Abstract
DesII from Streptomyces venezuelae is a radical SAM (S-adenosyl-l-methionine) enzyme that catalyzes the deamination of TDP-4-amino-4,6-dideoxy-d-glucose to form TDP-3-keto-4,6-dideoxy-d-glucose in the biosynthesis of TDP-d-desosamine. DesII also catalyzes the dehydrogenation of the non-physiological substrate TDP-d-quinovose to TDP-3-keto-6-deoxy-d-glucose. These properties prompted an investigation of how DesII handles SAM in the redox neutral deamination versus the oxidative dehydrogenation reactions. This work was facilitated by the development of an enzymatic synthesis of TDP-4-amino-4,6-dideoxy-d-glucose that couples a transamination equilibrium to the thermodynamically favorable oxidation of formate. In this study, DesII is found to consume SAM versus TDP-sugar with stoichiometries of 0.96 ± 0.05 and 1.01 ± 0.05 in the deamination and dehydrogenation reactions, respectively, using Na2S2O4 as the reductant. Importantly, no significant change in stoichiometry is observed when the flavodoxin/flavodoxin NADP+ oxidoreductase/NADPH reducing system is used in place of Na2S2O4. Moreover, there is no evidence of an uncoupled or abortive process in the deamination reaction, as indicated by the observation that dehydrogenation can take place in the absence of an external source of reductant whereas deamination cannot. Mechanistic and biochemical implications of these results are discussed.
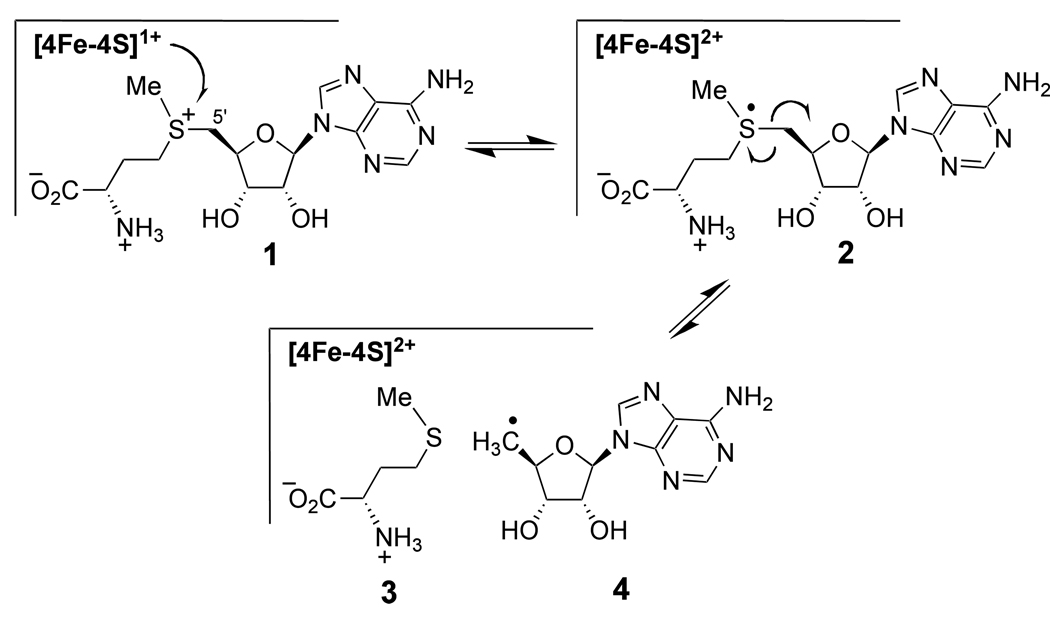
Radical SAM enzymes are an important class of biological catalysts involved in radical mediated transformations in both primary and secondary metabolic pathways.1–3 All of these enzymes contain a [4Fe-4S] cluster in the active-site and are S-adenosyl-l-methionine (1, SAM) dependent. The reactions are initiated by a single electron transfer from the reduced [4Fe-4S]1+ cluster to SAM (1 → 2) to induce the homolytic cleavage of the C5'-S bond in SAM, which yields a reactive 5'-deoxyadenosyl radical (4) along with l-methionine (3), as shown in Scheme 1.2,4–6 Utilization of a 5'-deoxyadenosyl radical (4) by the radical SAM enzymes is similar to the B12-dependent enzymes. In both cases, the 5'-deoxyadenosyl radical is used to generate a substrate radical intermediate that is subsequently converted to product.1,2 However, unlike the radical SAM enzymes, the 5'-deoxyadenosyl radical (4) in the B12-dependent enzymes is derived from an adenosylcobalamin cofactor.7–9 Moreover, the B12-dependent enzymes are primarily involved in redox neutral isomerization and lyase reactions, whereas the reactions catalyzed by radical SAM enzymes may or may not lead to a net change in the redox state of the substrate as it is converted to product. Thus, the fact that SAM can act as both a radical initiator as well as an oxidant highlights a clear difference between those enzymes utilizing adenosylcobalamin versus radical SAM chemistry.
