task based on the published work of Strupp’s group (Bunsey, M. et al.1990). On PND 19, rats were removed from the dams for 5 min and habituated to the testing environment, apparatus and the food reward. On PND 20 and 21, rats were habituated with Cheerios® and crackers. The task included a pre-training phase and 3 testing phases. In all phases, rats received 6 consecutive trials beginning at 11:00 am and 6 trials beginning at 3:00 pm, 7 days a week. A maximum of 5 min was allotted for a trial.
Pre-training
On PND 22, rats were trained in the home cage to remove a lid from a plain wooden box in order to retrieve a food reward, a third of a Cheerio®. Initially, a light-weight wooden lid was placed on the box diagonally. Once the rat learned to remove the diagonal lid and to retrieve the reward, the lid was placed squarely on the box. After the rat learned to lift the light lid, a heavy lid was used to maximize the cost of an error. Typically rats removed the heavy lid in less than 2 min by the end of the second pre-training session. Rats that did not consistently remove the lid after 36 trials (N=1) were excluded from further testing. No drug was administered during pre-training phase.
Training
On PND 23, rats were assigned semi-randomly to treatment x sex conditions (2 rats/condition/litter). MPD or sterile water was administered on a cracker 30 min prior to testing session. The black-rubber-covered box and the sandpaper-covered box were used. The black-rubber-covered box consistently contained the food reward. The lids were both plain wood and heavy. The location of the rewarded box was pseudorandom and on one side for no more than 4 trials in a given session. The same left-right sequence applied to all rats on any given day. A choice was defined as the lifting of the lid with the rat’s nose or paws, high enough to put its nose into the box. Failure to break at least one side of the contact between box and lid was not considered a choice. The criterion was 12 correct choices within 15 consecutive trials. After reaching the criterion, a rat entered the Extra-dimensional Shift phase.
Extra-Dimensional Shift
This phase is referred to below as the “Shift phase”. The salient material (black rubber) covered the lid instead of the sides of the box. The other lid was covered with sandpaper. The boxes were plain wood. The dosing and testing procedures, and the criterion were the same as used in the Training phase.
Distraction
Two novel materials (green paper and white rubber mesh) were introduced. The new materials covered the boxes and created a distraction, while the salient material on the lids continued to indicate the location of the reward. The rats had to ignore the new-material covering the boxes and continue paying attention to the predictive cues on the lids. The same dosing and testing procedures, and the same criterion were followed as in the previous 2 phases. Additionally, the location of the Green/White boxes was pseudorandom, that is, the same box was on one side for no more than 4 trials in a session.
Trials to reach criterion (Trials to Criterion) of each phase and trials to complete the entire task (Trials to Completion) were recorded. Age in days at the beginning of each phase was also recorded for further assessment of the effectiveness of MPD across the period of development.
2.5. Data Analysis
All analyses were carried out by SAS 9.1.3 software (SAS Institute, Cary, NC). Sex, treatment and the interaction of sex and treatment were considered fixed factors in all analyses. The dependent variables were transformed as necessary to reduce skew and to stabilize variance. few outlying observations (n ≤ 2 for a given analysis) were excluded.
Continuous dependent variables, such as Open Arm Time and total distance traveled, were analyzed in mixed linear models. Litter was introduced as a random factor. Satterthwaite adjustments were made to denominator degrees of freedom. Time interval after dosing in locomotor activity was considered a fixed factor and collapsed into 10-min blocks. Sum of baseline activity was analyzed and was considered a fixed continuous variable for the regression analysis of total distance traveled. An unstructured within-subject covariance matrix was fitted for analysis across time. For Margin Time, a proportional hazards model was fitted to allow for a right-censored dependent variable due to the “ceiling effect”. Litter was introduced as a stratification factor.
For count dependent variables, such as head dip and Trials to Criterion, generalized estimating equations models were applied. Litter effect was controlled as a cluster variable with an exchangeable covariance structure. A negative binomial distribution was specified for the dependent variable and over-dispersion parameter was introduced.
The effects of age differences in the Shift and the Distraction phase and Trials to Completion of the attention task were analyzed in mixed linear models. Litter was introduced as a random factor. Age at the beginning of each phase, if applicable, was considered a continuous predictor. Satterthwaite adjustments were applied to denominator degrees of freedom.
3. Results
3.1. Elevated Plus-Maze
No significant litter effect was found in any analysis for this test. Since the previous radial arm maze test was 5 min in duration, the behavior during the first 5 min on the elevated plus-maze was analyzed separately from the behaviors during the total 10 min. Open Arm Time revealed no significant effect of treatment, sex or interaction of treatment and sex (data not shown). Time Beyond Rails in the first 5 min (Fig 1A) showed a significant effect of treatment [F(1, 31)=12.04, p=0.0015] and an interaction of treatment and sex [F(1, 31)=8.19, p=0.0074]. A simple effects analysis revealed that MPD increased Time Beyond Rails significantly in females while it had little effect in males (Fig 1B). As illustrated in Fig 2, total number of stretch-attended postures showed a marginally significant treatment effect [Chi-Square=3.42, df=1, p=0.0644] as the MPD-treated group made fewer stretch-attended postures than the vehicles. The number of protected stretch-attended postures showed a significant treatment effect in the same direction [Chi-Square=4.41, df=1, p=0.0358]. Total number of head dips and number of unprotected head dips were significantly greater in the MPD-treated animals [Chi-Square=4.35, df=1, p=0.0371; Chi-Square=4.21, df=1, p=0.0401, respectively]. No effect of sex or interaction of treatment and sex was detected. Grid crossings (Fig 3) were significantly increased in the treated group [F(1, 31)=19.45, p=0.0001]. Sex and the interaction of treatment and sex were not significant. Increases in Time Beyond Rails, head dips and grid crossing, together with decreased stretch-attended postures on the elevated plus maze comprise a behavioral profile consistent with MPD acting as an anxiolytic agent in both sexes.
Figure 1.
