Abstract
Purpose of the Review
This review details the role of memory T cells in physiologic and allospecific immunity, and summarizes the effects of immunosuppressive agents used to manipulate their function in the context of organ transplantation.
Recent Findings
Memory T cells are lymphocytes with characteristics that are thought to promote anamnestic immune responses. They have a unique capacity to generate rapid effector functions upon secondary exposure to a pathogen, and this is achieved through truncated requirements for antigen presentation, reduced activation thresholds, and enhanced trafficking and adhesion mechanisms. In general, these same mechanisms also appear to evoke improved efficiency in mediating allograft rejection. The phenotype of these cells has been increasingly well defined and associated with a characteristic pattern of susceptibility to immunosuppressive agents. This knowledge is now being exploited in the development of immune therapeutic regimens to selectively mollify T memory cell effects.
Summary
A specific targeting of memory T cells has potential to prevent allograft rejection in a more precise manner that current means of immunosuppression. However, these benefits will be balanced by the reciprocal risk of susceptibility to recurrent infection.
Keywords: memory T cell, heterologous immunity, homeostatic proliferation, tolerance, allograft
Introduction
Immunological memory, or the ability to generate increasingly efficient antigen-specific protective immune responses with subsequent antigenic exposures, is a fundamental hallmark of adaptive immunity in higher vertebrates. The effect of an initial exposure to an environmental antigen is imprinted on a host organism’s immune cell repertoire in such a way so as to increase the magnitude and rapidity of antigen clearance following re-exposure to that antigen. In particular, antigen-experienced T cells take on characteristics indicative of prior activation and give rise to a population of cells collective referred to a memory T cells (TMs). These cells mediate enhanced protection against invading pathogens and are thought to convey an evolutionary survival advantage. However, in the context of transplantation, the presence of cells with prejudiced reactivity against donor antigens increases the likelihood of immune mediated rejection such that adaptive immunity becomes counter-adaptive. While the precise pathways and cellular interactions that shape TM function rejection remain to be fully elucidated, emerging evidence suggests that these cells play a critical role in rejection. In this review we describe fundamental characteristics of TMs, discuss their role in allograft rejection, and relate their unique traits to existing and emerging immune therapeutic agents.
Characteristics of Memory T Cells
T cells emerge from the thymus with a naïve or non-activated phenotype characterized by relatively high T cell receptor (TCR) density and limited adhesion molecule expression. This phenotype persists until the cell becomes primed. Priming requires repetitive binding of a cell’s TCRs to major histocompatibility complex (MHC) molecules presenting the T cell’s cognate peptide antigen in the context of sufficient costimulatory signals, accessory molecules and adhesion molecules to induce cell division. Following several rounds of division, naïve T cells differentiate into an activated, effector T cell population that then mediates antigen elimination. Most of these cells undergo apoptosis in the conduct of their effector function, leading to population contraction with antigen elimination. However, some cells persist as a pool of long-lasting antigen-specific TMs. Two models have been suggested to describe the generation of TMs from naïve precursors: a linear progression model postulating that memory populations arise from a pool of previously primed effectors, and a parallel progression model stipulating that memory populations develop as a separate lineage alongside the population of short-lived effectors [1–3]. In addition, recent evidence suggests that the development of TMs may be influenced by antigen-specific T cell precursor frequency, the extent of antigenic stimulation, and/or the cytokine milieu present at the time of priming [4–6]. Antigen-specific T cell memory is maintained within the host by a basal homeostatic turnover that is thought to be supported independent of antigen by cytokines including IL-15 [7–11].
As compared to naïve T cells, TMs possess distinct phenotypic, functional, and homing properties (Figure 1) [9, 10]. They produce cytokines faster than naïve T cells, potentially from decreased activation thresholds [12], and possess direct cytolytic function in vivo following reencounter with antigen [13, 14]. They also express higher levels of CD2, CD11a, and CD44 compared with their naïve counterparts and in humans express the RO isoform of CD45 as opposed to the RA isoform [10, 15–18]. Numerous groups have demonstrated that altered expression of selectins, integrins, and chemokine receptors on TMs are likely responsible for their unique homing properties including residence in peripheral tissues, allowing them more immediate access to peripheral antigen including alloantigen following transplantation [19–27]. While TMs are heterogeneous, two well-described subsets exist within most antigen-specific memory populations. Central memory T cells (TCM; CCR7+ CD62Lhi) migrate primarily to secondary lymphoid tissues (e.g. lymph node and spleen) and are responsible for generating a burst of new effectors following recall. Effector-memory T cells (TEM; CCR7- CD62Llo) migrate to non-lymphoid tissues and provide immediate effector function at peripheral sites [19, 26, 27]. Whether these two populations derive from one another or have distinct origins is unclear, and there is evidence to support both paradigms [4, 28, 29].
Figure 1.
