Abstract
Recent results suggest a paradigm shift from viewing inorganic phosphate as a passive requirement for basic cell functions to an active regulator of cell behavior. We have previously demonstrated that elevated concentrations of phosphate increased cell proliferation and expression of pro-tumorigenic genes such as Fra-1 and osteopontin in a pre-osteoblast cell line. Therefore, we hypothesized that elevated phosphate concentrations would promote cell transformation in vitro and tumorigenesis in vivo. Supplementation of medium with phosphate increased anchorage independent transformation and proliferation of Balb/C mouse JB6 epidermal cells, activation of N-ras, ERK1/2, and AP-1, and increased gene expression of Fra-1, COX-2, and osteopontin in a dose-dependent manner. These in vitro results led to the hypothesis that varying the levels of dietary inorganic phosphate would alter tumorigenesis in the mouse model of skin carcinogenesis. Female FVB/N mice were treated with 7,12-dimethylbenz[a]anthracene/12-O-tetradecanolyphorbol-13-acetate (DMBA/TPA) and fed high or low phosphate diets (1.2 versus 0.2% of the diet) for 19 weeks. The high phosphate diet increased skin papilloma number by approximately 50% without changing feed intake and body weights. High dietary phosphate increased serum concentrations of phosphate, parathyroid hormone, and osteopontin and decreased serum concentrations of calcium. Thus, we conclude that elevated phosphate promotes cell transformation and skin tumorigenesis partly by increasing the availability of phosphate for activation of N-ras and its downstream targets, which defines dietary phosphate as a novel target for chemoprevention.
Keywords: Dietary inorganic phosphate, skin carcinogenesis, osteopontin, N-ras, AP-1
Introduction
Tumorigenesis is a multistage, complex process often involving both genetic and environmental or lifestyle factors. Lifestyle factors, such as diet, can have a profound influence on the initiation, progression, and/or recovery from disease. This idea is supported by studies evaluating the effects of human migration on the incidence of particular diseases including cancer [reviewed in (1)]. A general estimate of the potential contribution of diet to cancer ranges from 10-70% (2). Diet represents an environmental factor that can be relatively easily manipulated and it is becoming increasingly apparent that diet can have profound effects on functional genomics (3). However, sufficient information does not currently exist to permit a comprehensive utilization of individual dietary components in the prevention, intervention, and treatment of disease. A number of in vitro studies have suggested that inorganic phosphate (Pi) and phosphate transport are necessary for cell growth, essentially acting as a mitogen (4-7), however little research has been directed at determining the mechanism and the consequences of elevated Pi in vivo. Our previous results in a pre-osteoblast cell line suggested that, in vitro, elevated Pi led to increased cell proliferation (8) as well as activation of ERK1/2, changes in protein and gene expression of AP-1 proteins, and increased expression of transformation associated proteins such as osteopontin (OPN), Fra-1, and cyclin D1 (8-12). In addition, elevated Pi promotes Akt-ERK1/2-Mnk1 signaling, cap-dependent protein translation, and growth in human lung cells (13). Taken together the data suggest that the elevated levels of available Pi may be an important driving factor to the growth and transformation potential of cells.
The transformation sensitive epidermal cell line JB6 is recognized as an excellent model to study multi-stage tumor promotion including the effects of bioactive food components such as Pi on the process of transformation (14). When treated with various tumor promoters these cells respond with anchorage independent growth in soft agar and tumorigenicity (15). Many key factors necessary for various steps of the transformation process have been identified and include: activation of members of the AP-1 transcription factor family, an increase in cyclins A, B1, and D1, activation of signaling proteins ERK1/2 and iNOS and increased expression of the extracellular matrix protein osteopontin [reviewed in (16)]. Members of the AP-1 transcription factor family (17) have also been identified as attractive targets in chemoprevention because their activation is linked to proliferation, transformation, and inflammation [reviewed in (18)] in a number of cell types and tissues including skin (19). A number of food factors have been demonstrated to reduce AP-1 activity and thereby reduce the associated transformation properties [reviewed in (18, 20)]. A gene/protein that is also tightly associated with transformation, inflammation, and metastasis is OPN, an extracellular matrix and circulating factor (21-23). Osteopontin influences cell function by acting as a cytokine through its ability to bind integrin receptors. Recently, OPN was found to be regulated by AP-1 under transforming conditions in the JB6 transformation model (24) and to be strongly regulated by Pi (10). The cellular and molecular events required for transformation in this model have been validated in vivo using the 7,12-dimethylbenz[a]anthracene/12-O-tetradecanolyphorbol-13-acetate (DMBA/TPA) two-stage skin carcinogenesis mouse model (23), which is commonly used to study the initiation, promotion, and progression of carcinogenesis (25). The defined molecular mechanisms make the JB6 and two-stage skin carcinogenesis models an excellent system to study the effect of Pi on cell transformation and tumorigenesis.
The ras family (H, K, N, R) of small GTP binding proteins (approximately 21 kDa) functions as important signaling proteins in controlling proliferation, transformation, and tumor invasion [reviewed in (26)]. Ras proteins are activated in the GTP bound state and are known to be central to many events related to proliferation, transformation, and tumor invasion through downstream effectors ERK1/2 and Akt. Ras regulated proliferation and transformation is linked to the regulation of proteins such as cyclin D1, the AP-1 transcription factor Fra-1, and osteopontin all of which we have previously demonstrated as responsive to elevated Pi (8, 10, 11). Investigations into the role of ras and tumorigenesis have focused on activating mutations, however, overexpression of ras without activating mutations is also capable of transforming cells (27, 28). Although in vivo papilloma formation is known to involve activating mutations in the H-ras isoform and activation of AP-1 transcription (29), the H-ras null mice do form papillomas with 62% having k-ras mutations and 38% having no ras mutations (30).
The objective of the current study was to test the hypothesis that elevated levels of Pi promote proliferation and anchorage independent growth of transformation sensitive epidermal cell line JB6 and skin tumorigenesis in DMBA/TPA treated female FVB/N mice. Furthermore, we explored the molecular mechanism by which Pi alters tumorigenesis and hypothesized that Pi promotes activity of ras, ERK1/2, and AP-1 and ultimately gene expression of Fra-1, COX-2, and osteopontin in a dose-dependent manner.
Materials and Methods
Cell culture
Transformation-sensitive JB6 (clone 41) mouse epidermal cells were cultured in monolayers at 37 °C and 5% CO2 using Eagle's minimal essential medium (EMEM; 1 mM Pi; Invitrogen, Carlsbad, CA) containing 4% fetal bovine serum (FBS) (Atlanta Biologicals, Atlanta, GA) supplemented with 2 mM l-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin (Invitrogen). GDPβS was purchased from Calbiochem (San Diego, CA). Unless noted all experiments were performed in EMEM which contains 1mM Pi and concentrations listed in the figures are final Pi medium concentrations. Added Pi is in the form of NaPO4, pH 7.4 (Sigma).
Cell proliferation assays
Cell viability was measured using XTT {2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide} assay according to manufacturer's protocol (Promega, Madison, WI). JB6 cells were plated at 5×103 cells /100 μL per well in 96 well plates. After 24 hr, plated cells were treated with 1 and 2mM Pi (1, 2, and 3mM final) for 36 hr. The change in absorbance was measured 1 hr after addition of XTT assay reagent on a BioRad Lumimark plate reader (BioRad Laboratories, Hercules, CA). The results are from 6 replicates/treatment. Cell counts were performed generally as described previously (8). JB6 cells were plated at 5×104 cells/well of a 12 well plate and cell counts were performed after 96 hours in medium containing 1 or 3 mM Pi (final). Cell cycle analysis was performed by Flow Cytometry. JB6 cells were subcultured on 10cM plates and after 48 hours, 2mM phosphate was added, or not, for a final concentration of 1 and 3mM. After 36 hours cells were lifted with Accutase (Innovative Cell Technologies Inc., San Diego, CA), washed with PBS, fixed in ethanol, and washed twice. Cells were stained with propidium iodide (Roche) at 0.1mg/ml final with RNase A (Invitrogen) 1-hour prior to analysis by flow cytometry (Accuri flow cytometer, Ann Arbor, MI).
Anchorage-independent transformation assay
Promotion of neoplastic transformation assays were performed as described previously (15). In a 60-mm tissue culture dish JB6 cells (1×104) were resuspended in 1.5 mL of 0.33% agar in EMEM (1 mM Pi) with 10% FBS and layered over 7 mL of 0.5% agar in EMEM with 10% FBS. Both layers of agar were supplemented with Pi (1.2, 2.2, or 3.7 mM NaPO4) in the presence of DMSO or 10 ng/mL phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA; Alexis, San Diego, CA) in DMSO. The cells were cultured at 36 °C for 14 d, stained with neutral red (Sigma) diluted 1:75 in PBS. Colonies were counted by an automated image analysis system supported by Image Pro-Plus (version 3.0.1) software (Media Cybernetics, Silver Spring, MD). Colonies of greater than 8 cells were scored. The transformation responses are presented as average number of colonies formed per 60-mm tissue culture dish (5 replicates/treatment).