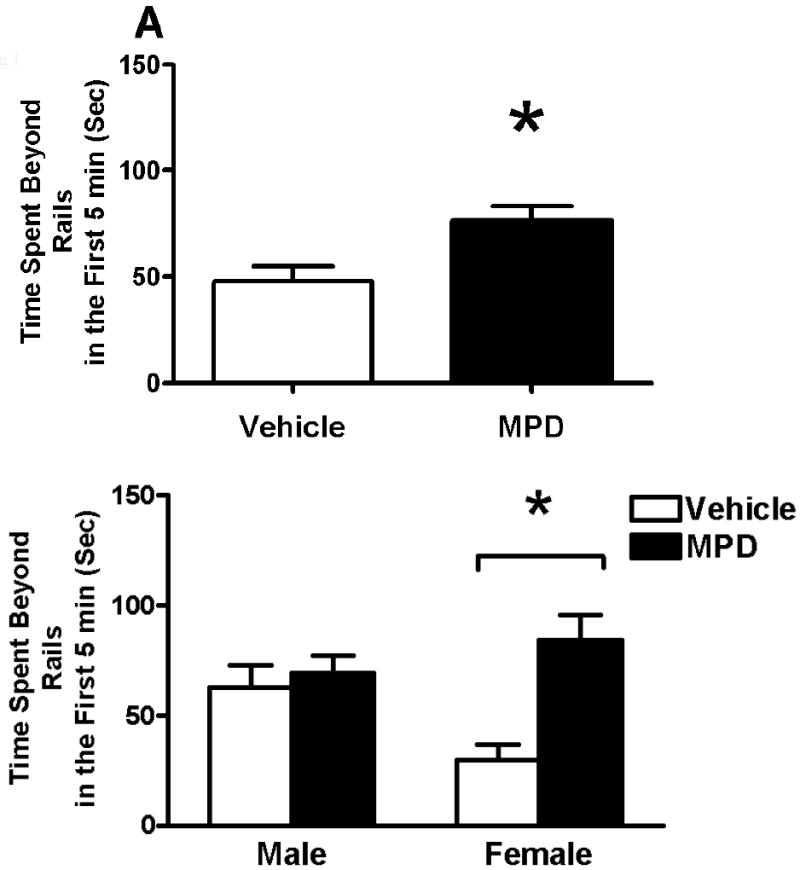
Effects of oral MPD and the interaction of MPD and sex on time spent beyond the rails during the first 5 min on the elevated plus maze. A. Mean (+ SEM) time spent beyond the rails of the MPD-treated rats and the controls. B. Means (+ SEM) of time spent beyond the rails are shown for the sex by treatment groups. * Significant difference from control (p<0.01).
Figure 2.
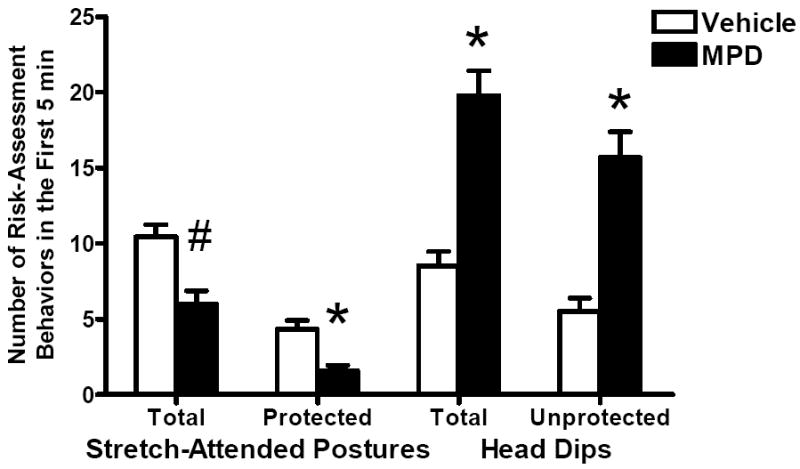
Effect of oral MPD on the risk-assessment behaviors during the first 5 min on the elevated plus maze. Mean numbers (+SEM) of stretch-attended postures, protected stretch-attended postures, head dips and unprotected head dips are shown for the treatment groups. The MPD-treated rats made fewer protected stretch-attended postures and tended to make fewer total stretch-attended postures than the controls did. Oral MPD increased total head dips and unprotected head dips. * Significant difference from the control (p<0.05). # Marginally significant difference from the control (p=0.0644).
Figure 3.
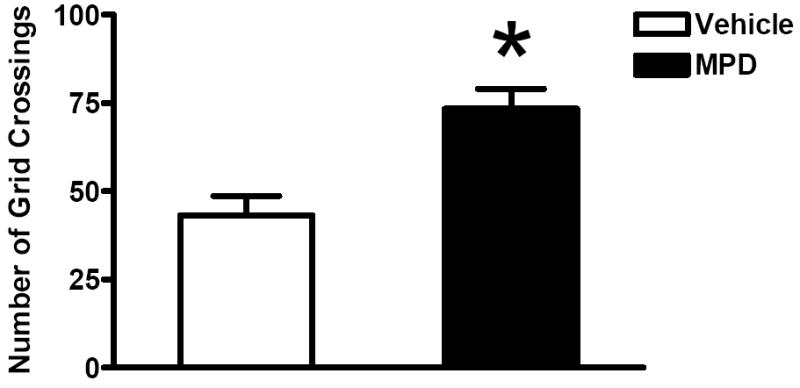
Mean numbers (+SEM) of grid crossings for the MPD-treated and the vehicle-treated groups during the first 5 min on the elevated plus maze. * Significant difference from the controls (p<0.001).
During the 10 min on the elevated plus-maze, a similar pattern of results was found as that during the first 5 min (data not shown) except that time Beyond Rails did not differ between treatment groups.