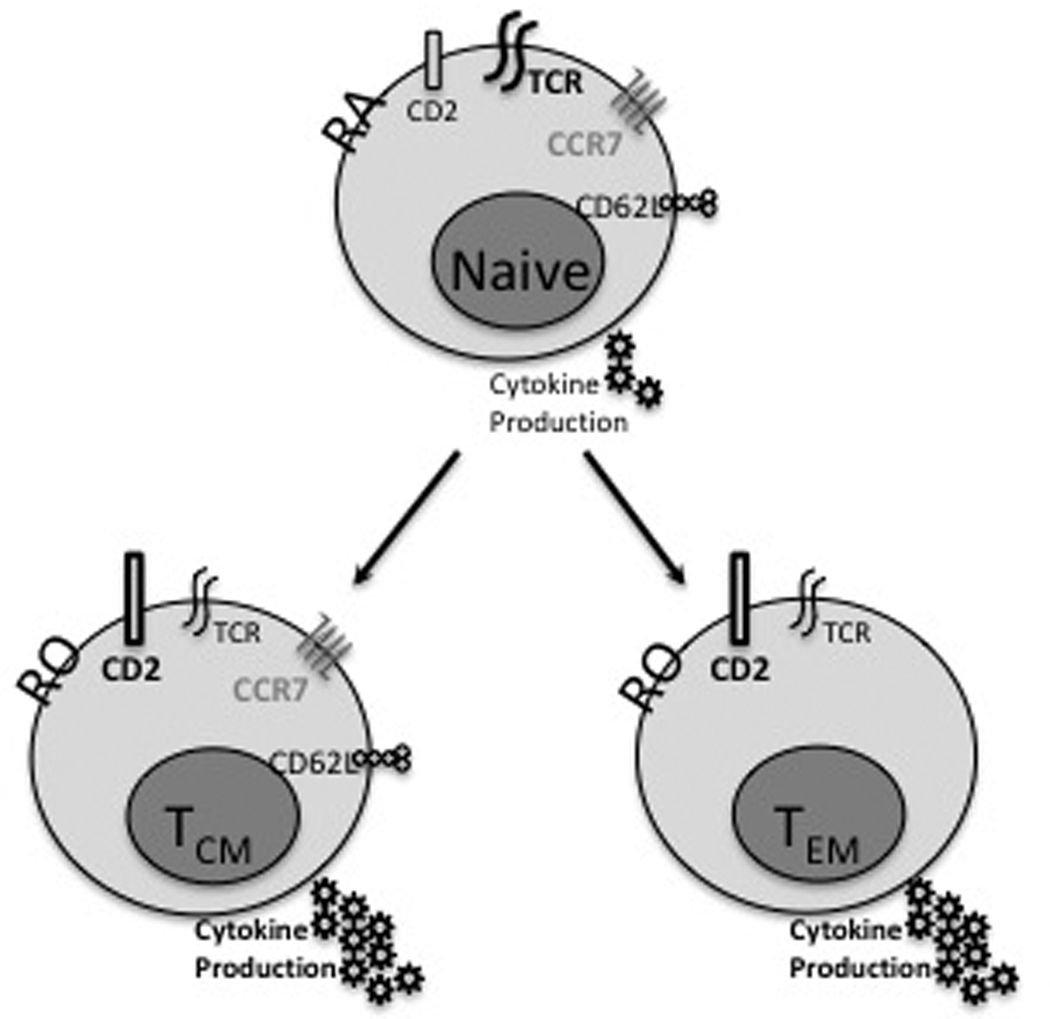
Schematic of the unique characteristics of naïve T cells, central memory T cells (TCM) and effector memory T cells (TEM). Naïve T cells express the CD45RA isoform, have relatively high expression of the T cell receptor (TCR), low CD2 expression, and require substantial stimulation to produce cytokines and other effector molecules. With memory differentiation, TEM and TCM assume the CD45RO isoform, increase their expression of CD2, and reduce their expression of the TCR. Their capacity to produce cytokines is augmented. TCM express CD62L and CCR7 facilitating their homing to secondary lymphoid tissues, while TEM lack these molecules and reside in the periphery.
In unsensitized transplant recipients, two unique mechanisms for the generation of donor-reactive TMs have been described. First, heterologous immunity is the phenomenon whereby previous exposures to environmental pathogens influence the course of future immune responses to seemingly unrelated antigens [30–32]. Once thought to be exquisitely specific for a single peptide:MHC complex, TCRs are now appreciated to possess inherent degeneracy with regard to their recognition of antigen, such that a T cell recognizing one antigen can also respond to other antigens, albeit with altered affinity. Heterologous alloimmunity thus results when a TM population primed by self-MHC presenting an environmental antigen generates cross-reactive TMs responsive to allo- or self-MHC presenting an allopeptide. Recently, dual receptor T cells have been described as being over-represented in alloreactive T cell populations, raising the possibility that if a dual receptor T cell is activated via one pathogen-specific TCR, it could later respond as a TM if its second, alloreactive TCR were to encounter donor antigen [33].
Donor-reactive TMs can also be generated via homeostatic proliferation, a process whereby transient lymphopenia caused by viral infection, or in the case of transplantation, therapeutic T cell depletion, induces the proliferation and differentiation of naïve T cells into cells with true phenotypic and functional characteristics of TMs as well as cells that appear to be TMs but fail to have robust effector functions [34–36]. Therefore, a proportion of the naïve alloreactive T cell pool is likely stochastically converted into alloreactive TMs throughout life, giving most patients some degree of allo-responsive memory, although they have not had exposure to alloantigen.
Role of Memory T cells in Allograft Rejection
A growing body of evidence exists to suggest that TMs may play a critical role in inhibiting allograft acceptance. For example, TMs have recently been shown to have the ability to migrate into allograft tissues and secrete inflammatory cytokines well before the 4–6 days required for donor-specific priming in the spleen [27]. Furthermore, tolerance achieved in cardiac allografts using anti-CD154 are rejected when alloreactive TMs are generated in recipients via sensitization with donor-type skin grafts [39, 40]. In addition, regimens to induce tolerance have been highly successful in rodents, but less so in non-human primates (NHPs). One possible explanation for this is that longer lived, socially housed animals such as NHPs have, in general, greater exposure to environmental pathogens as compared to rodents housed in specific-pathogen free facilities, and therefore possess an increased opportunity to generate a diverse repertoire of TMs [37, 38]. Evidence supporting this hypothesis includes experiments in rodents where tolerance was achievable in naïve animals treated with CTLA4-Ig, anti-CD40L mAb, busulfan and donor bone marrow, but not in animals infected with LCMV, vaccinia virus, and vesicular stomatitis virus. Even after anti-viral immune responses were allowed to resolve to memory, the mice resisted tolerance induction. Stimulation of splenocytes isolated with these recipients with donor tissue resulted in increased frequency of cytokine producing cells, thus implicating the virus-elicited allo-crossreactive TMs in the prevention of tolerance induction in this model [41].