Immunoblotting
JB6 cells were cultured as above and nuclear isolation and immunoblotting performed as described previously (8). Thirty μg of lysate was resolved on a 10% SDS-polyacrylamide gel. All antibodies were purchased from (Santa Cruz Biotechnologies Inc., Santa Cruz, CA), except ERK1/2 from (Promega, Madison, WI).
Northern blot assay
Total cell RNA was prepared using Trizol Reagent (Invitrogen) according to manufacturer's recommendations. A total of 10 μg of RNA was loaded per lane and separated by electrophoresis through a 1% formaldehyde-agarose gel. The RNA was transferred to a Hybond-N nylon membrane (Amersham Pharmacia Biotech, Inc., Piscataway, NJ), crosslinked by UV irradiation, and baked at 80 °C. The 32P-labelled probes were prepared using a random prime labeling kit (Roche Diagnostics Corp., Indianapolis, IN). Between successive probes, blots were stripped by treatment with boiling 0.1% SDS. Radiochemicals were obtained from Perkin Elmer Life Sciences Inc. (Boston, MA). The OPN, Fra-1, and actin probes used for Northern blotting have been described previously (8). The cDNA probe for Cox-2 (ptgs2) was created by RT-PCR using primers 5’-ATGCTCTTCCGAGCTGTGCT-3 ’ AND 5 ’-CAGCTCAGTTGAACGCCTTT-3’ and Egr1 using primers (5’-ATGGCAGCGGCCCAAGGCCGAGATGCAATT-3’) and (5’-GCAAATTTCAATTGTCCTGGG-3’) and cloned into vector TOPO.2.1 (Invitrogen).
Promoter-luciferase assay
Transformation-sensitive JB6 cells were seeded at 2×104 per well in a 24 well plate. After 24 hr, plated cells were transfected with 4×AP-1 luciferase construct (500 ng/well) and Renilla (50ng/well) (Promega) using Fugene (Roche). After another 24 hr the medium was replaced with DMEM and 0.2% FBS (6 replicates/treatment). After another 24 hr, the medium was supplemented with 1, 2, or 3 mM Pi for 6 hrs and DMSO (control) or 10ng/mL TPA in DMSO as indicated. The cells were washed in PBS and harvested in 1Xlysis buffer. Firefly luciferase and Renilla activities were measured with a luciferase kit from Promega (Madison, WI) according to manufacturer's recommendations on a Turner Biosystems Inc. microplate luminometer (Sunnyvale CA).
Electrophoretic mobility shift assay
Transformation-sensitive JB6 cells were serum starved overnight, supplemented with 2 mM NaPO4 for 0, 5, 10, 15, 30, and 60 min, and harvested in PBS. To separate nuclear and cytoplasmic fractions, cells were first lysed in a buffer of 0.25 mM HEPES, 50 mM KCl, 2 mM PMSF, 100 μM DTT, 0.5% NP-40, and protease inhibitors (at a final concentration of 2.0 μg/mL aprotinin, 2.0 μg/mL leupeptin, 1.0 μg/mL pepstatin, 100 μM sodium ortho vanadate, and 1 mM DTT). After centrifugation and removal of the cytoplasmic fraction, the nuclear pellet was lysed in an extraction buffer of 0.25 mM HEPES, 50 mM KCl, 2 mM PMSF, 100 μM DTT, 10% glycerol, and protease inhibitors at the concentrations indicated above. The protein concentration of cell lysates was determined with a BCA Protein Assay Reagent kit (Biorad). Radiolabeled consensus AP-1 oligonucleotides, purchased from Santa Cruz Biotechnology (Santa Cruz CA), were generated according to manufacturer's protocol (Promega). DNA binding reactions were performed using 5 μg of nuclear extract, 5X Gel Shift Binding Buffer (Promega), labeled oligonucleotide, and nuclease-free water. Reactions were separated on 6% DNA retardation gels (Invitrogen). After separation the gels were vacuum dried and exposed to film. The supershift assay was performed using nuclear lysate from a sample that was exposed for 5 min with 2 mM supplemented Pi. Labeled oligonucleotide and nuclear lysate were combined and incubated for 15 min followed by addition of antibodies of the specific AP-1 family members. All antibodies were purchased from Santa Cruz.
Ras activation assay
JB6 cells were serum starved overnight and supplemented with 0, 2, or 4 mM NaPO4 for 5 min (in addition to the 1 mM in the medium). Ras activity was measured using the Ras-Binding Domain of Raf-1 to pulldown active ras according to manufacturer's protocol (Cell BioLabs, San Diego, CA). Blots of whole cell lysate (50 μg) from the input were probed with antibodies to total ERK1/2 (Promega) and phospho-ERK1/2 (Santa Cruz). For time course experiments JB6 cells were supplemented with 2 mM Pi for 0, 2.5, 5, 10, or 15 min and ras activation assay performed. To investigate the requirement of phosphate transport 1 mM foscarnet (phosphonoformic acid) was added 1 hr prior to the supplementation of 2mM Pi. Following separation by SDS polyacrylamide gel electrophoresis, the resulting membrane was probed with a pan-ras antibody followed by N-ras, R-ras, H-ras, and K-ras specific antibodies (Pan-ras antibody was from Cell BioLabs, all isoform specific antibodies were purchased from Santa Cruz).
Animals and Diets
Mice (female FVB/N) were obtained at 4-5 wk of age from NCI-Frederick Animal Production Area, Frederick Cancer Research and Development Center (Frederick, MD). Mice were housed in a facility with controlled conditions (temperature: 21-24°C, humidity: 40-70%, and light/dark cycle: 12 hr light/12 hr dark). Until 8 wk of age, mice were fed ad libitum NIH-31 diet (1.1% phosphate; Harlan Teklad, Indianapolis, IN). At 8 wk of age, mice were randomly assigned to either a low phosphate diet (LPD; 0.2% phosphate) or a high phosphate diet (HPD; 1.2% phosphate) (Table 1) both manufactured by TestDiet (catalog # 58299 and # 25608, respectively, Purina Mills, Richmond, IN). For the two-stage skin carcinogenesis model, 14 mice/group were shaved at 8 wk of age. After 2 d, mice were topically treated with 400 nM of 7,12-dimethylbenz [a]anthracene (DMBA-Sigma) in 200 μL of acetone. Starting 14 d after DMBA initiation, the mice were topically treated with 10 nM of TPA (Alexis, San Diego, CA) in 200μL of acetone twice a week. Mice were visually examined weekly for skin papilloma and squamous cell carcinoma number and size. Every 3 wks individual body weights and food disappearance per cage (3 or 4 mice/cage) were measured. Mice were sacrificed at 27 wk of age.
Table 1.
Diet composition
| Diet composition | Low phosphate (LPD) | High phosphate (HPD) |
|---|---|---|
| Minerals | ||
| Phosphorus, % | 0.17 | 1.21 |
| Calcium, % | 0.60 | 0.60 |
| Magnesium, % | 0.07 | 0.07 |
| Sodium, % | 0.19 | 0.28 |
| Potassium | 0.39 | 0.39 |
| Nutrients | ||
| Protein, % | 19.0 | 19.0 |
| Amino acids, % | identical | identical |
| Fat, % | 10.0 | 10.0 |
| Cholesterol, ppm | 48 | 48 |
| Fiber (max), % | 4.9 | 4.7 |
| Carbohydrates, % | 60.6 | 58.1 |
| Energy, kcal/g | 4.09 | 3.99 |
| Calories provided by: | ||
| Protein, % | 18.623 | 19.094 |
| Fat, % | 22.036 | 22.593 |
| Carbohydrates, % | 59.341 | 58.312 |
Full details of diets can be obtained from www.testdiets.com; LPD (Cat#58299) and HPD (Cat#25608).
For the serum analysis, 5 mice/group were fed starting at 8 wks of age the experimental diets for 5 wk. Serum samples were collected from the mice by cardiac puncture. Serum concentrations of phosphorus, calcium, magnesium, creatinine, uric acid, and total protein were measured by the Laboratory of Experimental Immunology (LEI), National Cancer Institute-Frederick, MD. Serum concentrations of parathyroid hormone (PTH) and insulin growth factor I (IGF-I) were measured by AniLytics (Gaithersburg, MD). In addition, 6 mice/group were fed starting at 8 wks of age diets containing 0.2 or 1.2% of Pi for 2 wk and then topically treated with 200 μL of acetone (control) or TPA (10 nM) in 200 μL of acetone. Serum samples were collected by cardiac puncture 6 hr after TPA treatment. Serum concentrations of OPN were measured by ELISA (R&D Systems). Animal care and experimental procedures were conducted with approval of the NCI-Frederick Animal Care and Use Committee.
