Abstract
Resveratrol (trans-3,4’, 5-trihydroxystilbene) is a naturally occurring polyphenolic compound that has anti-inflammatory, antioxidant, neuroprotective properties and acts as a chemopreventive agent. Resveratrol causes cell cycle arrest and induces apoptotic cell death in various types of cancer cells. In the current studies, the effect of resveratrol on phosphoinositide kinase-3 (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signaling pathway was examined in human U251 glioma cells. Resveratrol decreased both the expression and phosphorylation of Akt. Inhibitors of PI3K (LY294002) and Akt (SH-6) enhanced resveratrol-induced LDH release and caspase-3 activation. Resveratrol reduced phosphorylation of ribosomal protein S6 and the mTOR inhibitor rapamycin further enhanced resveratrol-induced cell death. These results suggest that the downregulation of PI3K/Akt/mTOR signaling pathways may be an important mediator in resveratrol-induced apoptosis in glioma cells.
Keywords: resveratrol, glioma, Akt, caspases-3
INSTRUCTION
Glioma is the most common malignant brain tumors in adults. Despite advances in the currently available combined therapies, such as surgery, radiation and chemotherapy, the prognosis of patients has not been improved (1). Alteration of major signaling cascades has been demonstrated in the development of gliomas (2), such as overexpression and/or activation of growth factors and their receptors [e.g. transforming growth factor-alpha (TGF-α), EGF and EGFR], overexpression and/or activation of intracellular signaling proteins (e.g. PI3K/Akt), and mutation of tumor suppressor genes [e.g. p53 and phosphatase and tensin homolog deleted on chromosome 10 (PTEN)]. Development of therapeutic agents targeting these altered signaling cascades may provide alternative strategies in addition to conventional treatment procedures.
Resveratrol is a naturally occurring polyphenolic compound that is highly enriched in grapes, peanuts, red wine and a wide variety of other food sources (3). Resveratrol has anti-inflammatory, anti-oxidant, antileukemic, anti-viral, neuroprotective properties (4), and acts as a cancer chemopreventive and chemotherapeutic agent (5), inhibiting different stages of carcinogenesis, such as initiation, promotion and progression of the tumor (6). Resveratrol is implicated in the regulation of a variety of cellular responses such as cell cycle arrest, differentiation and apoptosis in various cancer cell lines and experimental tumor models (7).
Alteration of growth factor receptor expression often leads to the overactivation of PI3K/Akt (8). PI3K has been implicated in tumor progression, invasion and angiogenesis (9). Attenuation of the PIK3CB gene encoding the PI3K catalytic subunit p110β by siRNA in human U251 glioma cells suppresses cell proliferation, induces cell cycle arrest, reduces cell invasion and promotes apoptosis in vitro (10). In addition, the growth of the subcutaneous U251 glioma in the nude mice is significantly inhibited after treatment with siRNA targeting PIK3CB (10). Akt is a family of the serine/threonine protein kinases that are involved in cell proliferation and survival, as demonstrated by several downstream cellular targets it regulates, such as Bad, caspase-9, glycogen synthase kinase 3 (GSK3), Forkhead transcription factors and nuclear factor-kappa B (NF-κB) (11). The mTOR is one of the downstream signaling targets of PI3K/Akt that regulates the signaling proteins essential for protein synthesis, such as ribosomal p70S6 kinase (12). Phosphorylation of Akt and mTOR is significantly correlated, and deregulation PI3K/Akt/mTOR signaling may lead to uncontrolled protein synthesis and cell cycle progression (13).
Our previous study showed that resveratrol induced time- and dose-dependent apoptosis in human U251 glioma cells (14). The deregulation of cell cycle kinetics, alteration of expression of Bcl-2 family and activation of caspases are involved in the resveratrol-induced apoptotic cell death. In the current study, we further examined the effect of resveratrol on PI3K/Akt/mTOR signaling pathway. We demonstrate that resveratrol downregulates PI3K/Akt/mTOR signaling pathway and inhibitors of these signaling proteins further enhance the resveratrol-induced caspase-3 activation and cell death.
MATERIALS AND METHODS
Materials
Resveratrol (Sigma Chemical Co., St. Louis, MO) was prepared in dimethyl sulfoxide (DMSO) at the stock solution of 100 mM and further diluted to appropriate concentration with cell culture medium immediately before use. Control experiments contain DMSO only. PI3K inhibitor LY294002 (2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one), Akt inhibitor (SH-6, phosphoinositide analogue inhibitor), and mTOR inhibitor rapamycin were obtained from Calbiochem (La Jolla, CA). Antibodies against phospho-mTOR (ser2448), phospho-Akt (ser473), Akt, cleaved caspase-3 (Asp-175) and PathScan multiplex Western cocktail I kit (phospho-p90RSK, phospho-Akt, phospho-p44/42 MAPK and phospho-S6 ribosomal protein) were obtained from Cell Signaling Technology (Beverly, MA). Antibodies against cyclin D1 and actin (I-19) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and antibody against poly(ADP-ribose) polymerase (PARP) was purchased from Oncogene Research Products (Cambridge, MA).