Similar inhibition of tolerance has been demonstrated in rodents by donor-reactive TMs generated through homeostatic proliferation. In an experimental model where recipients were devoid of endogenous T cells, adoptive transfer of naïve syngeneic splenocytes resulted in the rapid homeostatic expansion of the transferred cells and acquisition of a memory phenotype. Importantly, when these animals were challenged with allogeneic skin grafts, the transplants were rejected despite treatment with costimulation blockade [34]. These data provide direct experimental evidence that donor-reactive memory T cells can be generated by homeostatic mechanisms.
Effects of Immunosuppressants on Memory T Cells
Given that TEM have unique properties, it is not surprising that they also have characteristic sensitivities to various immune therapeutics that distinguish the from naïve cells.
T cell depleting agents
Many agents are used in clinical transplantation to intentional evoke global T cell depletion. These include polyclonal antibody preparations such as anti-thymocyte globulins (ATGs) and monoclonal antibodies specific for CD3 (muromonab) or CD52 (alemtuzumab). These agents mediate depletion through a variety of mechanisms [42, 43] and while the T cell depletion that occurs following treatment with these drugs is profound, emerging evidence suggests that TMs may have some degree of resistance to depletional therapies. For example, treatment of human transplant recipients with alemtuzumab resulted in >90% T cell depletion; however, the cells that remain have been shown to contain a predominance of CD4+ CD45RO+ CD62Llo TEMs. The origin of the TM predominance likely stems from a combination of TM resistance to antibody mediated depletion and resultant homeostatic activation of non-depleted cells. The proliferating population is likely derived from naïve cells as these cells, being less terminally differentiated, should be expected to have greater proliferative capacity. Based on these and other studies, it is accepted that T cell depletional therapy will increase the overall frequency of TMs, both due to the relative resistance of TMs as well as the likely conversion of naïve to TMs via homeostatic activation [44, 45, 46]. Recent studies have also suggested that alemtuzumab may decrease the requirement for immunosuppression by down-regulating the CD4+ TEM population associated with rejection [46]. In a human cohort receiving alemtuzumab for kidney transplantation, CD8+ T cells recovered to their baseline population in 6 months, while the recovery of CD4+ T cells was delayed until approximately 15 months. Furthermore, the CD8+ T cells that repopulated the peripheral T cell compartment by homeostatic proliferation were of immunosenescent CD28−/CD8+ phenotype, which the investigators postulated may compete for space with or even may suppress the proliferation of CD4+ TEM cells [47]. Alternatively, the absence of CD28 may render these cells resistant to costimulation blockade based therapies while remaining sensitive to calcineurin inhibitors. Importantly, post-depletional T cells are TEM skewed and clearly capable of mediating rejection despite exceptionally low numbers of cells without some adjuvant immunosuppressive therapy [48, 49]. Thus, while their long-term characteristics may be altered in ways that influence their sensitivity to immunosuppressants, they remain immunocompetent.
Effects of T cell costimulatory pathway blockade
Costimulation is required for optimal activation of naïve antigen-specific T cells. The role of costimulation in the activation of TMs is dependent on both the type of TM and the costimulatory molecule. The CD28 pathway is one of the most important and well studied of the T cell costimulatory pathways. CD28 binds to its ligands CD80 or CD86, and propagates a positive costimulatory signal into the T cell. Agents have been developed to target this pathway. These include CTLA4-Ig, a fusion protein containing the extracellular domain of the CTLA-4 molecule, which associates tightly with CD80 and CD86 and therefore prevents CD28 ligation, and LEA29Y, a second-generation derivative of CTLA-4 Ig with increased affinity for CD86 [50]. However, evidence from several studies exists to suggest that TMs are relatively independent of CD28-mediated costimulation for recall responses, thus refractory to the effects of CD28 pathway blockers. In rodents deficient in CD28, this costimulatory pathway is not necessary for the generation or recall of TMs [7, 51, 52]. Furthermore, tolerance induction protocols based on CTLA-4 Ig have been found to be ineffective in recipients that possess cross-reactive virus-elicited donor-reactive TMs. Rejection in these recipients was characterized by a lack of attenuation of donor-reactive CD8+ T cell responses; however, CD4+ donor-reactive TM responses were significantly reduced following this costimulation blockade based regimen [41]. In another study, CTLA4-Ig was found to inhibit proliferation and expansion of memory CD4 T cells in response to peptide antigen challenge, with no effect on early activation [53]. Taken together, these data suggest that the type and character of the donor-reactive TM population may influence its relative requirement for CD28-mediated costimulation upon recall. In humans and NHPs, which contain a large population of TMs in their peripheral T cell repertoires, CTLA4-Ig and LEA29Y have been shown to prolong graft survival, but not to the extent evident in mouse models. Interestingly, the TEM population in NHPs is largely void of CD28 and thus unlikely to be sensitive to agents targeting this pathway [54]. In humans, many CD8+ TEMs are CD28− while CD4+ TEMs tend to retain CD28 expression. Although systematic measurement of the effect of CD28 pathway blockade in humans and NHPs has not been performed, it is clear that adjuvant immune modulatory agents are required to complement the effect of costimulation blockade [55, 56].