Statistical analysis
Statistical analysis was conducted using SAS Version 9.1 (SAS, Inc., Cary, NC). For the cell culture studies a one-way ANOVA was used. When significant effects were detected (F-test significant at P < 0.05) we used a two-sided t test to compare each of the two higher Pi concentrations with the lowest Pi concentration. For the animal studies, papilloma size and mouse weight data were analyzed as a repeated measures study using the mixed models procedure (PROC MIXED). Papilloma size data were transformed to the log (X+0.5) scale so that the data were approximately normal distributed. The fixed effects in the model were dietary Pi (low, high), time (week of age), and the interaction of dietary phosphate and time. A completely unrestricted variance-covariance structure was used to account for repeated measures taken on individual mice across time. The effects of dietary Pi were evaluated by comparing the estimated values at each time point with each other using two-sided t tests in the LSMEANS statements. Papilloma and squamous cell carcinoma incidence data of the two dietary Pi groups were compared by using Fisher's Exact test in PROC FREQ. Concentrations of serum variables of the two dietary Pi groups were compared using a two-sided Student's t test. Significance was declared at P ≤ 0.05 and trends toward significance were declared at P ≤ 0.10. Unadjusted means and standard errors of means (SEM) are presented.
Results and Discussion
It is becoming increasingly apparent that diet can have profound effects on functional genomics and represents an area of research that has yet to be exploited for potential health benefits. Inorganic phosphate (Pi) is a common dietary component that may directly alter cell and tissue behavior in just such a manner. Almost four decades ago studies noted that contact inhibited 3T3 cells responded to serum stimulation with a rapid increase in Pi transport (31) and described Pi as a limiting nutrient in proliferation (4-6) capable of actively altering cell growth properties (32) and transformation (7). Recent in vitro results suggest that Pi is capable of stimulating specific signal transduction pathways including ERK1/2 and Akt (8, 12, 13) and suggest a paradigm shift from viewing inorganic phosphate as a passive requirement in these processes to an active regulator, thereby defining a novel mitogenic signal. Based on these in vitro studies we hypothesized that Pi is in fact a mitogen. It would then follow that the level of available Pi would actively alter the transformation potential of cells in response to a tumor promoting event.
Elevated Phosphate increases proliferation and promotes transformation
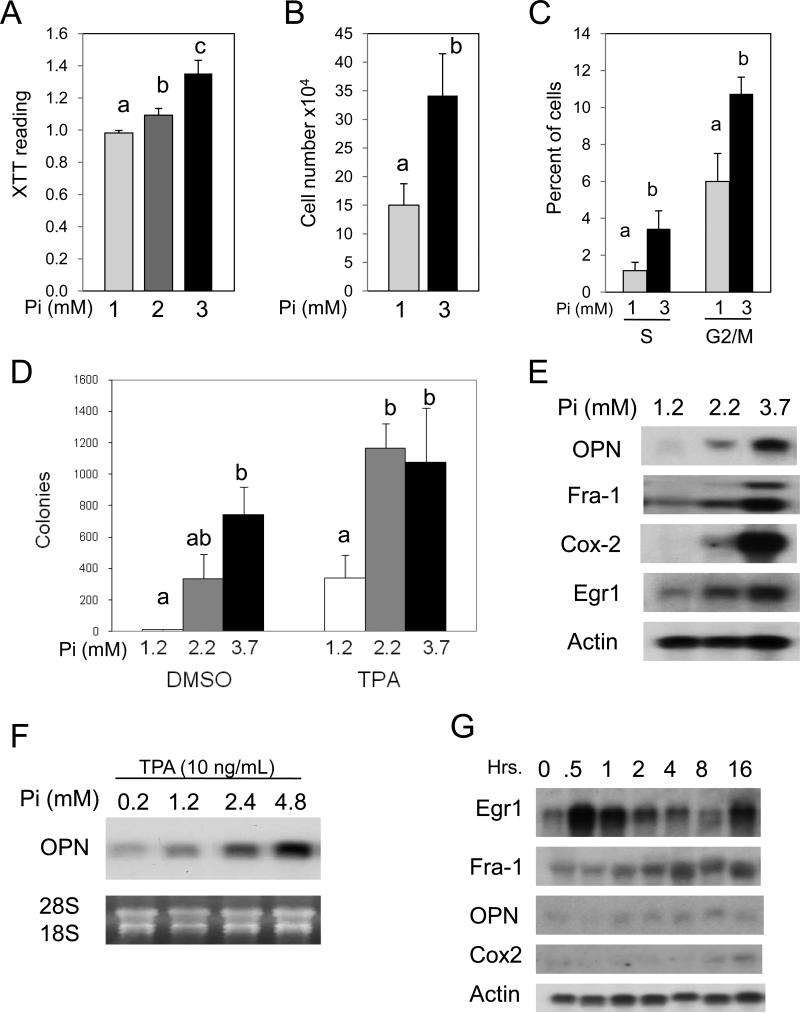
We utilized a number of assays to determine if elevated Pi promotes proliferation. Throughout our in vitro studies we have used a spectrum of final Pi concentrations to cover the physiological range of both mice and humans. Elevated Pi promoted cell proliferation as measured by cell viability (Figure 1A), cell number (Figure 1B), and the percent of cells in the S and G2/M phases of the cell cycle (Figure 1C). Additionally, elevation of Pi in a physiological range increased conversion of cells to anchorage independent growth in transformation sensitive JB6 cells (Figure 1D). Anchorage independent growth in transformation sensitive JB6 cells represents a well defined marker of transformation in vitro and correlates specifically with tumorigenicity in vivo (33). An increase of as little as 1 mM Pi alone increased growth and anchorage independent growth in soft agar (Figures 1A, D). Supplementation of 1 mM Pi is in addition to the 1mM Pi in the medium and indicates that elevated Pi alone is sufficient to enhance cell transformation. Furthermore, elevated Pi acted synergistically with TPA, which is commonly used to promote cell transformation in JB6 cells, to increase growth in soft agar (Figure 1D) indicating that Pi renders JB6 cells more sensitive to TPA induced transformation. The soft agar response correlated with a dose-dependent increase in gene expression of OPN, Fra-1, and Cox-2 (Figure 1E), which are linked to transformation in the JB6 model (22, 27). The augmentation of the TPA response by Pi was also correlated with the expression of osteopontin (Figure 1F). In particular, OPN (spp1) represents a gene/protein that is tightly associated with transformation, inflammation, and metastasis and is an extracellular matrix and circulating factor (21, 22). The growth of JB6 cells in soft agar has been shown to be dependent on OPN expression (34) and recently OPN was found to be regulated by AP-1 under transforming conditions in JB6 cells model (24). The expression of OPN is often considered a late response and to determine if elevated Pi resulted in an upstream response cells were analyzed for gene expression at earlier time points. The results revealed that, in fact, elevated Pi alters the Early growth response-1 (Egr1) gene within 30 minutes of exposure (Figure 1G). Egr1 is considered a marker of cell growth and proliferation and we have previously described it as a Pi responsive protein (35). Fra-1 and Cox-2 begin to rise later in the response and OPN does not change at these relatively early time points. In summary, our results suggest that high available Pi promotes proliferation and transformation as well as the gene expression changes necessary for transformation in JB6 cells.
Figure 1. High inorganic phosphate (Pi) concentrations increase proliferation, anchorage independent growth, and transformation-responsive gene expression in transformation-sensitive JB6 cells.