3.2. Locomotor Activity
We observed no significant litter effects in any measure. No difference was detected in baseline activity between treatment or sex groups. Figure 4A illustrates total distance traveled after dosing on PND 23 collapsed across sexes. A significant effect of treatment [F(1, 29)=14.68, p=0.0006] and a significant interaction of treatment and interval [F(3, 34)=4.16, p=0.0129] were seen. Simple effects analysis showed that the MPD-treated animals traveled more distance from 10 min to 40 min after dosing: 10 min to 20 min [F(1, 34)=7.38, p=0.0103], 20 min to 30 min [F(1, 28)=17.54, p=0.0003], and 30 min to 40 min [F(1, 27)=19.17, p=0.0002], compared to the vehicle rats. There were no significant effects of sex or interactions between sex and treatment and interval.
Figure 4.
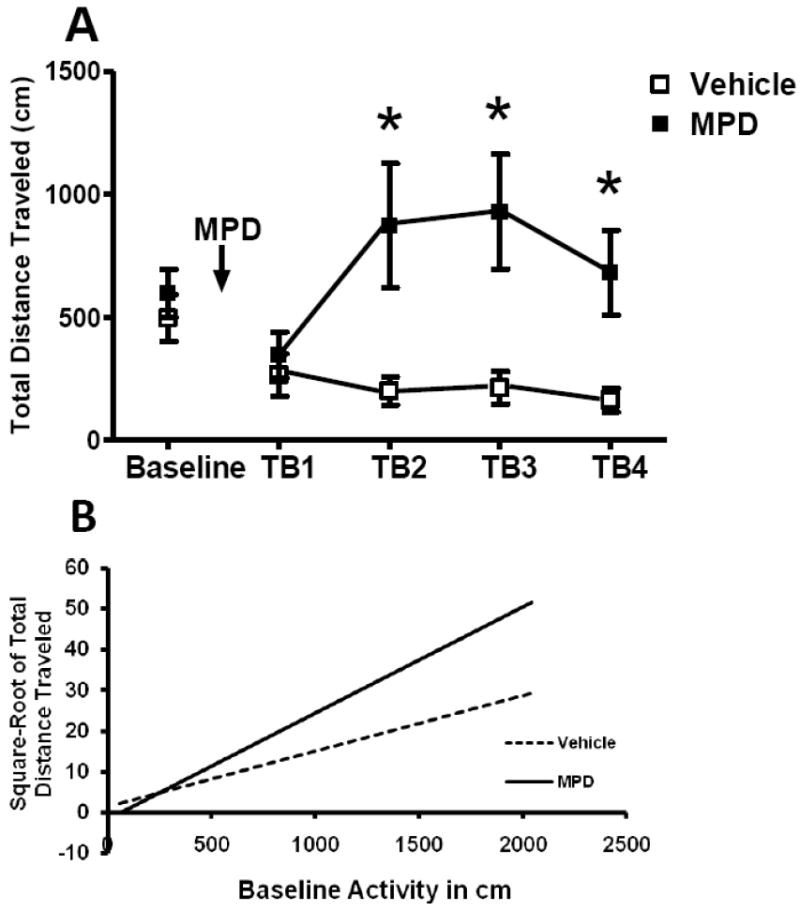
Effect of oral MPD on locomotor activity on PND 23. A. Means (±SEM) of total distance traveled are shown for the treatment groups during the baseline period and during 10-min time blocks (TB) after administration of MPD. From 10 min to 40 min after dosing, that is, time blocks 2 through 4, the MPD-treated group traveled more distance than did the vehicle-treated group, with a peak of activity approximately 30 min (TB3) after dosing. * Significant difference between the vehicle group and the MPD-treated group (p<0.05). B. Regression lines for the relationship between baseline activities and the square-root of individual total distance traveled are shown for the vehicle and the treated groups. Time blocks 2, 3 and 4 are collapsed for illustration. From 10 min to 40 min after dosing, MPD increased distance traveled in animals more active at baseline but not in animals with a low level of baseline activity. Slopes of the two regression lines were significantly different (p≤0.001).
Activity during habituation may reflect a rat’s response to novelty. Since animals often respond to psychostimulants differently depending on their responses to novelty (Klebaur, J. E. et al.2001; Bevins, R. A. and Peterson, J. L.2004), we analyzed the effects of MPD on total distance traveled using baseline activity as a continuous variable to probe individual differences in response to MPD. This control procedure did not alter the pattern of significant effects. A significant interaction of baseline activity and treatment and time blocks was found [F(3, 34)=8.40, p<0.001]. Simple effects analyses showed significant interaction of baseline and treatment only for time block 2, 3 and 4 (p<0.001 in each case). Oral administration of MPD increased distance traveled in animals more active at baseline but not in animals with a low level of baseline activity during 10 to 20 min, 20 to 30 min and 30 to 40 min after dosing. This point is illustrated in fig 4B with a regression line of locomotor activity from 10 to 40 min after dosing covaried for baseline activity.
Baseline Center Time (Fig 5) revealed a significant sex effect [F(1, 34)=4.49 , p=0.0414]. This effect was not significant after MPD. The female rats spent less time in the center of the Accuscan monitor than males before treatment, indicating a higher basal level of anxiety in females.
Figure 5.
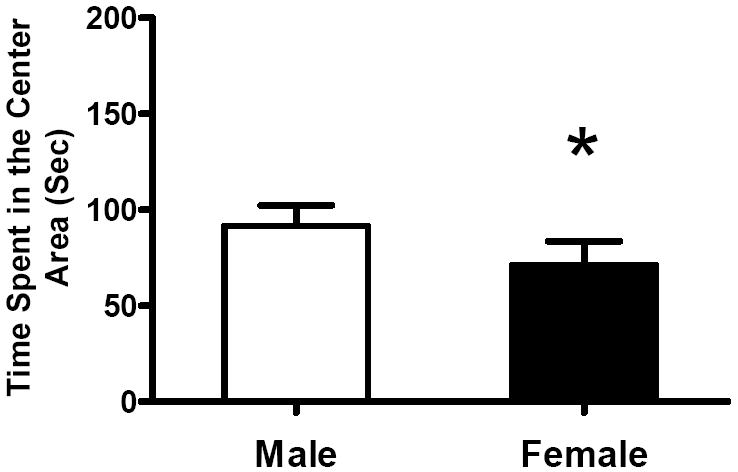
Means (+SEM) of time spent in center area of the activity monitor during baseline recording for the male and the female subjects are shown. Female rats spent less time in the center area compared with males. * Significant difference (p<0.05).