Cell Culture
U251 glioma cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Mediatech, Herndon, VA) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT), 100 units/ml of penicillin and 100 µg/ml streptomycin (Invitrogen, Carlsbad, CA) at 37°C in a humidified incubator containing 5% CO2 and 95% air, as previously described (14).
Lactate dehydrogenase (LDH) Release Assay
LDH release was measured using a CytoTox 96 non-radioactive cytotoxicity assay kit from Promega (Madison, WI). Cells (2×105 cells per well) were plated in 24-well plates the day before the experiments. After various treatments, medium from each well was collected to measure the amount of released LDH. Cells in separate sister wells were exposed to lysis buffer (9% Triton X100) and media were collected to measure the total amount of cellular LDH. The amount of LDH from each sample was measured at the wavelength of 490 nm by a BioTek EL-340 microplate reader. The percentage of released LDH vs. total intracellular LDH was calculated and reflected the amount of cell death.
Caspase-3 Activity Assay
Caspase-3 activity assay was performed using an Apo-Alert colorimetric caspase assay kit from BD Biosciences Clontech (Palo Alto, CA). Cells (1×106) were plated into 6-cm dishes the day before the experiments. After various treatments, cells were collected and cell lysates were prepared. Protein concentration was determined by using a BCA (bicinchoninic acid) protein assay kit (Pierce, Rockford, IL). Equal amounts of lysates were used for caspase-3 activity assay, measured at a wavelength of 405 nm using the detection of chromophore p-nitroaniline (pNA) after its cleavage by caspase-3 from the labeled caspase-3 specific substrate, DEVD-pNA. The data are presented as pmoles of pNA per µg of cell lysate per hour of incubation.
Western Blot Analysis
Western blot analysis was performed, as previously described (15). Cells were collected after various treatments and washed once with 1X phosphate-buffered saline (Mediatech) and lysed in lysis buffer [20 mM HEPES, pH 7.4, 100 mM NaCl, 1% Nonidet P-40, 0.1% SDS, 1% deoxycholic acid, 10% glycerol, 1 mM EDTA (ethylendiaminetetraacetic acid), 1 mM EGTA (ethylene glycol tetraacetic acid), 1 mM NaVO3, 50 mM NaF, and cocktail I of protease inhibitors from Calbiochem]. Soluble proteins were obtained by centrifugation at 13,000 rpm for 10 min at 4°C and protein concentration was determined. Equal amounts of cell lysate were subjected to SDS-polyacrylamide electrophoresis on Novex tris-glycine pre-cast gels (Invitrogen) and separated proteins were then electrotransferred to Immobilon polyvinylidene fluoride (PVDF) membranes (Whatman, Sanford, ME). After incubation with selective primary antibodies, proteins were visualized using SuperSignal West Pico chemiluminescence substrate system (Pierce). The band intensity was analyzed using Scion image software (Frederick, MD).
Statistical Analysis
Data were presented as means ± SD. Differences between different treatment groups were analyzed by using student’s t-test and a p-value < 0.05 was considered statistically significant.