(A) High Pi increases proliferation of JB6 cells: cells were plated in a 96 well plate at 1×104/100 μL and incubated for 36 hr in final medium concentrations of 1, 2, and 3 mM Pi. Cell growth was measured using XTT assay and results are expressed as the mean XTT reading ± SEM (6 replicates/treatment). Columns with different characters differ at p<0.001. (B) High PI increases cell number: JB6 cells were plated at 5×104 cells/ml and grown in 1 or 3 mM phosphate (final) for 96 hr and cell number recorded. Columns with different characters differ at p<0.05. (C) High Pi increases mitotic cells: JB6 cells were grown in 1 or 3mM final Pi for 36 hr and analyzed by flow cytometry. Columns with different characters differ at p<0.05. (D) High Pi increases anchorage independent growth of JB6 cells in soft agar: JB6 cells (1×104 cells/100 μL) were plated for 14 d in the presence of DMSO (control) or 10ng/mL TPA in agar containing 1.2, 2.2, or 3.5 mM Pi. Colony number, expressed as the mean colony number ± SEM (5 replicates/treatment), was quantified by staining with neutral red and analyzed by automated microscopy. Columns with different characters differ at p<0.05. (E) High Pi increases transformation responsive gene expression in JB6 cells: cells were grown for 7 days in medium supplemented with 1.2, 2.2, or 3.7 mM Pi. The RNA was harvested for Northern blotting and probed for osteopontin (OPN), Fra-1, Cox-2, and actin (representative of multiple experiments). (F) High Pi augments TPA-induced transformation responsive gene expression: JB6 cells were grown in the presence of 10 ng/mL TPA in DMSO for 24 hr in a medium containing 0.2, 1.2, 2.4, or 4.8 mM Pi. The resulting RNA samples were analyzed by Northern blotting for OPN, 18S rRNA, and 28S rRNA. (G) High Pi increases early gene expression: JB6 cells were cultured in growth medium (1mM Pi) followed by addition of 4mM Pi (5mM final) for the indicated times. The resulting RNA samples were analyzed by Northern blotting and probed as indicated.
Elevated Phosphate activates AP-1
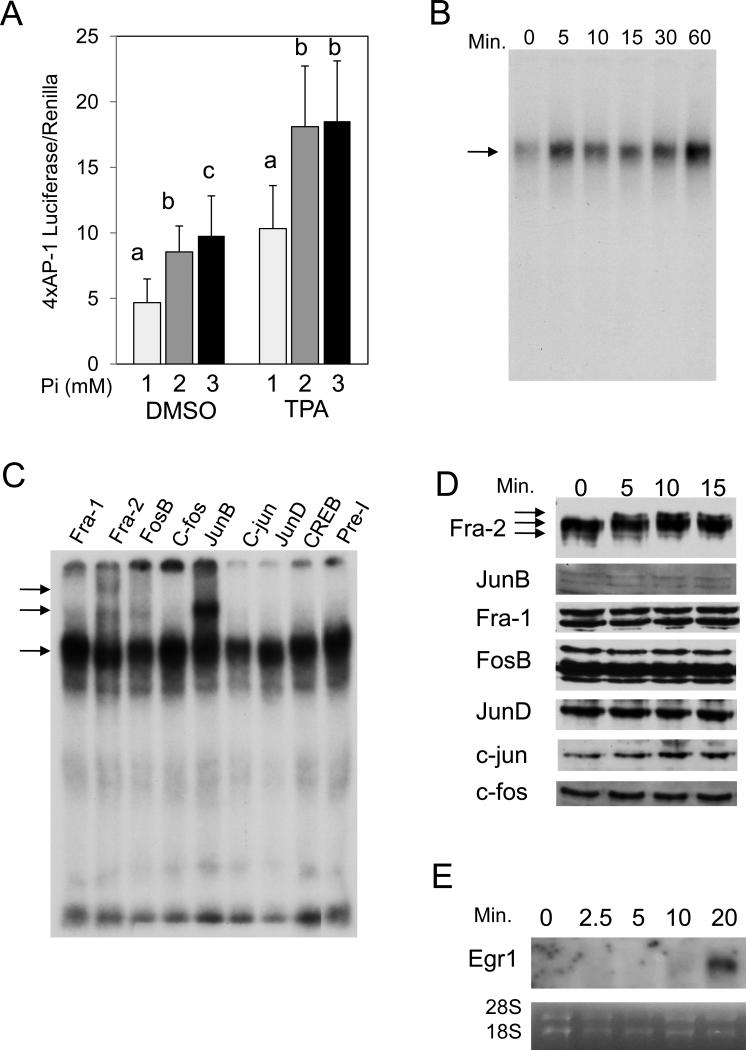
To elucidate the molecular mechanism by which high available Pi promotes cell proliferation, transformation, and changes in gene expression, we focused on AP-1 and ras activation. Our previous in vitro studies suggested that Pi is a molecule capable of regulating specific signal transduction pathways including ERK1/2 and Akt and subsequent gene expression leading to increased proliferation (8, 12, 13). The strong link between AP-1 transcription factor activation and JB6 transformation (14, 36-38) and the increased expression of the AP-1 transcription factor Fra-1 in response to Pi (Figure 1E) suggested that the AP-1 transcription factor family may be responsive to Pi. Parallel to the soft agar response (Figure 1D), elevated Pi alone was sufficient to promote AP-1 activity and augment TPA in promoting AP-1 activity (Figure 2A). The effect of elevated Pi on AP-1 DNA binding was rapid (within 5 min; Figure 2B) and supershifts suggest the presence of at least the AP-1 transcription factors Fra-2, JunB, and FosB at this early time point (Figure 2C). In agreement with the rapid stimulation of these AP-1 proteins, Fra-2 and JunB have been previously demonstrated to be regulated within 30 to 45 min in response to serum stimulation and suggests elevated Pi induces a common, early growth signaling response (39). Although, JunB has also been linked to senescence promoting activities (40), the strong induction of proliferation by Pi suggests a positive role in this case. It is possible that the dimerization partner may determine the positive or negative effect of JunB on proliferation. We performed a Western blot of nuclear lysates from JB6 cells to determine if high Pi results in changes in AP-1 protein levels or posttranslational modifications. Results suggest a change in the protein status of Fra-2 with a rapid shift to a slower migrating form in response to elevated Pi (Figure 2D). Other AP-1 proteins did not appear to be significantly altered at these time points. To confirm changes in gene expression under these conditions RNA was analyzed for Egr1 expression and demonstrated a strong increase within 20 minutes of elevated Pi exposure (Figure 2E). In summary, our results suggest that one potential mechanism by which high available Pi promotes cell proliferation and transformation in JB6 cells and the gene expression changes necessary for transformation is by promoting AP-1 DNA binding.
Figure 2. High inorganic phosphate (Pi) concentrations increase AP-1 activation in transformation-sensitive JB6 cells.
(A) High Pi increases AP-1 transcriptional activity in JB6 cells: cells were transfected with a 4xAP-1 luciferase and Renilla reporter constructs, and after 24 hrs serum starved overnight. Medium was then supplemented to a final concentration of 1, 2, or 3mM Pi in the presence of DMSO (control) or 10ng/ml TPA in DMSO for 6 hrs. AP-1 transcriptional activity was measured using a luciferase reporter assay (6 replicates/treatment) and Renilla by Stop-and-Glo. Luciferase activity was then normalized to Renilla and averaged. Columns with different characters differ at p<0.05. (B) High Pi increases within 5 min AP-1 DNA binding in JB6 cells: cells were supplemented with 2mM Pi for 0, 5, 10, 15, 30, or 60 min and EMSA was performed using an oligo containing a consensus AP-1 binding element. (C) High Pi primarily increases within 5 min DNA binding of the AP-1 proteins Fra-2, JunB, and FosB: cells were supplemented with 2 mM Pi for 5 min and EMSA was performed using an oligo containing a consensus AP-1 element and antibodies specific to the 7 individual AP-1 family members (Fra-1, Fra-2, c-fos, FosB, JunB, c-jun, JunD) and CREB (representative of multiple experiments). (D) High Pi alters AP-1 protein forms: JB6 cells were serum starved (1mM Pi) followed by addition of 2mM Pi (3mM final) for the indicated times. Nuclear lysates were analyzed by Western blotting and probed as indicated. (E) High Pi increases Egr1 expression within 20 minutes. JB6 cells were treated as in D and cells harvested for Northern analysis. Results are representative of multiple experiments.