The CD154/CD40 costimulation pathway is also intimately involved in the activation of T cells. In rodent models, inhibition of CD154 with anti-CD154 blocking antibodies has shown to be effective in the prevention of rejection in pre-sensitized hosts, but ineffective in sensitized hosts, indicating that CD154 may not be necessary for the activation of memory T cells [39, 40, 57]. Initial CD154 blocking agents did not undergo development, and their brief investigation in human transplantation suggested a lack of efficacy [58]. As such, other modes of blocking CD154, such as blocking CD40 have been attempted, and have shown promise in long-term kidney allograft tolerance in NHPs [59]. Recent success using a fully human CD40 specific monoclonal antibody suggest that this approach has promise, but remains dependent on adjuvant therapy [60, 61]. However, the direct effects of blocking CD40 and CD154 on donor-reactive TMs have varied depending on the model used. Using mice infected with LCMV, investigators have demonstrated that the CD4 TM response was downregulated compared to the CD8 response when given anti-CD154 agents [62]. In contrast, using a murine cardiac allograft model, the TM response was unchanged with anti-CD154 therapy [63]. These findings suggest that the type and rate of antigen exposure, combined with the heterogeneity of the host TM population may demonstrate variable sensitivity to blockade of the CD154/CD40 pathway.
Another costimulatory molecule that has been shown to be important in the activation of memory T cells is the OX40 pathway, a member of the TNF receptor superfamily. This pathway has been implicated in synergistically driving the proliferation of TMs along with CD28-mediated costimulation [64]. In murine models, TM mediated graft rejection was prevented when OX40 blockade was given combined with CD28/CD40 blockade, while grafts were rejected when OX40 blockade was given alone [65].
While there is a growing body of evidence to suggest that TMs are relatively resistant to costimulation blockade, other agents have been designed to specifically target and deplete the memory compartment based on unique enhanced expression of certain integrins, specifically CD11a and CD2 [66–68]. LFA-3-IgG1 fusion protein (Alefacept) binds to CD2 and has been shown to both prevent the activation of TMs and induce apoptosis, thereby decreasing the TM population [68, 69]. Alefacept is currently approved for clinical treatment of psoriasis, and its therapeutic effect is linked to its ability to deplete TM [69, 70]. Recently, alefacept has been shown to extend kidney allograft survival in NHPs when added to a CTLA4-Ig-based regimen [54]. In this study, CD4+ and CD8+ TEM were shown to be specifically depleted by alefacept, and this appeared to be related to the increased expression of CD2, the target of alefacept, on TEM populations. Further in vitro studies of this model examined the effect of alefacept on alloreactive cytokine producing cells and demonstrated responding alloreactive CD4+ and CD8+ T cells also exhibited increased CD2 expression, thus providing an increased available target for the effects of alefacept. This study was the first to specifically target TMs with the prospective intent on neutralizing cells resistant to costimulation blockade [54].
Effects of blocking signaling through the TCR and cytokine receptors
The most commonly used immunosuppressants, the calcineurin inhibitors (CNIs) (cyclosporine A and tacrolimus), target TCR-mediated signaling. These agents prevent the nuclear translocation of NFAT that is required for gene transcription of IL-2, which is important for optimal expansion and survival of T cells. The reduced requirements of TMs for costimulation focus a greater reliance on the TCR, and as such, CNIs have been shown to be unique among the clinically available immunosuppressants in preventing TM proliferation and cytokine production [45]. This has been suggested to be a predominant reason why CNIs have been such important contributors to the prevention of rejection in humans. However, while TCR signal inhibition prevents TM activation, it also may inhibit TM apoptosis and inhibit response contraction [71]. Thus, the CNI approach appears to have exquisite ability to prevent rejection, but may also prevent regulation and TM contraction through apoptosis.
Inhibiting non-TCR signals in the T cell may have paradoxical influence of TM function and in fact enhance some aspects of immunity. A recent example involves sirolimus, a clinically used immunosuppressant known to attenuate downstream signaling events through mTOR, thereby preventing G1→S transition required for T cell proliferation and population expansion. Sirolimus has been used as a replacement for CNIs with one rationale being to specifically allow TCR signaling to foster activation induced cell death. While there are few studies assessing the direct effects of these agents on the memory cell population despite their common use, it has recently been demonstrated in an infection model that sirolimus alone acts to actually increase the frequency of antigen-specific T cells that differentiate into the memory lineage [72]. In this study, mice given low-dose sirolimus following acute LCMV infection demonstrated enhanced quality and quantity of virus-specific TMs compared with untreated controls. Similarly, NHPs given sirolimus after modified vaccinia (MVA) vaccination exhibited an increased frequency of memory T cells.
Like sirolimus, the CD25-specific monoclonal antibodies daclizumab and basiliximab also interfere with the IL-2 pathway, by binding the high-affinity α chain of the IL-2 receptor (CD25) [73]. Although CD25 is up-regulated in both naïve and TMs upon encounter with antigen, TMs up-regulate CD25 faster, and have been shown to subvert the blocking effects of anti-CD25 monoclonal antibodies through higher expression of the low affinity IL-2 receptor subunits (CD122 and CD132) that may support TM proliferation and activation by ligating IL-15 in addition to IL-2 [74]. Thus, the extent to which TMs are relevant to an individual’s clinical outcome may significantly influence the relative success of CNI-sparing regimens in general, and sirolimus or anti-CD25-based regimens specifically.