RESULTS
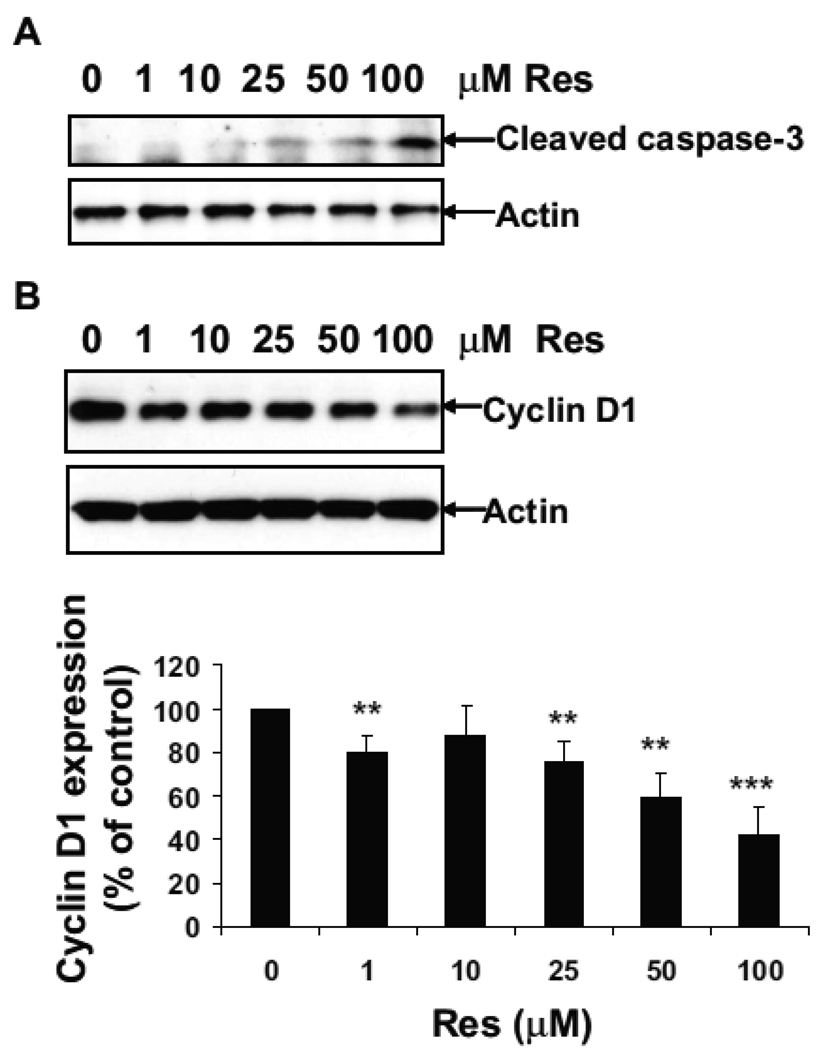
Resveratrol induces dose-dependent increase of caspase-3 activation and decrease of cyclin D1 expression
Our previous study has shown that resveratrol induces apoptotic cell death through activation of caspase-3 and decreases cyclin D1 expression at concentration of 100 µM (14). However, the dose-dependent effect of resveratrol on caspase-3 activation and cyclin D1 expression was not measured. In the current study, U251 cells were treated with 0, 1, 10, 25, 50 and 100 µM of resveratrol for 24 h. Western blot analysis showed that caspase-3 activation appeared at the concentration of 25 µM of resveratrol, with the highest activation of caspase-3 observed at the concentration of 100 µM (Fig. 1A). Resveratrol decreased the cyclin D1 expression to 80%, 87%, 76%, 59%, and 42% of the control levels at the concentrations of 1, 10, 25, 50 and 100 µM, respectively (Fig. 1B). Since 100 µM of resveratrol showed the maximal effect on caspase-3 activation and inhibition of cyclin D1 expression, subsequent experiments were carried out at this concentration.
Figure 1.
Resveratrol induces dose-dependent increase of caspase-3 activation and decrease of cyclin D1 expression. U251 cells were treated with 0, 1, 10, 25, 50 and 100 µM of resveratrol for 24 h. Western blot analysis was performed using antibodies against cleaved caspase-3 and actin (A) or Cyclin D1 and actin (B). For densitometric analysis of cyclin D1 expression, **, p < 0.01, ***, p < 0.001, vs. Con, n=3.
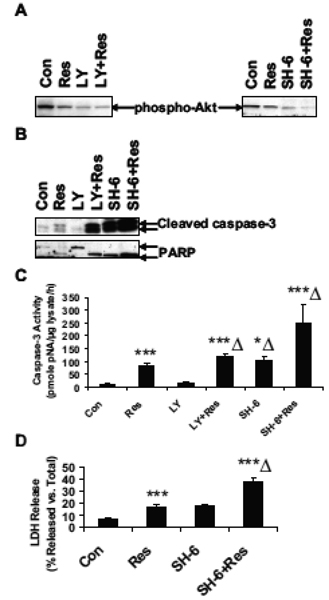
Inhibition of PI3K/Akt activation enhances resveratrol-induced apoptosis
PI3K/Akt signaling pathway is important for cell growth, differentiation and survival. Resveratrol has been shown to induce apoptotic cell death (14) and suppression of pro-survival PI3K/Akt signaling may be an important mediator in this process. To examine the effect of inhibition of PI3K on resveratrol-induced apoptosis, U251 cells were treated with a selective PI3K inhibitor LY294002 or a selective Akt inhibitor SH-6. Basal levels of phospho-Akt were greatly reduced after resveratrol treatment, and were further reduced in the presence of LY294002 or SH-6 (Fig. 2A). Resveratrol increased the cleavage of pro-caspase-3 to active caspase-3, as well as the cleavage of PARP from 110 kDa to 85 kDa, an effect which was further enhanced in the presence of LY 294002 (Fig. 2B). SH-6 itself induced a strong increase of activation of caspase-3 and cleavage of PARP, which was further enhanced in the presence of resveratrol (Fig. 2B). Caspase-3 activity assay showed that resveratrol induced a 9.3-fold increase of caspase-3 activation (Fig. 2C). LY294002 itself caused only a 1.6-fold increase of caspase-3 activity, which was increased 13.2-fold in the presence of resveratrol (Fig. 2C). SH-6 itself increased caspase-3 activity 11.4-fold, to the levels which were comparable to resveratrol treatment (9-fold as compared to control) and it further enhanced resveratrol-induced caspase-3 activity 27.7-fold (Fig. 2C). These results were consistent with results of Western blot analysis. To further examine the role of Akt in resveratrol-induced apoptosis, LDH release assay was performed (Fig. 2D). Resveratrol induced a 2.5-fold increase of LDH release, which is compatible with the treatment of SH-6 alone. Combination of SH-6 and resveratrol treatment further increased LDH release by 5.3- fold, indicating the additive effect of Akt inhibition on the resveratrol-induced apoptosis. These results suggest that resveratrol decreases the activation of pro-survival PI3K/Akt signaling pathway and inhibition of PI3K/Akt further enhances the resveratrol-induced caspase-3 activation and LDH release in U251 cells.