Elevated Phosphate activates N-ras
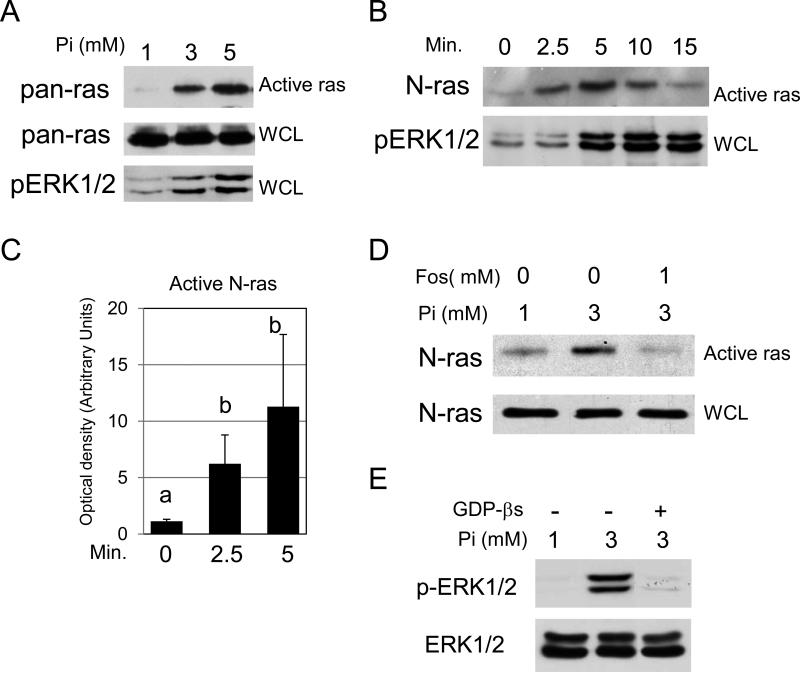
Transformation of JB6 cells is dependent on activation of the Ras-Raf-MEK pathway (41). Our previous studies combining proteomic and microarray analysis in the pre-osteoblast cell line predicted that the ras family was involved in Pi signaling (10). As hypothesized, elevated Pi promoted within 5 min ras activity and ERK1/2 phosphorylation in JB6 cells (Figure 3A). At this early time point, elevated Pi primarily activated N-ras (Figure 3B). Reprobing stripped blots with antibodies to H-, K-, and R-ras showed no detectable change in response to Pi (data not shown) suggesting elevated Pi selectively activates N-ras. Quantification of 3 experiments suggested a significant time dependent increase of N-ras activity in response to elevated Pi (Figure 3C). The Pi-induced increase in N-ras activity at this early time point can be inhibited by blocking phosphate transport into the cell (Figure 3D), as evaluated by pretreatment with 1 mM of the known phosphate transport inhibitor phosphonoformic acid, also known as foscarnet (42). This provides strong evidence that availability of Pi alters N-ras activity and identifies Pi as a novel regulator of ras activity. To determine if GTP signaling occurs upstream of ERK1/2 activation JB6 cells were serum starved and pretreated overnight with GDPβS, a stable, inactive analog of GDP, and treated with phosphate for 10 minutes. The resulting Western blot revealed that inhibition of GTP signaling blocked phosphorylation of ERK1/2, suggesting the GTP signaling event is upstream (Figure 3E).
Figure 3. High inorganic phosphate (Pi) concentrations activate N-ras in transformation-sensitive JB6 cells.
(A) High Pi increases within 5 min ras activity and phosphorylation of ERK1/2 in JB6 cells: cells were serum starved overnight and supplemented with 2 or 4 mM Pi for 5 min (1, 3, and 5 mM final). Ras activation of whole cell lysate blots was measured using the Ras-Binding Domain of Raf-1 and detected using pan-ras antibody. Phosphorylation of ERK1/2 was measured from a duplicate whole cell lysate blot using phospho-ERK1/2 antibody. (B) High Pi increases within 2.5 min N-ras activity and phosphorylation of ERK1/2 in JB6 cells: cells were serum starved overnight and treated with 2mM Pi (3mM final) for 0, 2.5, 5, 10, and 15 min. A ras activation assay was performed (Active ras) and whole cell lysate (WCL) from the same samples were probed with antibodies for N-ras and phospho-ERK1/2, respectively. (C) Quantitation of N-ras activity at 0, 2.5, and 5 minutes from 3 separate experiments: Results are expressed as arbitrary units obtained from densitometry analysis. Columns with different characters differ at p<0.05. (D) Phosphate transport is required for Pi to increase N-ras activity: cells were serum starved overnight and supplemented for 2.5 min with 0 or 2mM Pi and Vehicle (H2O) or 1mM of the phosphate transport inhibitor foscarnet (phosphonoformic acid). Ras activation (Active ras) and whole cell lysate (WCL) blots were probed with antibodies for N-ras. (E) Inhibition of GDP/GTP exchange blocks Pi induced ERK1/2 phosphorylation: JB6 cells were serum starved overnight (1mM Pi) and pretreated with 2.5mM GDPβS. The cells were treated with 2mM Pi (3mM final) for 10 minutes and resulting cell lysates analyzed by Western blotting.
The N-ras knockout mice are viable and grow normally (43), however, these mice are less responsive to carcinogen induced tumors than wild-type mice and over-expression of wild-type N-ras has been demonstrated to increase the incidence of lymphomas (44). To our knowledge the N-ras null mouse has not been tested in the two-stage model. Our data suggest that normal activated N-ras cooperates with activated H-ras to enhance tumor formation, progression, or both. A precedent for such a scenario has been suggested using the N-ras null mice. This study demonstrated the stimulation of Raf and Rhoa by N-ras and Akt and simultaneous activation of cdc42 by K-ras to cooperate in transformation (45). Another study using a colon cancer model demonstrated that expression of active K-Ras (G12D) altered proliferation while active N-ras (G12D) altered apoptosis, again suggesting unique, non-overlapping, and cooperative roles for the ras isoforms in transformation (46). In summary, our results suggest that another potential mechanism by which high available Pi promotes cell proliferation, transformation, and gene expression in JB6 cells is by activating a Ras signaling network.
A High Phosphate Diet Increases Sensitivity to Skin Papillomagenesis
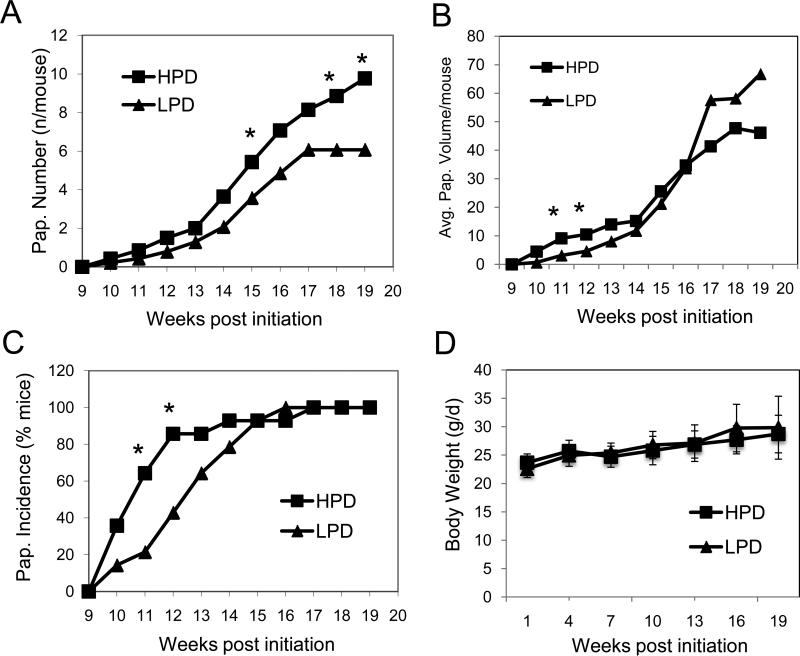
The JB6 transformation model has been demonstrated to be predictive of results in the two-stage skin carcinogenesis model. Consistent with the results in the JB6 model, high dietary phosphate (HPD, 1.2%Pi) increased skin papilloma number by 50% compared to low dietary phosphate (LPD, 0.2% Pi) in DMBA/TPA treated female FVB/N mice (Figure 4A&B). Similarly, in a recent study of lung tumorigenesis in mice, high dietary Pi (1.0%) increased tumor number and size compared to 0.5% Pi (47), suggesting a general promoting effect of high dietary phosphate level on tumorigenesis. Although all mice developed at least one papilloma and papillomas of similar size at the end of trial, there was a trend toward delayed incidence and growth (Figures 4B&C). Speculatively, mice on the HPD develop papillomas earlier and more papillomas may progress to squamous cell carcinoma than the mice on the LPD (HPD: 3.9% or 5 of 127 papillomas versus LPD: 2.3% or 2 of 86 papillomas), although more data is needed for statistical validation of these observations. However, the fact that the papillomas were smaller in size with the LPD and taken with a recent study that demonstrated that HPD stimulated increased cell proliferation and lung tumorigenesis of K-ras active mice (47) suggests that the HPD does accelerate proliferation. Neither of the Pi diets impacted body weight (Figure 4D) or feed consumption (not shown), which is consistent with the results in the lung tumorigenesis model (47). In summary, our results suggest that high dietary Pi promotes skin tumorigenesis in DMBA/TPA treated female FVB/N mice.
Figure 4. High phosphate diet promotes skin tumorigenesis in DMBA/TPA-treated FVB/N mice.