Janus kinases (JAKs) are a family of cytoplasmic tyrosine kinases that regulate gene expression after signaling by cytokines. JAK3, expressed primarily in hematopoietic cells, is downstream of the common γ chain (CD132). Therefore blocking JAK3 has the potential to interrupt the signals of a broader array of cytokines used by TMs such as IL-2, IL-7, and IL-15. Indeed, JAK3 kinase inhibitors have been shown to prevent allograft rejection in both murine and NHP models [75, 76]. Further studies have proposed that prolonged graft survival observed following administration of JAK3 inhibitors could be due to blockade of signaling through the IL-7 and IL-15 receptors and thus target TM methods for by-passing a requirement for CD25. For instance, recent investigations have suggested that IL-7 is critical both to the generation of TMs following homeostatic proliferation, to aid the survival of TMs, and that IL-15 is required for the generation and maintenance of anti-viral CD8 TMs [77]. Currently one such inhibitor of the JAK3 pathway (CP-690550) is in phase II clinical trials of renal transplantation [78]. It has yet to be specifically investigated for its role in thwarting allospecific memory, but may offer a means of targeting TMs without TCR inhibition.
Effects of blocking T cell trafficking
Given the role of TEMs in initiating effector function in the periphery and the role of TCMs in rapidly deriving new effectors, interruption of trafficking could have selectively potent effects of TM function. As discussed above, inhibition of LFA-3 interactions with CD2 appear to effectively target TMs and facilitate costimulation-based therapies. Several other agents currently under study for use in transplantation target similar processes. One such treatment is FTY720, which binds sphingosine-1-phosphate (S1P) receptor as an agonist and disables the SIP receptor from performing its function of allowing lymphocyte migration from the thymus and peripheral lymphoid tissues, effectively sequestering T cells in the lymph nodes and inhibiting them from trafficking to peripheral graft sites [26, 79, 80]. Blockade of CD11a also may hold promise as an agent that targets the trafficking of TMs. For example, recent work has demonstrated in murine models of transplantation that anti-LFA-1 monoclonal antibodies result in attenuation of donor-reactive memory recall responses and decreased T cell trafficking to the allograft following graft placement [81, 82]. The initial phase 2 testing of the anti-LFA-1 agent efalizumab in renal transplantation suggests that this agent indeed has potent inhibitory effects on protective memory in that its combination with a standard immunosuppressive regimen evoked a higher rate of EBV-associated malignancy [83]. Thus, memory inhibition is likely to require cautious paring with other agents to avoid pathologic inhibition of protective immunity.
Conclusion
Memory T cells can pose a critical barrier to successful organ transplantation. The population of TMs of a given individual may vary based on the prior immune history of that patient, including the type and frequency of environmental exposures to pathogens. The alloreactivity of the TM population may also vary based on heterologous cross-reactivity or direct prior alloantigen exposure. Regardless, the importance of a thorough understanding of the mechanisms of donor-specific TM generation and the effects of immunosuppressive agents on these heterogeneous populations is becoming increasingly apparent. Closer attention to memory responses will likely enhance the potential to tailor immunomodulatory strategies a given transplant recipient.
Acknowledgments
ADK is supported by the National Institutes of Health (1U01AI079223-01A1), the Georgia Research Alliance, the McKelvey Foundation, and the Juvenile Diabetes Research Foundation. MLF is supported by the National Institutes of Health (AI 079409).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272(5258):54. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 2.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nature Reviews Immunology. 2002;2(12):982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 3.Moulton VR, Farber DL. Committed to memory: lineage choices for activated T cells. TRENDS in Immunology. 2006;27(6):261–267. doi: 10.1016/j.it.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Marzo AL, et al. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. nature immunology. 2005;6(8):793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinhardt RL, et al. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410(6824):101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 6.Williams MA, Bevan MJ. T cell memory: fixed or flexible? nature immunology. 2005;6:752–754. doi: 10.1038/ni0805-752. [DOI] [PubMed] [Google Scholar]
- 7.Murali-Krishna K, et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8(2):177–188. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 8.Sprent J, Surh CD. T cell memory. Annual review of Immunology. 2002;20(1):551–579. doi: 10.1146/annurev.immunol.20.100101.151926. [DOI] [PubMed] [Google Scholar]
- 9.Surh CD, Sprent J. Regulation of naive and memory T-cell homeostasis. Microbes and Infection. 2002;4(1):51–56. doi: 10.1016/s1286-4579(01)01509-x. [DOI] [PubMed] [Google Scholar]
- 10.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nature Reviews Immunology. 2002;2(4):251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 11.Becker TC, et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. Journal of Experimental Medicine. 2002;195(12):1541. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farber DL. Biochemical signaling pathways for memory T cell recall. Seminars in Immunology. 2009 April;9(21):89–97. doi: 10.1016/j.smim.2009.02.003. ◦ This paper describes the unique mechanisms involved in memory T cell activation, including TCR signalling pathways, ZAP70 expression, CD28 costimulation, and nuclear transcription mechanisms.