Figure 2.
PI3K/Akt inhibitors enhance the resveratrol-induced apoptosis. U251 cells were treated with 0 or 100 µM of Res for 24 h, in the absence or presence of 20 µM of PI3K inhibitor LY294002 or 20 µM of Akt inhibitor, SH-6. (A) Western blot analysis of phospho-Akt (ser473). (B) Western blot analysis of cleaved caspase-3 and PARP. (C) Caspase-3 activity assay; ***, p < 0.001, vs. Con; ***Δ, p < 0.001, vs. Res; n=3. (D) LDH release assay; **, p < 0.01, vs. Con; **Δ, p < 0.01, vs. Res; n=3.
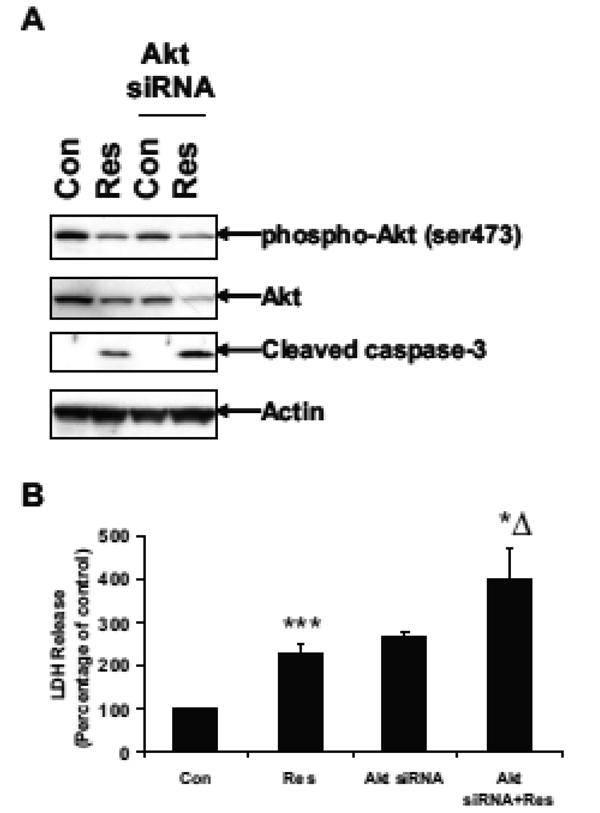
Downregulation of Akt enhances resveratrol-induced apoptosis
To further examine the role of Akt in resveratrolinduced apoptosis, U251 cells were transfected with Akt siRNA for 48 h followed by resveratrol treatment for 24 h. Western blot analysis showed that resveratrol reduced the Akt phosphorylation and expression in normal cells (Fig. 3A). Resveratrol decreased Akt expression to 55% of the control levels in non transfected cells. In transfected cells, Akt siRNA reduced Akt expression to 62% of the control levels. Resveratrol further decreased Akt expression to 19% of the control levels. Resveratrol also increased the cleavage of caspase-3 in Akt siRNA transfected cells compared to normal cells (Fig. 3A). LDH release assay showed that resveratrol induced a 2-fold increase of LDH release as compared to control, while Akt siRNA itself induced more than a 2-fold increase of LDH release (Fig. 3B). Resveratrol induced close to a 4-fold increase of LDH release in Akt siRNA transfected cells. These results again suggest that Akt siRNA transfected cells are more sensitive or susceptible than untreated cells to resveratrol-induced apoptosis and Akt is a critical mediator of resveratrol-induced apoptosis.
Figure 3.
Akt siRNA enhances resveratrol-induced apoptosis. U251 cells in 6-cm dishes were transfected with 50 nM of Akt siRNA for 48 h using a SignalSilence Akt siRNA kit (Cell Signaling), followed by treatment with 100 µM of Res for 24 h. (A) Western blot analysis of phospho-Akt (ser473), Akt, cleaved caspase-3 and actin. (B) LDH release assay; ***, p < 0.001, vs. Con; *Δ, p < 0.05, vs. Res; n = 3.