(A) A high phosphate diet increases skin papilloma number in DMBA/TPA-treated FVB/N mice: skin papilloma numbers were counted weekly from 14 mice/group, which were fed for 19 wk high (1.2% Pi, HPD) or low phosphate diets (0.2% Pi, LPD) starting with the DMBA initiation. Stars indicate differences between dietary groups at p < 0.05. (B) A high phosphate diet accelerates early skin papilloma growth: skin papilloma dimensions were measured weekly from 14 mice/group, which were fed for 19 wk HPD or LPD starting with the DMBA initiation. Stars indicate differences between dietary groups at p < 0.05. (C) A high phosphate diet accelerates first appearance of skin papilloma number in DMBA/TPA-treated FVB/N mice: skin papilloma numbers were counted weekly from 14 mice/group, which were fed for 19 wk high (1.2% Pi, HPD) or low phosphate diets (0.2% Pi, LPD) starting with the DMBA initiation. Stars indicate differences between dietary groups at p ≤0.05. (D) The low phosphate diet does not affect body weight in DMBA/TPA-treated FVB/N mice: Individual body weight was measured every 3 weeks from 14 mice/group, which were fed for 19 wk HPD or LPD starting with the DMBA initiation.
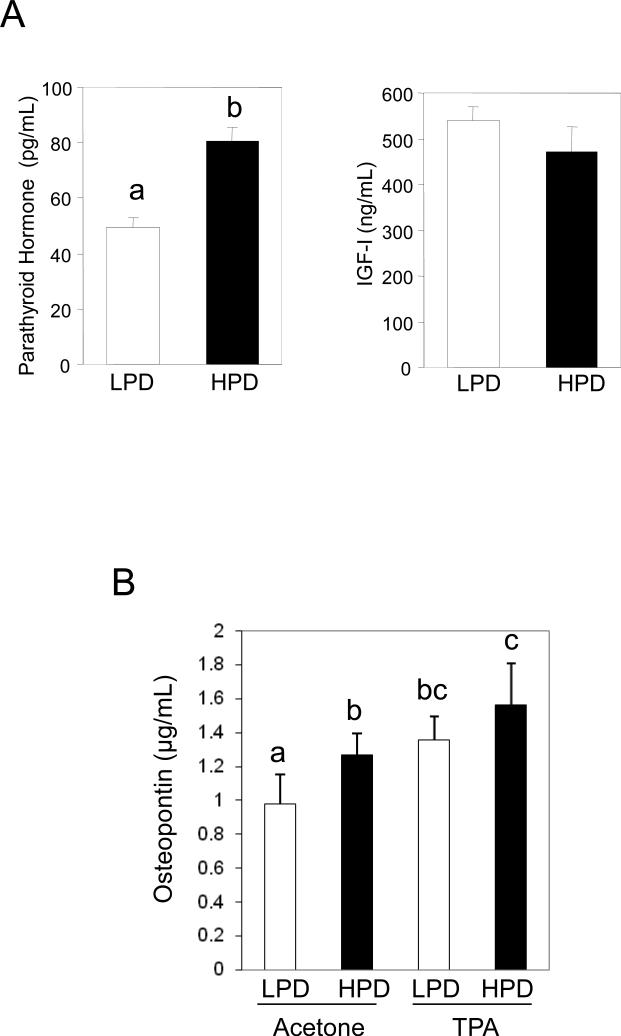
A High Phosphate Diet Increases Serum Osteopontin Levels
Altered serum phosphate might not only influence cell and tissue behavior by cell autonomous effects but also paracrine and endocrine effects. Levels of serum phosphate in mice and humans have been demonstrated to be positively correlated to parathyroid hormone (PTH) levels (48, 49). Although the receptor for PTH has been identified in rodent keratinocytes (50), the presence in human keratinocytes is not clear (51) and little is known about potential effects on cell behavior. We found that HPD increased serum concentrations of phosphate and PTH and decreased concentrations of calcium and uric acid, whereas serum IGF-I, magnesium, creatinine, and total protein concentrations were not affected (Table 2; Figure 5A). This suggests a specific rather than a general effect of dietary Pi on circulating factors. Here we report an additional circulating serum factor that is Pi responsive, OPN (Figure 5B). Osteopontin is known as both a survival factor and as a mediator of macrophage infiltration to the tumor site (52). Therefore, elevated circulating OPN concentrations might promote tumor initiation and progression. Consistent with our results in JB6 cells (Figure 1), high dietary Pi increased synergistically with TPA serum concentrations of OPN (Figure 5B). In summary, our results suggest that an additional mechanism by which changes in serum Pi might promote tumorigenesis is by increasing the levels of circulating OPN.
Table 2.
High inorganic phosphate (Pi) consumption (in % of diet) increases serum concentrations of phosphate and decreases concentrations of calcium and uric acid in FVB/N mice (n = 5/group)
| Diet |
Phosphorus mg/dL |
Calcium mg/dL |
Magnesium mg/dL |
Creatinine mg/dL |
Uric acid mg/dL |
Total protein g/dL |
|---|---|---|---|---|---|---|
| 0.2% Pi | 6.71 ± 0.60 | 10.38 ± 0.36 | 2.44 ± 0.17 | 0.14 ± 0.02 | 1.84 ± 0.11 | 5.70 ± 0.27 |
| 1.2% Pi | 10.60 ± 1.81# | 9.77 ± 0.24* | 2.59 ± 0.13 | 0.18 ± 0.03 | 1.38 ± 0.28* | 5.84 ± 0.37 |
p≤0.01
p≤0.001.
Figure 5. High phosphate diets increase serum concentrations of parathyroid hormone (PTH) in FVB/N mice and osteopontin (OPN) in TPA-treated FVB/N mice.
(A) A high phosphate diet increases serum concentrations of parathyroid hormone (PTH), but not IGF-I in FVB/N mice: serum samples were taken from 5 mice per group, which consumed for 5 wk high (1.2% Pi, HPD) or low phosphate diets (0.2% Pi, LPD), and analyzed for PTH and IGF-I. Columns with different characters differ at p < 0.05. (B) A high phosphate diet increases serum concentrations of OPN in TPA-treated FVB/N mice: serum samples were taken from 6 mice per group, which consumed for 2 wk high (1.2% Pi, HPD) or low phosphate diets (0.2% Pi, LPD), and then were treated topically 6 hr before sample collection with acetone (control) or 10nM of TPA in acetone. Columns with different characters differ at p < 0.01.
Dietary Phosphate can Effect Steady State Serum Phosphate Levels
Our in vitro studies were performed using a spectrum of Pi concentrations physiologically relevant to both humans and mice. In humans steady-state serum phosphate concentrations generally range from 0.70 to 1.55 mM although levels differ slightly with sex and age. However, serum levels in humans can vary by as much as 1.2 mM following a high phosphate meal and remain stably altered from diet alone (53-55). Although basal serum phosphate levels are different between mice and humans the percent change we achieved in our study is in line with what is achievable in humans. The diets used herein resulted in a change in serum phosphate from 2.17 mM (LPD) to 3.42 mM (HPD), a 37% difference (Table 2). The change is proportional to differences found in humans in response to altered Pi diets (53, 54, 56). Results presented here suggest that differences in dietary Pi can cause significant long-term differences in steady-state serum phosphate levels in agreement with other published long-term diet studies in both rodents (57, 58) and humans (48, 53, 56, 59, 60). Due in part to the increased consumption of processed foods, the amount of Pi in the American diet continues to rise above levels already considered high by the FDA (59). The current dietary recommended allowance for Pi is 700-800 mg/day and the tolerable upper intake limit is 4,000 mg/d for adults. It is important to note that the diets reported here have increased Pi without corresponding changes in calcium as would be the case with a diet high in dairy (59). A human dietary equivalent of the daily Pi consumption of our mice is 500 mg/d for the LPD and 1,800 mg/d for the HPD. More than 50% of young and middle aged men consume greater than 1,600 mg/d and these calculations largely reflect “natural” sources of Pi and are therefore likely an underestimate (61).
In conclusion, the in vitro and in vivo results reveal that elevated Pi promotes cell transformation and skin tumorigenesis and suggest at least two different means. First, elevated Pi may act in a cell autonomous manner as a mitogen and promote proliferation and transformation through activation of a signaling pathway consisting of ras and ERK1/2. Elevated Pi may promote AP-1 transcriptional activation early in the response and ultimately changes in gene expression such as OPN, a known autocrine, paracrine, and endocrine factor later in the response. Secondly, elevated Pi increases circulating concentrations of PTH and OPN, both of which can act as endocrine factors and promote tumorigenesis. The significance of OPN in papilloma formation in the two-stage model has been recently demonstrated using the OPN null mice in which the lack of osteopontin markedly suppressed papilloma development, possibly through the prevention of apoptosis (62). As the amount of Pi in the human diet, and in particular the western diet, continues to rise (59), it will be important to fully understand the influence of Pi on cell and tissue function and the relationship to tumorigenesis (63). Furthermore, these studies identify dietary Pi as a novel target for chemoprevention.