Scheme 1.
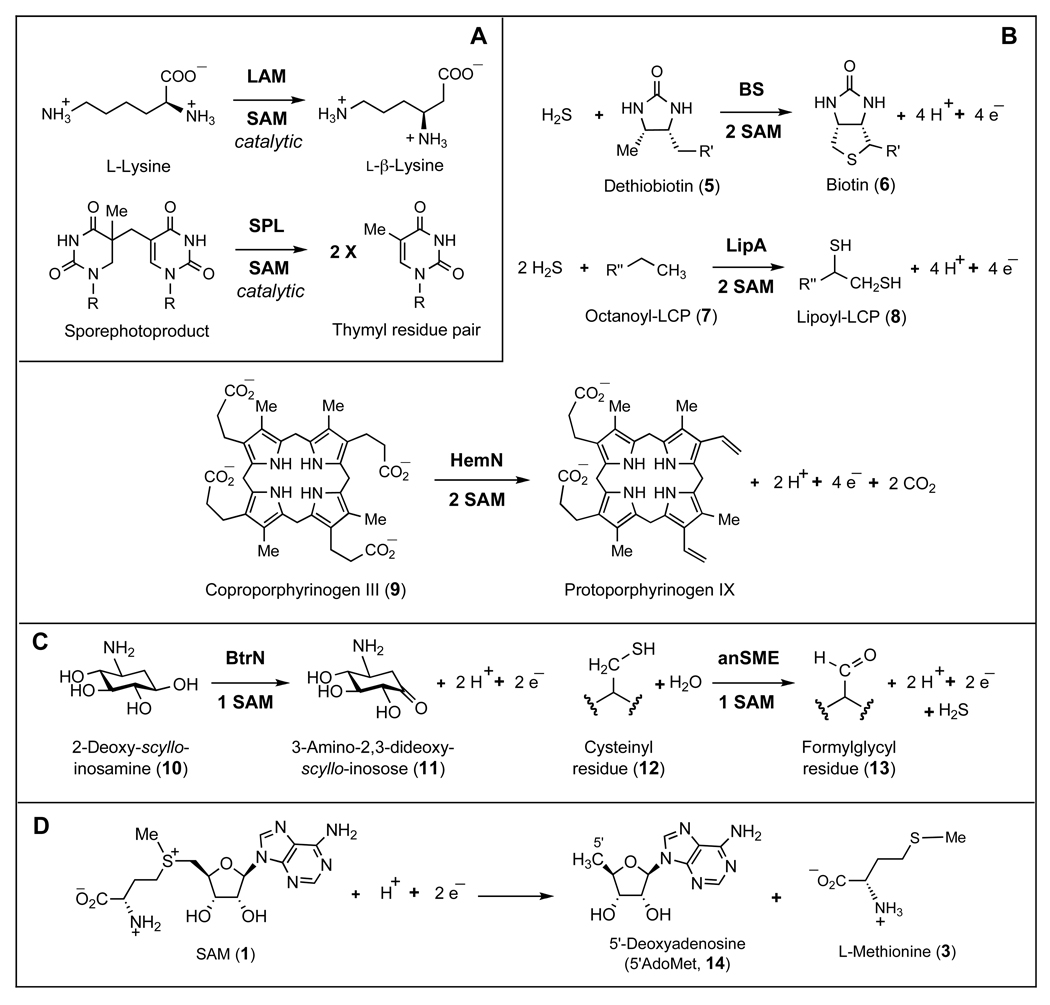
In the case of the adenosylcobalamin-dependent enzymes, isomerization reactions are typically accompanied by the regeneration of the adenosylcobalamin cofactor. Similarly, SAM, acting as a coenzyme, is also regenerated in the redox neutral transformations catalyzed by radical SAM dependent lysine 2,3-aminomutase (LAM)5,10,11 and spore photoproduct lyase (SPL) (see Scheme 2A).12,13 However, for the radical SAM enzymes catalyzing transformations that lead to an overall oxidation of the substrate, there is a net consumption of SAM. Examples include the sulfur insertion reactions catalyzed by biotin synthase (BS)14–16 and lipoyl synthase (LipA) (see Scheme 2B).17–19 These reactions involve four-electron oxidation of dethiobiotin (5) to biotin (6) and octanoyl-LCP (7) to lipoyl-LCP (8), where LCP denotes a lipoyl carrier protein. A net four-electron oxidation/decarboxylation of coproporphyrinogen III (9) by HemN has also been studied (Scheme 2B).20,21 The dehydrogenation of 2-deoxy-scyllo-inosamine (10) to 3-amino-2,3-dideoxy-scyllo-inosose (11) by BtrN,22 and the oxidative