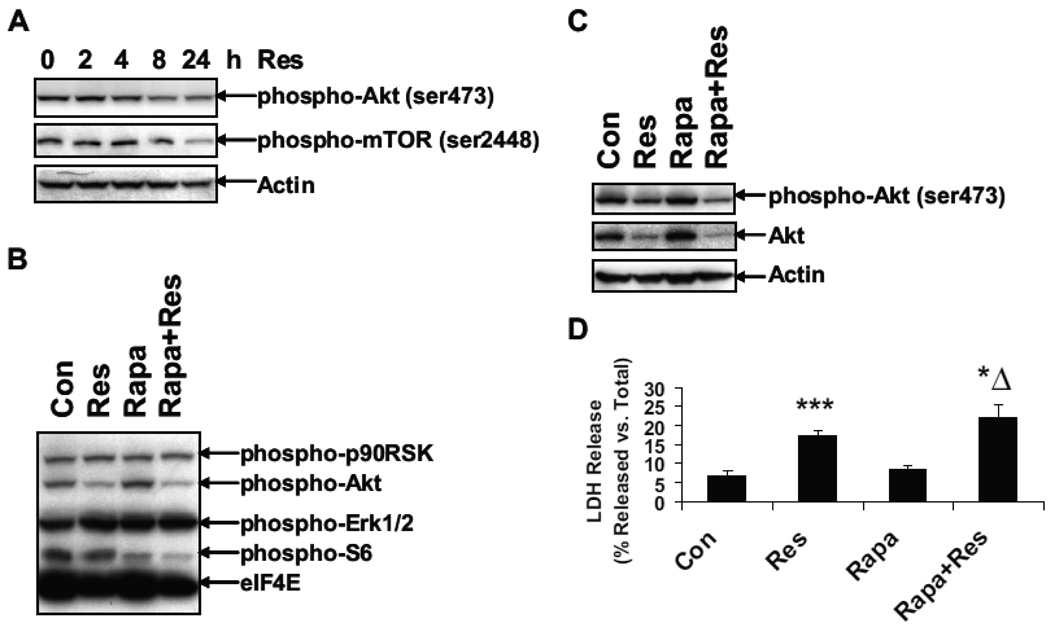
Inhibition of mTOR enhances resveratrol-induced apoptosis
The mTOR signaling protein is one of the downstream targets of PI3K/Akt. To further examine the effect of resveratrol on mTOR, U251 cells were treated with resveratrol for 0, 2, 4, 8 and 24 h. Levels of phospho-Akt and phospho-mTOR were examined by Western blot using phospho-specific antibodies. Resveratrol decreased phosphorylation of Akt and mTOR after 24 h of treatment (Fig. 4A). U251 cells were treated with 0 or 100 µM of resveratrol for 24 h, in the absence or presence of rapamycin, an inhibitor of S6 kinase. Western blot analysis was performed using the antibodies from Pathscan Multiplex cocktail kit (Fig. 4B). S6 phosphorylation was slightly decreased after resveratrol treatment, but was greatly decreased after rapamycin treatment, suggesting that resveratrol enhances rapamycin-mediated decrease of S6 phosphorylation. Both phosphorylation and expression of Akt was decreased after resveratrol treatment (Fig. 4C). Rapamycin itself had no effect on the phosphorylation and expression of Akt, but further enhanced resveratrol-mediated decrease. LDH release assay showed that rapamycin enhanced the resveratrol-induced cell death (Fig. 4D). These results suggest that resveratrol decreases the activation of Akt/mTOR and inhibition of mTOR by rapamycin further enhances the resveratrol-induced apoptosis.
Figure 4.
The mTOR inhibitor rapamycin enhances resveratrol-induced apoptosis. (A) U251 cells were treated with 100 µM of resveratrol for 0, 2, 4, 8 and 24 h. Western blot analysis was performed using antibodies against phospho-Akt (ser473), phospho-mTOR (ser2448) and actin. In a separate experiment, U251 cells were treated with 100 µM of resveratrol for 24 h, in the absence or presence of 10 nM rapamycin (Rapa). Western blot analysis was performed using PathScan multiplex Western cocktail I antibodies (B) or antibodies against phospho-Akt (ser473), Akt and actin (C). (D) LDH release assay was performed in U251 cells treated with vehicle or 100 µM of resveratrol for 24 h, in the absence or presence of 10 nM rapamycin (Rapa); **, p < 0.01, Vs. Con;* Δ, p < 0.05, vs. Res; n = 3.