Acknowledgements
GRB.Jr. and CEC are supported in part by grants from NIH/NCI (CA136059), NIH/NIAMS (AR056090), and an Emory University Research Committee grant. This project has been funded in part with Federal funds from the National Cancer Institute, National Institutes of Health (MRY, GB, CMP and NHC).
References
- 1.Newberne PM, Conner MW. Dietary modifiers of cancer. Prog Clin Biol Res. 1988;259:105–129. [PubMed] [Google Scholar]
- 2.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66:1191–1308. [PubMed] [Google Scholar]
- 3.Go VL, Wong DA, Wang Y, Butrum RR, Norman HA, Wilkerson L. Diet and cancer prevention: evidence-based medicine to genomic medicine. J Nutr. 2004;134:3513S–3516S. doi: 10.1093/jn/134.12.3513S. [DOI] [PubMed] [Google Scholar]
- 4.Holley RW, Kiernan JA. Control of the initiation of DNA synthesis in 3T3 cells: low-molecular weight nutrients. Proc Natl Acad Sci U S A. 1974;71:2942–2945. doi: 10.1073/pnas.71.8.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber MJ, Edlin G. Phosphate transport, nucleotide pools, and ribonucleic acid synthesis in growing and in density-inhibited 3T3 cells. J Biol Chem. 1971;246:1828–1833. [PubMed] [Google Scholar]
- 6.Hilborn DA. Serum stimulation of phosphate uptake into 3T3 cells. J Cell Physiol. 1976;87:111–121. doi: 10.1002/jcp.1040870114. [DOI] [PubMed] [Google Scholar]
- 7.Rubin H, Sanui H. Complexes of inorganic pyrophosphate, orthophosphate, and calcium as stimulants of 3T3 cell multiplication. Proc Natl Acad Sci U S A. 1977;74:5026–5030. doi: 10.1073/pnas.74.11.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conrads KA, Yi M, Simpson KA, Lucas DA, Camalier CE, Yu LR, et al. A combined proteome and microarray investigation of inorganic phosphate-induced pre-osteoblast cells. Mol Cell Proteomics. 2005;4:1284–1296. doi: 10.1074/mcp.M500082-MCP200. [DOI] [PubMed] [Google Scholar]
- 9.Beck GR, Jr., Moran E, Knecht N. Inorganic phosphate regulates multiple genes during osteoblast differentiation, including Nrf2. Exp Cell Res. 2003;288:288–300. doi: 10.1016/s0014-4827(03)00213-1. [DOI] [PubMed] [Google Scholar]
- 10.Beck GR, Jr., Zerler B, Moran E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc Natl Acad Sci U S A. 2000;97:8352–8357. doi: 10.1073/pnas.140021997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conrads KA, Yu LR, Lucas DA, Zhou M, Chan KC, Simpson KA, et al. Quantitative proteomic analysis of inorganic phosphate-induced murine MC3T3-E1 osteoblast cells. Electrophoresis. 2004;25:1342–1352. doi: 10.1002/elps.200405892. [DOI] [PubMed] [Google Scholar]
- 12.Beck GR, Jr., Knecht N. Osteopontin regulation by inorganic phosphate is ERK1/2-, protein kinase C-, and proteasome-dependent. J Biol Chem. 2003;278:41921–41929. doi: 10.1074/jbc.M304470200. [DOI] [PubMed] [Google Scholar]
- 13.Chang SH, Yu KN, Lee YS, An GH, Beck GR, Jr., Colburn NH, et al. Elevated inorganic phosphate stimulates Akt-ERK1/2-Mnk1 signaling in human lung cells. Am J Respir Cell Mol Biol. 2006;35:528–539. doi: 10.1165/rcmb.2005-0477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernstein LR, Colburn NH. AP1/jun function is differentially induced in promotion-sensitive and resistant JB6 cells. Science. 1989;244:566–569. doi: 10.1126/science.2541502. [DOI] [PubMed] [Google Scholar]
- 15.Colburn NH, Former BF, Nelson KA, Yuspa SH. Tumour promoter induces anchorage independence irreversibly. Nature. 1979;281:589–591. doi: 10.1038/281589a0. [DOI] [PubMed] [Google Scholar]
- 16.Dhar A, Hu J, Reeves R, Resar LM, Colburn NH. Dominant-negative c-Jun (TAM67) target genes: HMGA1 is required for tumor promoter-induced transformation. Oncogene. 2004;23:4466–4476. doi: 10.1038/sj.onc.1207581. [DOI] [PubMed] [Google Scholar]
- 17.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Gupta S, Mukhtar H. Chemoprevention of skin cancer: current status and future prospects. Cancer Metastasis Rev. 2002;21:363–380. doi: 10.1023/a:1021275330385. [DOI] [PubMed] [Google Scholar]
- 20.Dong Z. Effects of food factors on signal transduction pathways. Biofactors. 2000;12:17–28. doi: 10.1002/biof.5520120104. [DOI] [PubMed] [Google Scholar]
- 21.Denhardt DT, Mistretta D, Chambers AF, Krishna S, Porter JF, Raghuram S, et al. Transcriptional regulation of osteopontin and the metastatic phenotype: evidence for a Ras-activated enhancer in the human OPN promoter. Clin Exp Metastasis. 2003;20:77–84. doi: 10.1023/a:1022550721404. [DOI] [PubMed] [Google Scholar]
- 22.Rittling SR, Chambers AF. Role of osteopontin in tumour progression. Br J Cancer. 2004;90:1877–1881. doi: 10.1038/sj.bjc.6601839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Tanani MK, Campbell FC, Kurisetty V, Jin D, McCann M, Rudland PS. The regulation and role of osteopontin in malignant transformation and cancer. Cytokine Growth Factor Rev. 2006;17:463–474. doi: 10.1016/j.cytogfr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Matthews CP, Birkholz AM, Baker AR, Perella CM, Beck GR, Jr., Young MR, et al. Dominant-negative activator protein 1 (TAM67) targets cyclooxygenase-2 and osteopontin under conditions in which it specifically inhibits tumorigenesis. Cancer Res. 2007;67:2430–2438. doi: 10.1158/0008-5472.CAN-06-0522. [DOI] [PubMed] [Google Scholar]
- 25.Slaga TJ, Budunova IV, Gimenez-Conti IB, Aldaz CM. The mouse skin carcinogenesis model. J Investig Dermatol Symp Proc. 1996;1:151–156. [PubMed] [Google Scholar]
- 26.Malumbres M, Pellicer A. RAS pathways to cell cycle control and cell transformation. Front Biosci. 1998;3:d887–912. doi: 10.2741/a331. [DOI] [PubMed] [Google Scholar]
- 27.Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 28.Mangues R, Seidman I, Gordon JW, Pellicer A. Overexpression of the N-ras proto-oncogene, not somatic mutational activation, associated with malignant tumors in transgenic mice. Oncogene. 1992;7:2073–2076. [PubMed] [Google Scholar]
- 29.Wasylyk C, Imler JL, Wasylyk B. Transforming but not immortalizing oncogenes activate the transcription factor PEA1. Embo J. 1988;7:2475–2483. doi: 10.1002/j.1460-2075.1988.tb03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ise K, Nakamura K, Nakao K, Shimizu S, Harada H, Ichise T, et al. Targeted deletion of the H-ras gene decreases tumor formation in mouse skin carcinogenesis. Oncogene. 2000;19:2951–2956. doi: 10.1038/sj.onc.1203600. [DOI] [PubMed] [Google Scholar]
- 31.Cunningham DD, Pardee AB. Transport changes rapidly initiated by serum addition to “contact inhibited” 3T3 cells. Proc Natl Acad Sci U S A. 1969;64:1049–1056. doi: 10.1073/pnas.64.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engstrom W, Zetterberg A. Phosphate and the regulation of DNA replication in normal and virus-transformed 3T3 cells. Biochem J. 1983;214:695–702. doi: 10.1042/bj2140695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin SI, Freedman VH, Risser R, Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci U S A. 1975;72:4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang PL, Cao M, Hicks P. Osteopontin induction is required for tumor promoter-induced transformation of preneoplastic mouse cells. Carcinogenesis. 2003;24:1749–1758. doi: 10.1093/carcin/bgg138. [DOI] [PubMed] [Google Scholar]