- 13.Byers AM, et al. Cutting Edge: Rapid In Vivo CTL Activity by Polyoma Virus-Specific Effector and Memory CD8+ T Cells 1. Am Assoc Immnol. 2003:17–21. doi: 10.4049/jimmunol.171.1.17. [DOI] [PubMed] [Google Scholar]
- 14.Barber DL, Wherry EJ, Ahmed R. Cutting Edge: Rapid In Vivo Killing by Memory CD8 T Cells 1. Am Assoc Immnol. 2003:27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 15.Budd RC, et al. Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. The Journal of Immunology. 1987;138(10):3120–3129. [PubMed] [Google Scholar]
- 16.Wallace DL, Beverley PC. Phenotypic changes associated with activation of CD45RA+ and CD45RO+ T cells. Immunology. 1990;69(3):460. [PMC free article] [PubMed] [Google Scholar]
- 17.Slifka MK, Whitton JL. Activated and Memory CD8+ T Cells Can Be Distinguished by Their Cytokine Profiles and Phenotypic Markers 1. The Journal of Immunology. 2000;164(1):208–216. doi: 10.4049/jimmunol.164.1.208. [DOI] [PubMed] [Google Scholar]
- 18.Chandok MR, Farber DL. Signaling control of memory T cell generation and function. Elsevier; 2004. [DOI] [PubMed] [Google Scholar]
- 19.Sallusto F, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999:34–38. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 20.Usherwood EJ, et al. Functionally heterogeneous CD8+ T-cell memory is induced by Sendai virus infection of mice. Journal of Virology. 1999;73(9):7278–7286. doi: 10.1128/jvi.73.9.7278-7286.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. 2004 doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 22.Unsoeld H, et al. Cutting Edge: CCR7+ and CCR7-Memory T Cells Do Not Differ in Immediate Effector Cell Function 1. Journal of Immunology. 2002:638–641. doi: 10.4049/jimmunol.169.2.638. [DOI] [PubMed] [Google Scholar]
- 23.Bingaman AW, et al. Novel phenotypes and migratory properties distinguish memory CD4 T cell subsets in lymphoid and lung tissue. European Journal of immunology. 2005;35(11) doi: 10.1002/eji.200526004. [DOI] [PubMed] [Google Scholar]
- 24.Champagne P, et al. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410(6824):106–111. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 25.Masopust D, et al. Preferential localization of effector memory cells in nonlymphoid tissue. 2001:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Q, et al. Lymphoid Sequestration of Alloreactive Memory CD4 T Cells Promotes Cardiac Allograft Survival 1. The Journal of Immunology. 2006;176(2):770–777. doi: 10.4049/jimmunol.176.2.770. [DOI] [PubMed] [Google Scholar]
- 27. Schenk AD, et al. Donor-reactive CD8 Memory T Cells Infiltrate Cardiac Allografts Within 24 Hours Post-Transplant in Naïve Recipients. American Journal of Transplantation. 2008;8(8):1652. doi: 10.1111/j.1600-6143.2008.02302.x. ◦ Series of murine models demonstrating that inflammation can mediate donor-reactive CD8 memory T cells and augment T cell infiltration into cardiac allografts.
- 28.Wherry EJ, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nature Immunology. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 29.Baron V, et al. The repertoires of circulating human CD8+ central and effector memory T cell subsets are largely distinct. Immunity. 2003;18(2):193–204. doi: 10.1016/s1074-7613(03)00020-7. [DOI] [PubMed] [Google Scholar]
- 30.Mason D. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunology today. 1998;19(9):395–404. doi: 10.1016/s0167-5699(98)01299-7. [DOI] [PubMed] [Google Scholar]
- 31.Chen HD, et al. Memory CD8 T cells in heterologous antiviral immunity and immunopathology in the lung. nature immunology. 2001;2:1067–1076. doi: 10.1038/ni727. [DOI] [PubMed] [Google Scholar]
- 32.Aichele P, et al. Immunopathology or organ-specific autoimmunity as a consequence of virus infection. Immunological reviews. 1996;152:21. doi: 10.1111/j.1600-065x.1996.tb00909.x. [DOI] [PubMed] [Google Scholar]
- 33. Morris GP, Allen PM. Cutting Edge: Highly Alloreactive Dual TCR T Cells Play a Dominant Role in Graft-versus-Host Disease. The Journal of Immunology. 2009;182(11):6639. doi: 10.4049/jimmunol.0900638. ◦◦ Seminal study demonstrating that there is a small population of alloreactive T cells that have escaped thymic exclusion because of incomplete allelic exclusion of TCRα. The dual receptor T cells play an as of yet undefined role in allograft rejection
- 34.Wu Z, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nature medicine. 2003;10(1):87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor DK, Neujahr D, Turka LA. Heterologous immunity and homeostatic proliferation as barriers to tolerance. Current opinion in immunology. 2004;16(5):558–564. doi: 10.1016/j.coi.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Ndejembi MP, Tang AL, Farber DL. Reshaping the past: Strategies for modulating T-cell memory immune responses. Clinical Immunology. 2007;122(1):1–12. doi: 10.1016/j.clim.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 37.Adams AB, Pearson TC, Larsen CP. Heterologous immunity: an overlooked barrier to tolerance. Immunological Reviews. 2003;196(1):147. doi: 10.1046/j.1600-065x.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 38.Brook MO, Wood KJ, Jones ND. The Impact of Memory T Cells on Rejection and the Induction of Tolerance. Transplantation. 2006;82(1):1. doi: 10.1097/01.tp.0000226082.17507.da. [DOI] [PubMed] [Google Scholar]