DISCUSSION
Understanding the molecular signaling mechanisms of resveratrol-induced apoptosis may facilitate the development of additional therapeutic interventions for the prevention and treatment of glioma. The effect of resveratrol on PI3K/Akt/mTOR signaling pathway in glioma cells is not extensively studied. Our current study has demonstrated that resveratrol induces dose-dependent activation of caspase-3 and decrease of cyclin D1 expression. Resveratrol downregulates PI3K/Akt/mTOR-mediated signaling pathway and inhibitors to these signaling proteins further enhance resveratrol-induced caspase-3 activation and cell death. Akt is a critical mediator of resveratrol-induced apoptosis as demonstrated by using both chemical inhibitor and siRNA to reduce the Akt activation and expression, respectively. Our study suggests that resveratrol-induced apoptosis may be mediated through the induction of pro-apoptotic (e.g. caspase-3) and suppression of pro-survival (e.g. PI3K/Akt) signaling pathways.
Resveratrol has been shown to induce apoptotic cell death in a number of cancer cell lines, including glioma cells (14,16,17). Multiple apoptotic signaling cascades may be activated by resveratrol, such as mitochondria-mediated activation of caspases and alteration of expression of Bcl-2 family of proteins (18). Our previous study suggests that resveratrol-induced apoptosis involves cell cycle arrest and mitochondria-mediated activation of caspases (14). Resveratrol-induced cytochrome C, Bax translocation and activation of caspase-9 have been observed, which is consistent with other reported studies (19).
Inhibition of cell proliferation by resveratrol is likely mediated by the modulation of cell cycle-related proteins, such as cyclin D1 (3). It has been reported that PI3K/Akt/mTOR signaling may play a critical role in cell cycle progression in human prostate cancer cells (20). In addition, resveratrol has been shown to inhibit cell proliferation and induce apoptosis through the downregulation of Stat3- and NF-κB-mediated signaling proteins including cyclin D1 (21).
Akt belongs to a family of serine/threonine protein kinases that can be activated in response to various stimuli, including growth factor stimulation, stress, or protein phosphatase inhibitors, in a PI3K-dependent manner (11). Considerable evidence suggests that Akt plays a critical role in tumorigenesis. It has been reported that Akt1 is amplified and overexpressed in gastric adenoma, ductal breast carcinoma and ovarian cancer and cancer cell lines (22). Akt2 is amplified and/or overexpressed in ovarian, pancreatic, breast and thyroid carcinomas (22,23,24). Inhibition of Akt2 by anti-sense approach has been reported to inhibit malignant glioma cell growth and invasion (25,26). Furthermore, total Akt activity is increased in non small cell lung cancer, breast and prostate cancer (27,28,29,30). These observations suggest that targeting Akt may present a valuable therapeutic approach. Resveratrol has been reported to inhibit Akt activity and to induce apoptosis in human uterine cancer cells (31). Our results demonstrate that resveratrol downregulates Akt expression which may contribute to the reduction of Akt phosphorylation. We observed that the Akt inhibitor SH6 greatly enhances the activation of caspase-3, the cleavage of PARP and the induction of LDH release, as compared to the PI3K inhibitor LY294002. The combination of resveratrol and PI3K/Akt inhibitors further enhances the apoptotic cell death in glioma cells suggesting that they act on two different aspects of the signaling protein, expression and phosphorylation.
The mTOR is one of the major downstream signaling targets of PI3K/Akt and plays a critical role in regulating various cellular functions, including cell proliferation, differentiation and survival (32). Rapamycin is a mTOR inhibitor that has been implicated in regulation of cell cycle progression and apoptosis (33) and induces autophagy, a type II apoptotic cell death (34). It has been well established that activation of PI3K/Akt/mTOR pathway leads to phosphorylation of p70S6 kinase, which subsequently induces the phosphorylation of ribosomal protein S6. Our results show that resveratrol decreases the S6 phosphorylation, suggesting that resveratrol may have a role in regulation of protein translational machinery, as observed by others in human breast cell line (35).
In conclusion, our current study suggests that resveratrol downregulates and inactivates the pro-survival PI3K/Akt/mTOR signaling pathway, which may play a critical role in resveratrol-induced apoptosis in glioma cells and PI3K/Akt/mTOR signaling pathway may represent a potential therapeutic candidate for treatment of glioma.
ACKNOWLEDGEMENT
Supported by NIH R21 AT003463-01A2.