3.3. Attention Task
We found no significant litter effects in any dependent variable. Trials to Criterion of each phase of the task showed no significant effects of treatment, sex, or their interaction. Overall Trials to Completion however revealed a significant main effect of treatment [F(1, 31)=4.86, p=0.0351; Fig 6A], as well as a significant sex effect [F(1, 31)=4.45, p=0.0431; Fig 6B], but no significant interaction. The MPD-treated rats required fewer trials compared to the vehicles to accomplish the entire task. Females took fewer trials compared to males to complete the entire task as well.
Figure 6.
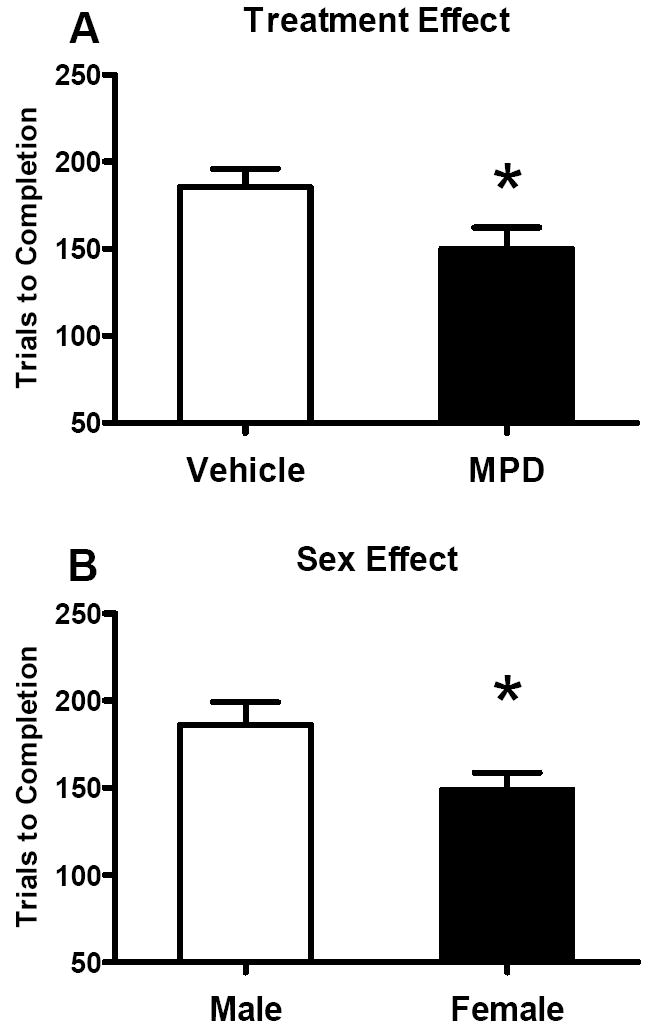
Effect of oral MPD on Trials to Completion of the entire attention task (see text for details). A. Mean numbers (+SEM) of Trials to Completion are shown for treatment groups. The MPD-treated rats learned the entire task significantly faster than the controls did. B. Mean numbers (+SEM) of Trials to Completion are shown for sex groups. The female rats took fewer trials to complete the entire attention task. * Significant
The ages at the beginning of the Shift and of the Distraction phase varied since the age at which animals started these phases depended on performance in the previous phase. Thus an age factor was possibly introduced into the experiment. We conducted age-controlled analyses of Trials to Criterions for the Shift and the Distraction phases. Ages at the beginning of each phase in days were considered as fixed continuous variables. No significant effect of sex or interaction of treatment and sex was detected. Hence we analyzed data without sex as a factor. Significant effects of treatment [F(1, 32)=5.26, p=0.0286], age [F(1, 6)=7.23, p=0.0386] and an interaction of treatment and age [F(1, 32)=4.56, p=0.0406] were seen in the Shift phase (Fig 7). In the Distraction phase, a significant age effect [F(1, 34)=4.61, p=0.0390] and a marginally significant treatment effect [F(1, 33)=3.97, p=0.0546] were found as in the Shift phase (data not shown). The interaction of age and treatment seen in the shift phase suggests that the response of juvenile rats to MPD is age-dependent (see discussion below).
Figure 7.
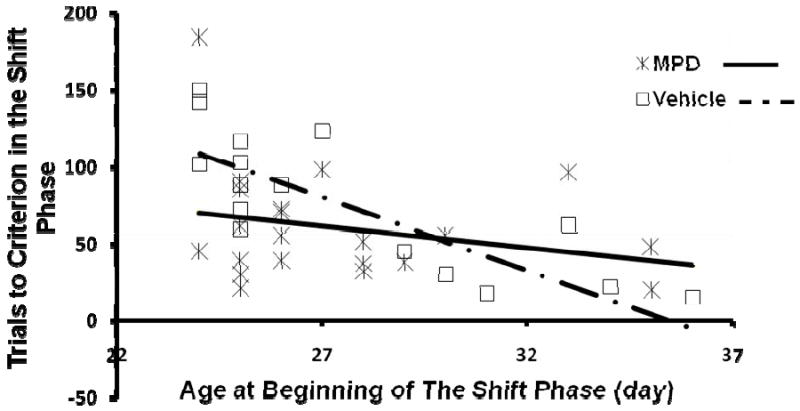
The effect of oral MPD on Trials to Criterion in the Shift phase of the attention task, controlled for age-differences at the beginning of the phase. Trials to Criterion of each rat is plotted against its own age at the beginning of the Shift phase. MPD reduced Trials to Criterion when age differences were controlled (p<0.05). Age effect emerged as the earlier a rat entered the Shift phase, the more trials this animal took to learn the Shift task (p<0.05). MPD interacted with age since the drug only improved performance in animals that started the Shift phase early, compared to the controls (p<0.05).