- 39.Valujskikh A, Pantenburg B, Heeger P. Primed Allospecific T Cells Prevent the Effects of Costimulatory Blockade on Prolonged Cardiac Allograft Survival in Mice. American Journal of Transplantation. 2002;2(6):501. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhai Y, et al. Allograft rejection by primed/memory CD8+ T cells is CD154 blockade resistant: therapeutic implications for sensitized transplant recipients. The Journal of Immunology. 2002;169(8):4667–4673. doi: 10.4049/jimmunol.169.8.4667. [DOI] [PubMed] [Google Scholar]
- 41.Adams AB, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. Journal of Clinical Investigation. 2003;111(12):1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Préville X, et al. Mechanisms Involved in Antithymocyte Globulin Immunosuppressive Activity In A Nonhuman Primate Model 1. Transplantation. 2001;71(3):460. doi: 10.1097/00007890-200102150-00021. [DOI] [PubMed] [Google Scholar]
- 43.Genestier L, et al. Induction of Fas (Apo-1, CD95)-mediated apoptosis of activated lymphocytes by polyclonal antithymocyte globulins. Blood. 1998;91(7):2360. [PubMed] [Google Scholar]
- 44.Kwun J, Knechtle SJ, Hu H. Determination of the Functional Status of Alloreactive T Cells by Interferon-[gamma] Kinetics. Transplantation. 2006;81(4):590. doi: 10.1097/01.tp.0000196353.04494.14. [DOI] [PubMed] [Google Scholar]
- 45.Pearl JP, et al. Immunocompetent T-Cells with a Memory-Like Phenotype are the Dominant Cell Type Following Antibody-Mediated T-Cell Depletion. American Journal of Transplantation. 2005;5(3):465. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 46.Trzonkowski P, et al. Recipient memory-like lymphocytes remain unresponsive to graft antigens after CAMPATH-1H induction with reduced maintenance immunosuppression. Transplantation. 2006;82(10):1342–1351. doi: 10.1097/01.tp.0000239268.64408.84. [DOI] [PubMed] [Google Scholar]
- 47. Trzonkowski P, et al. Homeostatic repopulation by CD28-CD8+ T cells in alemtuzumab-depleted kidney transplant recipients treated with reduced immunosuppression. Am J Transplant. 2008;8(2):338–347. doi: 10.1111/j.1600-6143.2007.02078.x. ◦◦ This study conducted in humans demonstrates a mechanism to account for the both depletional effects of anti-CD52 agents, and why the memory T cell response is blunted against allograft. Further, this study shows that depletional agents can decrease the requirement for chronic immunosuppression.
- 48.Kirk A, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H) Transplantation. 2003;76(1):120–129. doi: 10.1097/01.TP.0000071362.99021.D9. [DOI] [PubMed] [Google Scholar]
- 49.Kirk A, et al. Results from a Human Renal Allograft Tolerance Trial Evaluating T-Cell Depletion with Alemtuzumab Combined with Deoxyspergualin. Transplantation. 2005;80(8):1051–1059. doi: 10.1097/01.tp.0000174341.49741.8f. [DOI] [PubMed] [Google Scholar]
- 50.Larsen CP, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. The American Journal of Transplantation. 2005;5(3):443–453. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 51.London CA, Lodge MP, Abbas AK. Functional Responses and Costimulator Dependence of Memory CD4+ T Cells 1. The Journal of Immunology. 2000;164(1):265–272. doi: 10.4049/jimmunol.164.1.265. [DOI] [PubMed] [Google Scholar]
- 52.Croft M, Bradley LM, Swain SL. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. The Journal of Immunology. 1994;152(6):2675–2685. [PubMed] [Google Scholar]
- 53.Ndejembi MP, et al. Control of memory CD4 T cell recall by the CD28/B7 costimulatory pathway. The Journal of Immunology. 2006;177(11):7698. doi: 10.4049/jimmunol.177.11.7698. [DOI] [PubMed] [Google Scholar]
- 54. Weaver T, et al. Alefacept promotes costimulation blocakde based allograft survival in non-human primates. Nature Medicine. 2009;15:746–749. doi: 10.1038/nm.1993. ◦◦ This paper describes the use of LFA3-Ig, alefacept, to specifically target memory T cells and in doing so eliminate CD28 negative costimulation-blockade resistent T cells.
- 55.Larsen C, et al. A New Look at Blockade of T-cell Costimulation: A Therapeutic Strategy for Long-term Maintenance Immunosuppression. American Journal of Transplantation. 2006;6(5):876. doi: 10.1111/j.1600-6143.2006.01259.x. [DOI] [PubMed] [Google Scholar]
- 56.Vincenti F, et al. Costimulation blockade with belatacept in renal transplantation. 2005:770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 57.Rabant M, et al. Donor-specific memory CD4 T cells provide help for alloantibody production in the absence of CD40/CD154 interactions and germinal center formation. The FASEB Journal. 2008;22:862.5. (1_MeetingAbstracts) [Google Scholar]
- 58.Kirk A, et al. Preliminary results of the use of humanized anti-CD154 in human renal allotransplantation. American Journal of Transplantation. 2001;1 Supplement:191. [Google Scholar]
- 59.Haanstra K, et al. Costimulation Blockade followed by a 12-Week Period of Cyclosporine A Facilitates Prolonged Drug-Free Survival of Rhesus Monkey Kidney Allografts. Transplantation. 2005;79(11):1623. doi: 10.1097/01.tp.0000158426.64631.ed. [DOI] [PubMed] [Google Scholar]
- 60. Aoyagi T, et al. A Human Anti-CD40 Monoclonal Antibody, 4D11, for Kidney Transplantation in Cynomolgus Monkeys: Induction and Maintenance Therapy. American Journal of Transplantation. 2009 Jun 10; doi: 10.1111/j.1600-6143.2009.02693.x. [Epub ahead of print] ◦ This paper describes the use of a CD40 specific monoclonal antibody in NHPs with results suggesting that T memory cells may still present a barrier in this type of therapy.