REFERENCES
- 1.Kondo Y, Hollingsworth EF, Kondo S. Molecular targeting for malignant gliomas (Review) Int J Oncol. 2004;24:1101–1109. [PubMed] [Google Scholar]
- 2.Lassman AB. Molecular biology of gliomas. Curr Neurol Neurosci Rep. 2004;4:228–233. doi: 10.1007/s11910-004-0043-3. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 2004;24:2783–2840. [PubMed] [Google Scholar]
- 4.Das S, Das DK. Anti-inflammatory responses of resveratrol. Inflamm Allergy Drug Targets. 2007;6:168–173. doi: 10.2174/187152807781696464. [DOI] [PubMed] [Google Scholar]
- 5.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 6.Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21:381–406. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- 7.Joe AK, Liu H, Suzui M, Vural ME, Xiao D, Weinstein IB. Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin Cancer Res. 2002;8:893–903. [PubMed] [Google Scholar]
- 8.Mischel PS, Cloughesy TF. Targeted molecular therapy of GBM. Brain Pathol. 2003;13:52–61. doi: 10.1111/j.1750-3639.2003.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brader S, Eccles SA. Phosphoinositide 3-kinase signaling pathways in tumor progression, invasion and angiogenesis. Tumori. 2004;90:2–8. doi: 10.1177/030089160409000102. [DOI] [PubMed] [Google Scholar]
- 10.Pu P, Kang C, Zhang Z, Liu X, Jiang H. Downregulation of PIK3CB by siRNA suppresses malignant glioma cell growth in vitro and in vivo. Technol Cancer Res Treat. 2006;5:271–280. doi: 10.1177/153303460600500308. [DOI] [PubMed] [Google Scholar]
- 11.Testa JR, Bellacosa A. AKT plays a central role in tumorigenesis. Proc Natl Acad Sci U S A. 2001;98:10983–10985. doi: 10.1073/pnas.211430998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kesari S, Ramakrishna N, Sauvageot C, Stiles CD, Wen PY. Targeted molecular therapy of malignant gliomas. Curr Neurol Neurosci Rep. 2005;5:186–197. doi: 10.1007/s11910-005-0046-8. [DOI] [PubMed] [Google Scholar]
- 13.Choe G, Horvath S, Cloughesy TF, Crosby K, Seligson D, Palotie A, Inge L, Smith BL, Sawyers CL, Mischel PS. Analysis of the phosphatidylinositol 3’-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 2003;63:2742–2746. [PubMed] [Google Scholar]
- 14.Jiang H, Zhang L, Kuo J, Kuo K, Gautam SC, Groc L, Rodriguez AI, Koubi D, Hunter TJ, Corcoran GB, Seidman MD, Levine RA. Resveratrol-induced apoptotic death in human U251 glioma cells. Mol Cancer Ther. 2005;4:554–561. doi: 10.1158/1535-7163.MCT-04-0056. [DOI] [PubMed] [Google Scholar]
- 15.Jiang H, Movsesyan V, Fink DW, Jr., Fasler M, Whalin M, Katagiri Y, Monshipouri M, Dickens G, Lelkes PI, Guroff G, Lazarovici P. Expression of human p140trk receptors in p140trk-deficient, PC12/endothelial cells results in nerve growth factor-induced signal transduction and DNA synthesis. J Cell Biochem. 1997;66:229–244. [PubMed] [Google Scholar]
- 16.Tseng SH, Lin SM, Chen JC, Su YH, Huang HY, Chen CK, Lin PY, Chen Y. Resveratrol suppresses the angiogenesis and tumor growth of gliomas in rats. Clin Cancer Res. 2004;10:2190–2202. doi: 10.1158/1078-0432.ccr-03-0105. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Fei Z, Zhen HN, Zhang JN, Zhang X. Resveratrol inhibits cell growth and induces apoptosis of rat C6 glioma cells. J Neurooncol. 2007;81:231–240. doi: 10.1007/s11060-006-9226-x. [DOI] [PubMed] [Google Scholar]
- 18.Park JW, Choi YJ, Suh SI, Baek WK, Suh MH, Jin IN, Min DS, Woo JH, Chang JS, Passaniti A, Lee YH, Kwon TK. Bcl-2 overexpression attenuates resveratrol-induced apoptosis in U937 cells by inhibition of caspase-3 activity. Carcinogenesis. 2001;22:1633–1639. doi: 10.1093/carcin/22.10.1633. [DOI] [PubMed] [Google Scholar]
- 19.Zhuang S, Ouedraogo GD, Kochevar IE. Downregulation of epidermal growth factor receptor signaling by singlet oxygen through activation of caspase-3 and protein phosphatases. Oncogene. 2003;22:4413–4424. doi: 10.1038/sj.onc.1206604. [DOI] [PubMed] [Google Scholar]