4. Discussion
Previously we found that oral MPD at 3 mg/kg from PND 22 to 25 improved performance on a radial arm maze in rats, using a win-shift paradigm (Zhu, N. et al.2007). That is, the MPD-treated animals entered more arms before entering an arm previously explored. The goal of the present study was to elucidate the multifaceted effects of oral MPD in juvenile rats during their active phase of circadian cycle to better understand the previous results on the radial arm maze. We found that oral MPD increased time spent beyond the rails on the elevated plus maze, increased locomotor activity and enhanced attention, which all probably contribute to the improved performance we previously observed on the radial arm maze.
4.1. Anxiety and Oral Methylphenidate
We hypothesized that oral MPD at 3 mg/kg acts as an anxiolytic drug and facilitated the movement of the rat onto the open and elevated arms of the radial maze when the vehicle-treated rats were not yet exploring the maze. The results of the elevated plus maze experiment confirmed our hypothesis. The female MPD-treated animals spent more time beyond the rails compared to the vehicle-treated females during the first 5 min of the test. The MPD-induced increase in Time Beyond Rails was not seen during the latter half of the test, perhaps due to habituation of all groups of rats to the maze in general. Furthermore, MPD decreased stretch-attended postures but increased head dips (Fig 2), which supports the idea that oral MPD at 3 mg/kg reduces anxiety (Weiss, S. M. et al.1998; Albrechet-Souza, L. et al.2007). Stretch-attended postures and head dips, measures of risk-assessment behaviors, are more sensitive to anxiety than time spent in the open arms on the elevated plus maze (Rodgers, R. J. and Cole, J. C.1993; Rodgers, R. J. et al.1999), and are disassociated with locomotor activity and exploration (Fernandes, C. and File, S. E.1996; Doremus, T. L. et al.2006). Another measure of anxiety, thigmotaxis in the locomotor activity box (Treit, D. and Fundytus, M.1989) was not affected by MPD, which probably is due to the differences between the recording box and the elevated/open areas of the plus maze or the radial arm maze. Altogether MPD appears to reduce anxiety in juvenile rats at least on the plus maze. This is consistent with human data demonstrating that MPD reduces anxiety in patients with ADHD (Barrickman, L. L. et al.1995; Bouffard, R. et al.2003).
Interestingly, females displayed higher anxiety than males did both on the elevated plus maze and in the locomotor recording. The vehicle-treated females spent less time beyond the rails than the other groups (Fig 1B); females spent less time in the center area of the activity chamber during habituation (Fig 5). This is consistent with the higher level of anxiety in the human female, both healthy children and ADHD patients (see Kudielka, B. M. et al.2004; Sonuga-barke, E. J. et al.2007; Else-Quest, N. et al.2009 for reviews). The robust anxiolytic effect of MPD in female rats may suggest an advantage of MPD over other psychostimulants since anxiety has been a complication in treating female ADHD patients (Quinn, P. O.2005). Further human studies on sex differences in the effects of MPD on anxiety may be helpful to understand this facet of the drug effect.
4.2. Locomotor Activity and Oral Methylphenidate
Initially the radial arm maze task requires a rat to explore the maze to find the food rewards. Exploratory behavior around age of PND 25 is normally low (for reviews see Spear, L. et al.1980; Galef Jr., 1981) while MPD often increases locomotor activity in animals (Yang, P. B. et al.2003; Yang, P. B. et al.2006). Accordingly oral MPD could have elevated locomotor activity prompting the rats to explore the radial arm maze while the control rats naturally explored little at this age. The present study demonstrated that oral MPD at 3 mg/kg increased locomotor activity (Fig 4A). After 15 min of habituation to the recording box, MPD increased locomotor activity from 10 to 40 min, which coincides with the testing period of the radial maze task used previously. The elevated grid crossings on the plus maze in MPD-treated animals also suggested that the drug increased locomotor activity. Thus the locomotor-enhancing effect of oral MPD at 3 mg/kg probably did play a role in the improved performance on the radial arm maze in our previous study (Zhu, N. et al.2007).
Suppression of hyperactivity is a main effect of MPD treatment in children with ADHD. Kuczenski’s group (2002) found reduced locomotor activity in adolescent rats (PND 41) during the dark (active) phase with 3 daily dosages of oral MPD. Their recording boxes had two distinct compartments and access to food and water. The relatively complex environment alone might yield a high level of locomotor activity while the recording box in our study was simple and elicited minimal movement after habituation. It is possible that MPD suppresses locomotor activity when the environment promotes activity and increases locomotor activity in rats in simple apparatuses. Similarly, amphetamine reduces activity in the running-wheel while it increases locomotor activity in open field (Maggiore, V. D. and Ralph, M. R.2000). A recording of activity after oral dosing of MPD at 3 mg/kg to rats in the running wheel or in the figure-8 maze may replicate the decreased locomotor activity that Kuczenski and Segal found with multiple doses of 3 mg/kg MPD in adolescent rats. Within the present recording environment, locomotor responses to oral MPD were correlated with individual baseline activities (Fig 4B). That is, MPD increased locomotor activity in animals with higher baseline activity while it had minimal effects in the low baseline group. The levels of activity during habituation may reflect the responsiveness to novelty. Thus our data suggest a positive correlation of the locomotor response to novelty with the locomotor response to MPD as seen in other reports (Klebaur, J. E. et al.2001; Bevins, R. A. and Peterson, J. L.2004).
4.3. Attention and Oral Methylphenidate
Lastly, MPD improves attention in both clinical and social settings (for a review see Challman, T. D. and Lipsky, J. J.2000). In our previous study (Zhu, N. et al.2007), enhanced attention to the task and to the extra-maze cues could have facilitated the performance in the MPD-treated rats. Since oral MPD altered anxiety responses and locomotor activity as shown above, we chose an attention task with minimal stress and locomotor demands to examine the effects of MPD on attention. Performance on this task required the rat to discriminate between two boxes for food reward solely based on the salient material (black rubber) covering the sides or the lid of the box.