- 61.Imai A, et al. A novel fully human anti-CD40 monoclonal antibody, 4D11, for kidney transplantation in cynomolgus monkeys. Transplantation. 2007;84(8):1020–1028. doi: 10.1097/01.tp.0000286058.79448.c7. [DOI] [PubMed] [Google Scholar]
- 62.Whitmire JK, et al. Antiviral CD4 and CD8 T-cell memory: differences in the size of the response and activation requirements. Philosophical Transactions of the Royal Society B: Biological Sciences. 2000;355(1395):373. doi: 10.1098/rstb.2000.0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen Y, Heeger PS, Valujskikh A. In Vivo Helper Functions of Alloreactive Memory CD4+ T Cells Remain Intact Despite Donor-Specific Transfusion and Anti-CD40 Ligand Therapy 1. The Journal of Immunology. 2004;172(9):5456–5466. doi: 10.4049/jimmunol.172.9.5456. [DOI] [PubMed] [Google Scholar]
- 64.Rogers PR, et al. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15(3):445–455. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 65.Vu MD, et al. Critical, but Conditional, Role of OX40 in Memory T Cell-Mediated Rejection 1. The Journal of Immunology. 2006;176(3):1394–1401. doi: 10.4049/jimmunol.176.3.1394. [DOI] [PubMed] [Google Scholar]
- 66.Semnani R, et al. Costimulation by purified intercellular adhesion molecule 1 and lymphocyte function-associated antigen 3 induces distinct proliferation, cytokine and cell surface antigen profiles in human" naive" and" memory" CD4+ T cells. Journal of Experimental Medicine. 1994;180(6):2125–2135. doi: 10.1084/jem.180.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sanders M, et al. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. The Journal of Immunology. 1988;140(5):1401–1407. [PubMed] [Google Scholar]
- 68.da Silva A, et al. Alefacept, an Immunomodulatory Recombinant LFA-3/IgG1 Fusion Protein, Induces CD16 Signaling and CD2/CD16-Dependent Apoptosis of CD2+ Cells 1. The Journal of Immunology. 2002;168(9):4462–4471. doi: 10.4049/jimmunol.168.9.4462. [DOI] [PubMed] [Google Scholar]
- 69.Ortonne JP, et al. Alefacept-induced decreases in circulating blood lymphocyte counts correlate with clinical response in patients with chronic plaque psoriasis. The European Journal of Dermatology. 2003;13(2):117–123. [PubMed] [Google Scholar]
- 70.Larsen R, et al. Changes in circulating lymphocyte subpopulations following administration of the leucocyte function-associated antigen-3 (LFA-3)/IgG1 fusion protein alefacept. Clinical and Experimental Immunology. 2007;149(1):23. doi: 10.1111/j.1365-2249.2007.03380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Y, et al. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nature Medicine. 1999;5(11):1298–1302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 72. Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009:23. doi: 10.1038/nature08155. Epub ahead of print. ◦◦ This paper describes the paradoxical immunostimulant effect of rapamycin on memory CD8 T cells production when used alone in a murine infection model and NHP vaccination model.
- 73.Church AC. Clinical advances in therapies targeting the interleukin-2 receptor. Qjm. 2003;96(2):91. doi: 10.1093/qjmed/hcg014. [DOI] [PubMed] [Google Scholar]
- 74.Ku C, et al. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288(5466):675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 75.Borie DC, et al. Immunosuppression by the JAK3 inhibitor CP-690,550 delays rejection and significantly prolongs kidney allograft survival in nonhuman primates. Transplantation. 2005;79(7):791–801. doi: 10.1097/01.tp.0000157117.30290.6f. [DOI] [PubMed] [Google Scholar]
- 76.Changelian PS, et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. American Association for the Advancement of Science. 2003:875–878. doi: 10.1126/science.1087061. [DOI] [PubMed] [Google Scholar]
- 77.Schluns KS, et al. Cutting Edge: Requirement for IL-15 in the Generation of Primary and Memory Antigen-Specific CD8 T Cells 1. Am Assoc Immnol. 2002:4827–4831. doi: 10.4049/jimmunol.168.10.4827. [DOI] [PubMed] [Google Scholar]
- 78. Vincenti F, Kirk AD. What's Next in the Pipeline. American Journal of Transplantation. 2008;8(10):1972. doi: 10.1111/j.1600-6143.2008.02403.x. ◦ A recent review summarizing the state of clinical development of new immunomodulatory therapeutics.
- 79.Matloubian M, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427(6972):355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 80.Pinschewer D, et al. FTY720 Immunosuppression Impairs Effector T Cell Peripheral Homing Without Affecting Induction, Expansion, and Memory 1. The Journal of Immunology. 2000;164(11):5761–5770. doi: 10.4049/jimmunol.164.11.5761. [DOI] [PubMed] [Google Scholar]
- 81.Ford M, et al. Overcoming the memory barrier: role of anti-LFA-1 in promoting long-term graft survival in recipients possessing donor-reactive memory T cells. American Journal of Transplantation. 2009;9 Supplement 2:536. [Google Scholar]
- 82.Setoguchi K, et al. Anti-Lymphocyte Function Assoicated Antigen-1 (LFA-1) Monoclonal Antibody Prevents Graft-Infiltrating CD8+ Memory Cells Early Post-Transplant. The American Journal of Transplantation. 2009;9 Supplement 2:209. [Google Scholar]
- 83.Vincenti F, et al. A Phase I/II randomized open-label multicenter trial of efalizumab, a humanized anti CD11a, anti-LFA-1 inrenal transplantation. The American Journal of Transplantation. 2007;7(7):1770–1777. doi: 10.1111/j.1600-6143.2007.01845.x. [DOI] [PubMed] [Google Scholar]