- 20.Gao N, Zhang Z, Jiang BH, Shi X. Role of PI3K/AKT/mTOR signaling in the cell cycle progression of human prostate cancer. Biochem Biophys Res Commun. 2003;310:1124–1132. doi: 10.1016/j.bbrc.2003.09.132. [DOI] [PubMed] [Google Scholar]
- 21.Bhardwaj A, Sethi G, Vadhan-Raj S, Bueso-Ramos C, Takada Y, Gaur U, Nair AS, Shishodia S, Aggarwal BB. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007;109:2293–2302. doi: 10.1182/blood-2006-02-003988. [DOI] [PubMed] [Google Scholar]
- 22.Yuan ZQ, Sun M, Feldman RI, Wang G, Ma X, Jiang C, Coppola D, Nicosia SV, Cheng JQ. Frequent activation of AKT2 and induction of apoptosis by inhibition of phosphoinositide-3-OH kinase/Akt pathway in human ovarian cancer. Oncogene. 2000;19:2324–2330. doi: 10.1038/sj.onc.1203598. [DOI] [PubMed] [Google Scholar]
- 23.Arboleda MJ, Lyons JF, Kabbinavar FF, Bray MR, Snow BE, Ayala R, Danino M, Karlan BY, Slamon DJ. Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res. 2003;63:196–206. [PubMed] [Google Scholar]
- 24.Ruggeri BA, Huang L, Wood M, Cheng JQ, Testa JR. Amplification and overexpression of the AKT2 oncogene in a subset of human pancreatic ductal adenocarcinomas. Mol Carcinog. 1998;21:81–86. [PubMed] [Google Scholar]
- 25.Pu P, Kang C, Li J, Jiang H. Antisense and dominant-negative AKT2 cDNA inhibits glioma cell invasion. Tumour Biol. 2004;25:172–178. doi: 10.1159/000081099. [DOI] [PubMed] [Google Scholar]
- 26.Pu P, Kang C, Li J, Jiang H, Cheng J. The Effects of Antisense AKT2 RNA on the Inhibition of Malignant Glioma Cell Growth in vitro and in vivo. J Neurooncol. 2006;76:1–11. doi: 10.1007/s11060-005-3029-3. [DOI] [PubMed] [Google Scholar]
- 27.Mukohara T, Kudoh S, Yamauchi S, Kimura T, Yoshimura N, Kanazawa H, Hirata K, Wanibuchi H, Fukushima S, Inoue K, Yoshikawa J. Expression of epidermal growth factor receptor (EGFR) and downstream-activated peptides in surgically excised non-small-cell lung cancer (NSCLC) Lung Cancer. 2003;41:123–130. doi: 10.1016/s0169-5002(03)00225-3. [DOI] [PubMed] [Google Scholar]
- 28.Liao Y, Grobholz R, Abel U, Trojan L, Michel MS, Angel P, Mayer D. Increase of AKT/PKB expression correlates with gleason pattern in human prostate cancer. Int J Cancer. 2003;107:676–680. doi: 10.1002/ijc.11471. [DOI] [PubMed] [Google Scholar]
- 29.Bellacosa A, de Feo D, Godwin AK, Bell DW, Cheng JQ, Altomare DA, Wan M, Dubeau L, Scambia G, Masciullo V, Fernandina G, Benedetti Panici P, Mancuso S, Neri G, Testa JR. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer. 1995;64:280–285. doi: 10.1002/ijc.2910640412. [DOI] [PubMed] [Google Scholar]
- 30.Cheng JQ, Ruggeri B, Klein WM, Sonoda G, Altomare DA, Watson DK, Testa JR. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci U S A. 1996;93:3636–3641. doi: 10.1073/pnas.93.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sexton E, Van Themsche C, LeBlanc K, Parent S, Lemoine P, Asselin E. Resveratrol interferes with AKT activity and triggers apoptosis in human uterine cancer cells. Mol Cancer. 2006;5:45. doi: 10.1186/1476-4598-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang S, Houghton PJ. Targeting mTOR signaling for cancer therapy. Curr Opin Pharmacol. 2003;3:371–377. doi: 10.1016/s1471-4892(03)00071-7. [DOI] [PubMed] [Google Scholar]
- 33.Dutcher JP. Mammalian target of rapamycin inhibition. Clin Cancer Res. 2004;10:6382S–6387S. doi: 10.1158/1078-0432.CCR-050008. [DOI] [PubMed] [Google Scholar]
- 34.Iwamaru A, Kondo Y, Iwado E, Aoki H, Fujiwara K, Yokoyama T, Mills GB, Kondo S. Silencing mammalian target of rapamycin signaling by small interfering RNA enhances rapamycin-induced autophagy in malignant glioma cells. Oncogene. 2007;26:1840–1851. doi: 10.1038/sj.onc.1209992. [DOI] [PubMed] [Google Scholar]
- 35.Alkhalaf M. Resveratrol-induced growth inhibition in MDA-MB-231 breast cancer cells is associated with mitogen-activated protein kinase signaling and protein translation. Eur J Cancer Prev. 2007;16:334–341. doi: 10.1097/01.cej.0000228413.06471.4c. [DOI] [PubMed] [Google Scholar]