The initial phase of the task relies on associative learning. MPD in fact can impair associative learning by widening the “attention window” to include non-associated cues (Horsley, R. R. and Cassaday, H. J.2007). Nonetheless, twice oral MPD at 3 mg/kg on a cracker significantly reduced the number of total trials to complete the 3-phase attention task in juvenile rats (Fig 6A). Therefore, while MPD had no apparent effect in the initial training, the drug significantly improved performance in the later stages of testing which required the rat to pay attention to changes in the presentation of the cues in order to obtain the reward. It is highly likely that a similar enhancement in attention contributed to the improvement in performance seen on the radial arm maze in our earlier work (Zhu, N. et al.2007). Also females completed the entire task with significantly fewer trials than the males (Fig 6B). This is consistent with human data showing that females exceed males in cognitive tasks and learning, especially incidental learning (Eals, M. and Silverman, I.1994; Mcgivern, R. F. et al.1997).
Individual performances in the Shift and the Distraction phases were further examined by controlling for the age at which the animals started each phase. We obtained a significant enhancing effect of MPD in the Shift phase (Fig 7) and a marginally significant effect in the Distraction phase. A significant interaction of treatment and age indicated that MPD reduced the trials to reach criterion in animals that started the Shift phase at early ages whereas both the vehicle-treated and the MPD-treated animals that started later, finished the task rather quickly. Animals must have finished the Training phase quickly in order to start the Shift phase early. Though mainly associative learning, the Training phase demanded attention to the cues for the rats to perform accurately. Therefore animals that finished the Training phase early may hypothetically represent rats with optimal responses to MPD at 3 mg/kg whereas those that finished after PND 30 may perhaps have optimal responses to other doses. In support of this possibility, optimal doses in humans can vary across individuals with an average of 0.7±0.4 mg/kg/day (Greenhill, L. et al.2006). On the other hand, rats that finished the Training phase after PND 30 had more experience with the procedure and were more mature when they started the Shift phase, compared to those rats that started the phase prior to PND 30. Human studies showed that extensive training improved cognitive performance following placebo as much as MPD did (Rapport, M. D. et al.1989). Also Chambers et al. (Chambers, R. A. et al.1996) reported that older animals performed better in cognitive tests compared to younger animals. Age effects in the Shift and the Distraction phases demonstrated as well that rats that started the phase at later ages took fewer trials to reach the criterion. Altogether it is highly possible that all rats that started the Shift phase after PND 30 completed the phase quickly due to the extensive training and maturation, although they did not benefit from MPD at 3 mg/kg. While our data support the idea that MPD is less effective following extensive training or once a level of maturity is reached, a full dose-response curve is necessary to demonstrate this conclusively.
4.4. Conclusion and Implications
The present study demonstrated the multifaceted, age-dependent, and sex-related effects of oral administration of MPD at a clinically relevant dose in juvenile rats during the active phase of the circadian cycle. MPD at 3 mg/kg on a small cracker given to juvenile rats improves performance on tests of spatial cognition through enhancement of attention and locomotor activity as well as through its anxiolytic effects. The 3 facets of the drug action together likely contributed to the improved performance on the radial arm maze during PND 22 to 25 that our previous work demonstrated (Zhu, N. et al.2007). Oral administration of MPD in juvenile rats replicated the therapeutic effects on attention and anxiety in humans. Particularly, female rats benefited more from the anxiolytic-like effect of MPD in the test for anxiety, given a higher level of response to the anxiogenic setting. Hence MPD may be the preferred treatment for female ADHD patients who are perhaps more sensitive to stressors. Consistent with comparable responses to MPD in humans (for a review see Gray, J. R. and Kagan, J.2000), we found no sex differences in the locomotor and attentional effects of MPD. Since MPD appeared to be most effective on cognitive tasks during a restricted portion of the juvenile period, further studies on the therapeutic window in humans is warranted. The multifaceted effects of MPD on performance in cognitive tasks in rats suggest that MPD may also produce improvement in academic performance in children by similar mechanisms.
Acknowledgments
We thank the technical expertise of Lauren Harte, Maiko Iijima, April Jackson, Nora Siegal, Stacy Stephenson, and Dr. Ning Zhao; and the proof-reading of the manuscript by Nora Siegal.
Role of funding source This study was supported by National Institute of Mental Health Grant (R21) MH066852 and National Institute on Drug Abuse Grant P50 DA024584. These institutes had no further role in study design; in the collection, analysis and interpretation of data; in the preparation of the manuscript; and in the decision to submit the paper for publication.
Footnotes
Contributors Authors Ning Zhu and Diana L. Dow-Edwards designed the experiment and wrote the manuscript. Author Ning Zhu conducted the experiment. Author Jeremy Weedon performed the statistical analyses. Author Diana L. Dow-Edwards oversaw the project, the data collection and the writing of the report. All authors contributed to and have approved the final manuscript.