- 35.Meng Z, Camalier CE, Lucas DA, Veenstra TD, Beck GR, Jr., Conrads TP. Probing early growth response 1 interacting proteins at the active promoter in osteoblast cells using oligoprecipitation and mass spectrometry. J Proteome Res. 2006;5:1931–1939. doi: 10.1021/pr060009l. [DOI] [PubMed] [Google Scholar]
- 36.Bernstein LR, Bravo R, Colburn NH. 12-O-tetradecanoylphorbol-13-acetate--induced levels of AP-1 proteins: a 46-kDa protein immunoprecipitated by anti-fra-1 and induced in promotion-resistant but not promotion-sensitive JB6 cells. Mol Carcinog. 1992;6:221–229. doi: 10.1002/mc.2940060308. [DOI] [PubMed] [Google Scholar]
- 37.Dong Z, Lavrovsky V, Colburn NH. Transformation reversion induced in JB6 RT101 cells by AP-1 inhibitors. Carcinogenesis. 1995;16:749–756. doi: 10.1093/carcin/16.4.749. [DOI] [PubMed] [Google Scholar]
- 38.Young MR, Nair R, Bucheimer N, Tulsian P, Brown N, Chapp C, et al. Transactivation of Fra-1 and consequent activation of AP-1 occur extracellular signal-regulated kinase dependently. Mol Cell Biol. 2002;22:587–598. doi: 10.1128/MCB.22.2.587-598.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kovary K, Bravo R. Existence of different Fos/Jun complexes during the G0-to-G1 transition and during exponential growth in mouse fibroblasts: differential role of Fos proteins. Mol Cell Biol. 1992;12:5015–5023. doi: 10.1128/mcb.12.11.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piechaczyk M, Farras R. Regulation and function of JunB in cell proliferation. Biochem Soc Trans. 2008;36:864–867. doi: 10.1042/BST0360864. [DOI] [PubMed] [Google Scholar]
- 41.Kang NJ, Lee KW, Kwon JY, Hwang MK, Rogozin EA, Heo YS, et al. Delphinidin attenuates neoplastic transformation in JB6 Cl41 mouse epidermal cells by blocking Raf/mitogen-activated protein kinase kinase/extracellular signal-regulated kinase signaling. Cancer Prev Res (Phila Pa) 2008;1:522–531. doi: 10.1158/1940-6207.CAPR-08-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szczepanska-Konkel M, Yusufi AN, VanScoy M, Webster SK, Dousa TP. Phosphonocarboxylic acids as specific inhibitors of Na+-dependent transport of phosphate across renal brush border membrane. J Biol Chem. 1986;261:6375–6383. [PubMed] [Google Scholar]
- 43.Umanoff H, Edelmann W, Pellicer A, Kucherlapati R. The murine N-ras gene is not essential for growth and development. Proc Natl Acad Sci U S A. 1995;92:1709–1713. doi: 10.1073/pnas.92.5.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diaz R, Lopez-Barcons L, Ahn D, Garcia-Espana A, Yoon A, Matthews J, et al. Complex effects of Ras proto-oncogenes in tumorigenesis. Carcinogenesis. 2004;25:535–539. doi: 10.1093/carcin/bgh026. [DOI] [PubMed] [Google Scholar]
- 45.Fotiadou PP, Takahashi C, Rajabi HN, Ewen ME. Wild-type NRas and KRas perform distinct functions during transformation. Mol Cell Biol. 2007;27:6742–6755. doi: 10.1128/MCB.00234-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haigis KM, Kendall KR, Wang Y, Cheung A, Haigis MC, Glickman JN, et al. Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon. Nat Genet. 2008;40:600–608. doi: 10.1038/ngXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin H, Xu CX, Lim HT, Park SJ, Shin JY, Chung YS, et al. High dietary inorganic phosphate increases lung tumorigenesis and alters Akt signaling. Am J Respir Crit Care Med. 2009;179:59–68. doi: 10.1164/rccm.200802-306OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reiss E, Canterbury JM, Bercovitz MA, Kaplan EL. The role of phosphate in the secretion of parathyroid hormone in man. J Clin Invest. 1970;49:2146–2149. doi: 10.1172/JCI106432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calvo MS, Kumar R, Heath H. Persistently elevated parathyroid hormone secretion and action in young women after four weeks of ingesting high phosphorus, low calcium diets. J Clin Endocrinol Metab. 1990;70:1334–1340. doi: 10.1210/jcem-70-5-1334. [DOI] [PubMed] [Google Scholar]
- 50.Errazahi A, Bouizar Z, Lieberherr M, Souil E, Rizk-Rabin M. Functional type I PTH/PTHrP receptor in freshly isolated newborn rat keratinocytes: identification by RT-PCR and immunohistochemistry. J Bone Miner Res. 2003;18:737–750. doi: 10.1359/jbmr.2003.18.4.737. [DOI] [PubMed] [Google Scholar]
- 51.Sharpe GR, Dillon JP, Durham B, Gallagher JA, Fraser WD. Human keratinocytes express transcripts for three isoforms of parathyroid hormone-related protein (PTHrP), but not for the parathyroid hormone/PTHrP receptor: effects of 1,25(OH)2 vitamin D3. Br J Dermatol. 1998;138:944–951. doi: 10.1046/j.1365-2133.1998.02259.x. [DOI] [PubMed] [Google Scholar]
- 52.Duff MD, Mestre J, Maddali S, Yan ZP, Stapleton P, Daly JM. Analysis of gene expression in the tumor-associated macrophage. J Surg Res. 2007;142:119–128. doi: 10.1016/j.jss.2006.12.542. [DOI] [PubMed] [Google Scholar]
- 53.Portale AA, Halloran BP, Murphy MM, Morris RC., Jr. Oral intake of phosphorus can determine the serum concentration of 1,25-dihydroxyvitamin D by determining its production rate in humans. J Clin Invest. 1986;77:7–12. doi: 10.1172/JCI112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Portale AA, Halloran BP, Morris RC., Jr. Physiologic regulation of the serum concentration of 1,25-dihydroxyvitamin D by phosphorus in normal men. J Clin Invest. 1989;83:1494–1499. doi: 10.1172/JCI114043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kemi VE, Karkkainen MU, Lamberg-Allardt CJ. High phosphorus intakes acutely and negatively affect Ca and bone metabolism in a dose-dependent manner in healthy young females. Br J Nutr. 2006;96:545–552. [PubMed] [Google Scholar]
- 56.Nishida Y, Taketani Y, Yamanaka-Okumura H, Imamura F, Taniguchi A, Sato T, et al. Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int. 2006;70:2141–2147. doi: 10.1038/sj.ki.5002000. [DOI] [PubMed] [Google Scholar]
- 57.Katsumata S, Masuyama R, Koshihara M, Matsuzaki H, Uehara M, Suzuki K. High phosphorus diet changes phosphorus metabolism regardless of PTH action in rats. Biosci Biotechnol Biochem. 2004;68:243–246. doi: 10.1271/bbb.68.243. [DOI] [PubMed] [Google Scholar]
- 58.Czarnogorski M, Woda CB, Schulkin J, Mulroney SE. Induction of a phosphate appetite in adult male and female rats. Exp Biol Med (Maywood) 2004;229:914–919. doi: 10.1177/153537020422900907. [DOI] [PubMed] [Google Scholar]
- 59.Calvo MS. Dietary phosphorus, calcium metabolism and bone. J Nutr. 1993;123:1627–1633. doi: 10.1093/jn/123.9.1627. [DOI] [PubMed] [Google Scholar]
- 60.Huttunen MM, Tillman I, Viljakainen HT, Tuukkanen J, Peng Z, Pekkinen M, et al. High dietary phosphate intake reduces bone strength in the growing rat skeleton. J Bone Miner Res. 2007;22:83–92. doi: 10.1359/jbmr.061009. [DOI] [PubMed] [Google Scholar]
- 61.Uribarri J, Calvo MS. Hidden sources of phosphorus in the typical American diet: does it matter in nephrology? Semin Dial. 2003;16:186–188. doi: 10.1046/j.1525-139x.2003.16037.x. [DOI] [PubMed] [Google Scholar]
- 62.Hsieh YH, Juliana MM, Hicks PH, Feng G, Elmets C, Liaw L, et al. Papilloma development is delayed in osteopontin-null mice: implicating an antiapoptosis role for osteopontin. Cancer Res. 2006;66:7119–7127. doi: 10.1158/0008-5472.CAN-06-1002. [DOI] [PubMed] [Google Scholar]
- 63.McCarty MF. A moderately low phosphate intake may provide health benefits analogous to those conferred by UV light - a further advantage of vegan diets. Med Hypotheses. 2003;61:543–560. doi: 10.1016/s0306-9877(03)00228-7. [DOI] [PubMed] [Google Scholar]