Conflict of interests None for any author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albrechet-Souza L, de Carvalho MC, Franci CR, Bradao ML. Increases in plasma corticosterone and stretched-attend postures in rats naive and previously exposed to the elevated plus-maze are sensitive to the anxiolytic-like effects of midazolam. Hormones and Behavior. 2007;53:267–273. doi: 10.1016/j.yhbeh.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Dumont NL, Teicher MH. Developmental differences in dopamine synthesis inhibition by (+/-)-7-OH-DPAT. Naunyn-Schmiedeberg’s Archives of Pharmacology. 1997;356:173–181. doi: 10.1007/pl00005038. [DOI] [PubMed] [Google Scholar]
- Aoyama T, Kotaki H, Iga T. Dose-dependent kinetics of methylphenidate enantiomers after oral administration of racemic methylphenidate to rats. Journal of Pharmacobio-dynamics. 1990;13:647–652. doi: 10.1248/bpb1978.13.647. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through alpha2 adrenoceptor and dopamine D1 receptor actions: Relevance to therapeutic effects in attention deficit hyperactivity disorder. Behavioral and Brain Functions. 2005;1:1–9. doi: 10.1186/1744-9081-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asan E. The catecholaminergic innervation of the rat amygdala. Advances in Anatomy, Embryology, and Cell Biology. 1998;142:L1–118L. doi: 10.1007/978-3-642-72085-7. [DOI] [PubMed] [Google Scholar]
- Barrickman LL, Perry PJ, Allen AJ, Kuperman S, Arndt SV, Herrmann KJ, Schumacher E. Bupropion versus methylphenidate in the treatment of attention-deficit hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1995;34:649–657. doi: 10.1097/00004583-199505000-00017. [DOI] [PubMed] [Google Scholar]
- Bethancourt JA, Camarena ZZ, Britton GB. Exposure to oral methylphenidate from adolescence through young adulthood produces transient effects on hippocampal-sensitive memory in rats. Behavioral Brain Research. 2009;202:50–57. doi: 10.1016/j.bbr.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Peterson JL. Individual differences in rats’ reactivity to novelty and the unconditioned and conditioned locomotor effects of methamphetamine. Pharmacology, Biochemistry and Behavior. 2004;79:65–74. doi: 10.1016/j.pbb.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Bouffard R, Hechtman L, Minde K, Iaboni-Kassab F. The efficacy of 2 different dosages of methylphenidate in treating adults with attention-deficit hyperactivity disorder. Canadian Journal of Psychiatry. 2003;48:546–554. doi: 10.1177/070674370304800806. [DOI] [PubMed] [Google Scholar]
- Britton GB, Bethancourt JA. Characterization of anxiety-related responses in male rats following prolonged exposure to therapeutic doses of oral methylphenidate. Pharmacology Biochemistry and Behavior. 2009;93:451–459. doi: 10.1016/j.pbb.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Brown RM, Goldman PS. Catecholamines in neocortex of rhesus monkeys: regional distribution and ontogenetic development. Brain Research. 1977;124:576–580. doi: 10.1016/0006-8993(77)90960-x. [DOI] [PubMed] [Google Scholar]
- Bunsey M, Kramer D, Kesler M, Strupp BJ. A vasopressin metabolite increases attentional selectivity. Behavioral Neuroscience. 1990;104:277–287. [PubMed] [Google Scholar]
- Challman TD, Lipsky JJ. Methylphenidate: its pharmacology and uses. Mayo Clinic Proceedings. 2000;75:711–721. doi: 10.4065/75.7.711. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Moore J, McEvoy JP, Levin ED. Cognitive effects of neonatal hippocampal lesions in a rat model of schizophrenia. Neuropsychopharmacology. 1996;15:587–594. doi: 10.1016/S0893-133X(96)00132-7. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Varlinskaya EI, Spear LP. Factor analysis of elevated plus-maze behavior in adolescent and adult rats. Pharmacology, Biochemistry and Behavior. 2006;83:570–577. doi: 10.1016/j.pbb.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Dow-Edwards DL, Weedon JC, Hellmann E. Methylphenidate improves performance on the radial arm maze in periadolescent rats. Neurotoxicology and Teratology. 2008;30:419–427. doi: 10.1016/j.ntt.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eals M, Silverman I. the hunter-gather theory of spatial sex differences: Proximate factors mediating the female advantage in recall of object arrays. Ethology and Sociobiology. 1994;15:95–105. [Google Scholar]
- Else-Quest N, Hyde J, Goldsmith HH, Van Hulle CA. Gender differences in temperament: A meta-analysis. Psychological Bulletin. 2009;132:33–72. doi: 10.1037/0033-2909.132.1.33. [DOI] [PubMed] [Google Scholar]
- Fernandes C, File SE. The influence of open arm ledges and maze experience in the elevated plus-maze. Pharmacology, Biochemistry and Behavior. 1996;54:31–40. doi: 10.1016/0091-3057(95)02171-x. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Brown RM. Postnatal development of monoamine content and synthesis in the cerebral cortex of rhesus monkeys. Developmental Brain Research. 1982;4:339–349. doi: 10.1016/0165-3806(82)90146-8. [DOI] [PubMed] [Google Scholar]
- Gray JR, Kagan J. The challenge of predicting which children with ADHD will respond positively to methylphenidate. Journal of Applied Developmental Psychology. 2000;21:471–489. [Google Scholar]
- Greenhill L, Kollins S, Abikoff H, McCracken J, Riddle M, Swanson J, McGough J, Wigal S, Wigal T, Vitiello B, Skrobala A, Posner K, Ghuman J, Cunningham C, Davies M, Chuang S, Cooper T. Efficacy and safety of immediate-release methylphenidate treatment for preschoolers with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:1284–1293. doi: 10.1097/01.chi.0000235077.32661.61. [DOI] [PubMed] [Google Scholar]
- Horsley RR, Cassaday HJ. Methylphenidate can reduce selectivity in associative learning in an aversive trace conditioning task. Journal of Psychopharmacology. 2007;25:492–500. doi: 10.1177/0269881106067381. [DOI] [PubMed] [Google Scholar]
- Klebaur JE, Bevins RA, Segar TM, Bardo MT. Individual differences in behavioral responses to novelty and amphetamine self-administration in male and female rats. Behavioural Pharmacology. 2001;12:267–275. doi: 10.1097/00008877-200107000-00005. [DOI] [PubMed] [Google Scholar]
- Konkol RJ, Bendeich EG, Breese GR. A biochemical and morphological study of the altered growth pattern of central catecholamine neurons following 6-hydroxydopamine. Brain Research. 1978;140:125–135. doi: 10.1016/0006-8993(78)90242-1. [DOI] [PubMed] [Google Scholar]
- Koob GF, Swerdlow NR. The functional output of the mesolimbic dopamine system. Annuals New York Academy of Sciences. 1988;537:216–227. doi: 10.1111/j.1749-6632.1988.tb42108.x. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. Journal of Neuroscience. 2002 Aug 15;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. Differential heart rate reactivity and recovery after psychosocial stress (TSST) in healthy children, younger adults, and elderly adults: The impact of age and gender. International Journal of Behavioral Medicine. 2004;11:116–121. doi: 10.1207/s15327558ijbm1102_8. [DOI] [PubMed] [Google Scholar]
- Levitt P. Structural and functional maturation of the developing primate brain. The Journal of Pediatrics. 2003;143:S35–S45. doi: 10.1067/s0022-3476(03)00400-1. [DOI] [PubMed] [Google Scholar]
- Loizou LA, Salt P. Regional changes in monoamines of the rat brain during postnatal development. Brain Research. 1970;20:467–470. doi: 10.1016/0006-8993(70)90177-0. [DOI] [PubMed] [Google Scholar]
- Maggiore VD, Ralph MR. The effect of amphetamine on locomotion depends on the motor device utilized: The open field vs. the running wheel. Pharmacology, Biochemistry and Behavior. 2000;65:585–590. doi: 10.1016/s0091-3057(99)00260-9. [DOI] [PubMed] [Google Scholar]
- Markowitz JS, DeVane CL, Pestreich LK, Patrick KS, Muniz R. A comprehensive in vitro screening of d-, l-, and dl-threo-methylphenidate: An exploratory study. Journal of Child and Adolescent Psychopharmacology. 2006;16:687–698. doi: 10.1089/cap.2006.16.687. [DOI] [PubMed] [Google Scholar]
- Mcgivern RF, Huston JP, Byrd D, King T, Siegle GJ, Reilly J. Sex differences in visual recognition memory: Support for a sex-related difference in attention in adults and children. Brain and Cognition. 1997;34:323–336. doi: 10.1006/brcg.1997.0872. [DOI] [PubMed] [Google Scholar]
- Morris MJ, Dausse JP, Devynck MA, Meyer P. Ontogeny of a1 and a2-adrenoceptors in rat brain. Brain Research. 1980;190:268–271. doi: 10.1016/0006-8993(80)91178-6. [DOI] [PubMed] [Google Scholar]
- Olton DS, Collison C, Werz MA. Spatial memory and radial arm maze performance of rats. Learning and Motivation. 1977;8:289–314. [Google Scholar]
- Olton DS, Samuelson RJ. Remembrance of places. passed: spatial memory in rats. Journal of Experimental Psychology. Animal Behavior Processes. 1976;2:97–116. doi: 10.1037/0097-7403.33.3.213. [DOI] [PubMed] [Google Scholar]
- Quinn PO. Treating adolescent girls and women with ADHD: Gender-specific issues. Journal of Clinical Psychology. 2005;61:579–587. doi: 10.1002/jclp.20121. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Quinn SO, DuPaul GJ, Quinn EP, Kelly KL. Attention deficit disorder with hyperactivity and methylphenidate: the effects of dose and mastery level on children’s learning performance. Journal of Abnormal Child Psychology. 1989;17:669–689. doi: 10.1007/BF00917730. [DOI] [PubMed] [Google Scholar]
- Robison LM, Sclar DA, Skaer TL, Galin RS. National trends in the prevalence of attention-deficit/hyperactivity disorder and the prescribing of methylphenidate among school-age children: 1990-1995. Clinical Pediatrics. 1999;38:209–217. doi: 10.1177/000992289903800402. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Cole JC. An ethological analysis of chlordiazepoxide and bretazenil (Ro 16-6028) in the murine elevated plus-maze. Behavioural Pharmacology. 1993;4:573–580. [PubMed] [Google Scholar]
- Rodgers RJ, Haller J, Holmes A, Halasz J, Walton TJ, Brain PF. Corticosterone response to the plus-maze: high correlation with risk assessment in rats and mice. Physiology & Behavior. 1999;68:47–53. doi: 10.1016/s0031-9384(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Safer DJ, Zito JM, Fine EM. Increased methylphenidate usage for attention deficit disorder in the 1990s. Pediatrics. 1996;98:1084–1088. [PubMed] [Google Scholar]
- Schildkraut JJ. The catecholamine hypothesis of affective disorders. A review of supporting evidence. International Journal of Psychiatry. 1967;4:203–217. [PubMed] [Google Scholar]
- Sonuga-barke EJ, Coghill D, Markowitz JS, Swanson JM, Vandenberghe M, Hatch SJ. Sex differences in the response of children with ADHD to once-daily formulations of methylphenidate. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:701–710. doi: 10.1097/chi.0b013e31804659f1. [DOI] [PubMed] [Google Scholar]
- Spear L, Shalaby IA, Brick J. Chronic administration of haloperidol during development: Behavioral and psychopharmacological effects. Psychopharmacology. 1980;70:47–58. doi: 10.1007/BF00432369. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Volkow ND. Pharmacokinetic and pharmacodynamic properties of stimulants: Implications for the design of new treatments for ADHD. Behavioural Brain Research. 2002;130:73–78. doi: 10.1016/s0166-4328(01)00433-8. [DOI] [PubMed] [Google Scholar]
- Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacology, Biochemistry and Behavior. 1989;31:959–962. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- Weiss SM, Wadsworth G, Fletcher A, Dourish CT. Utility of ethological analysis to overcome locomotor confounds in elevated maze models of anxiety. Neuroscience and Biobehavioral Reviews. 1998;23:265–271. doi: 10.1016/s0149-7634(98)00027-x. [DOI] [PubMed] [Google Scholar]
- Yang PB, Amini B, Swann AC, Dafny N. Strain differences in the behavioral responses of male rats to chronically administered methylphenidate. Brain Research. 2003;971:139–152. doi: 10.1016/s0006-8993(02)04240-3. [DOI] [PubMed] [Google Scholar]
- Yang PB, Swann AC, Dafny N. Acute and chronic methylphenidate dose-response assessment on three adolescent male rat strains. Brain Research Bulletin. 2006;71:301–310. doi: 10.1016/j.brainresbull.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N, Weedon J, Dow-Edwards DL. Oral methylphenidate improves spatial learning and memory in pre- and periadolescent rats. Behavioral Neuroscience. 2007;121:1272–1279. doi: 10.1037/0735-7044.121.6.1272. [DOI] [PubMed] [Google Scholar]