desulfuration of cysteinyl (12) to formylglycyl residues (13) by anaerobic sulfatase maturating enzymes (anSMEs) are examples of two-electron oxidations catalyzed by radical SAM enzymes (see Scheme 2C).23,24 In these reactions, SAM not only serves as a radical initiator but also acts as a two-electron oxidant (see Scheme 2D) that is consumed in a two-to-one ratio versus substrate in the oxidation of dethiobiotin (5),16,25,26 octanoyl-LCP (7),17,27 and coproporphyrinogen III (9);28 or in a one-to-one ratio in the oxidation of 2-deoxy-scyllo-inosamine (10)22 and cysteinyl residues (12).29
Scheme 2.
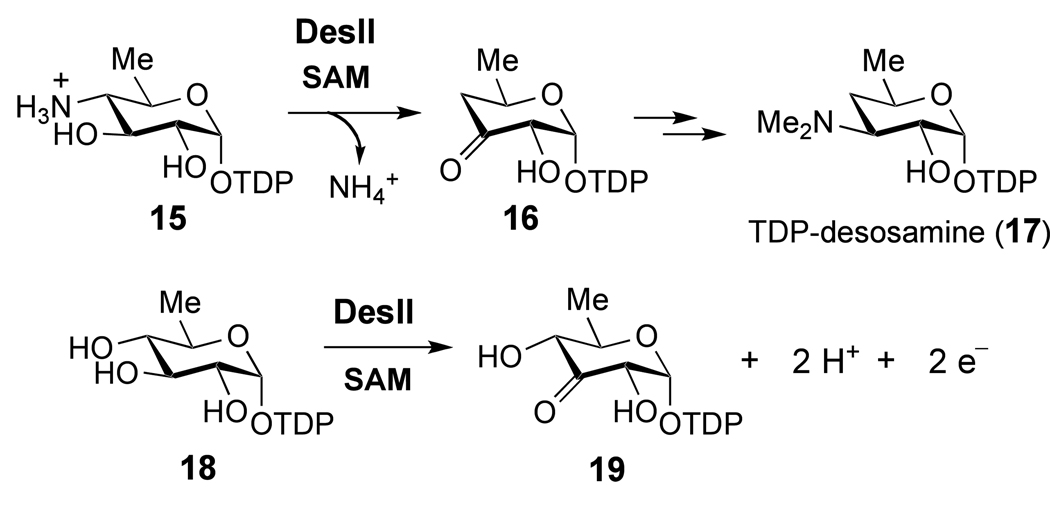
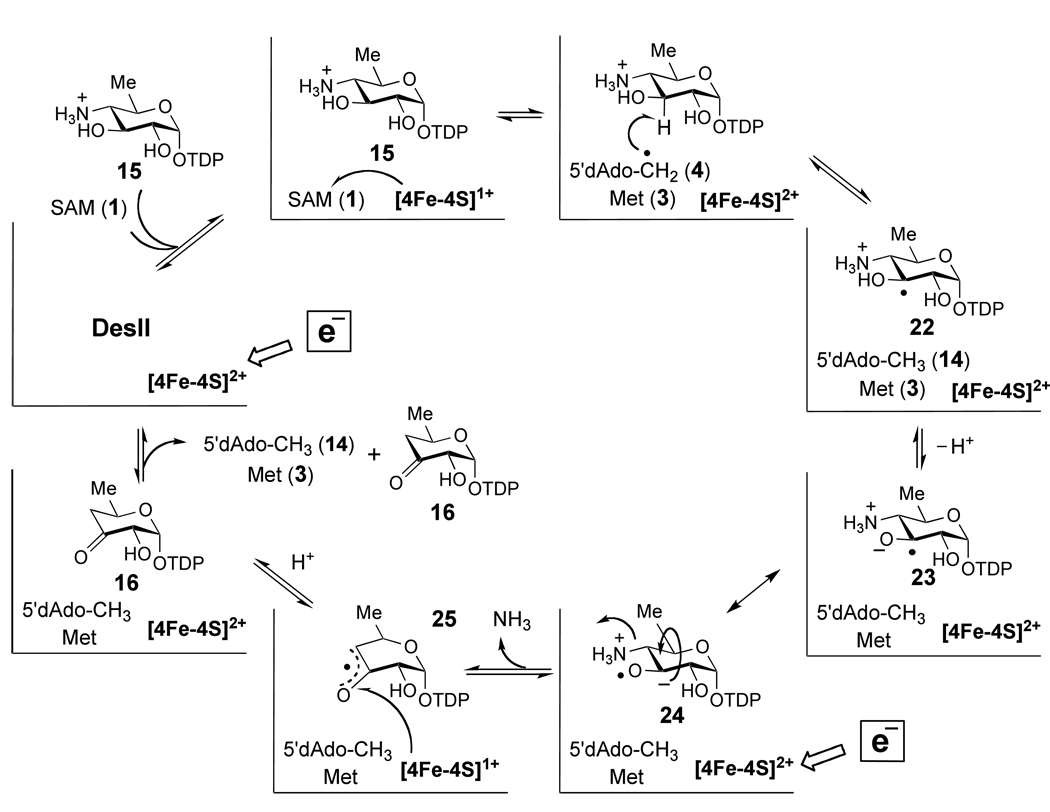
The radical SAM enzyme DesII, responsible for the deamination of TDP-4-amino-4,6-dideoxy-d-glucose to give TDP-3-keto-4,6-dideoxy-d-glucose (15 → 16, Scheme 3) in the biosynthetic pathway of desosamine, is a particularly noteworthy example.19,30 Desosamine (see 17) is a 3-dimethylamino-3,4,6-trideoxysugar derivative important for the antimicrobial activity of a number of macrolide antibiotics including erythromycin, methymycin and pikromycin.31,32 DesII, which contains the CxxxCxxC consensus sequence, has been verified to be a radical SAM enzyme on the basis of its dependence on SAM (1) and a reduced [4Fe-4S] cluster for enzymatic activity.30,33 Consistent with the established mechanisms for this class of enzymes, the DesII reaction is initiated with a hydrogen atom abstraction by the 5'-deoxyadenosyl radical (4). Using [3-2H]-15 as substrate, the transfer of the C-3 hydrogen atom from 15 to the C-5' position of SAM and/or 5'-deoxyadenosine (14) during turnover has been demonstrated.33 Subsequent radical mediated deamination of intermediate 22 (shown in Scheme 5) to generate 16 may involve a 1,2-migration of the amino group (from C-4 to C-3), reminiscent of the catalytic mechanisms of ethanolamine ammonia lyase34–37 and the dioldehydratases.9,38–43 The latter two enzymes rely on adenosylcobalamin instead of SAM to facilitate the radical induced 1,2-amino or 1,2-hydroxyl migration during the conversion of ethanolamine or ethylene glycol to acetaldehyde. DesII, therefore, represents an ideal system for detailed analysis and direct comparison of the chemistry underlying radical SAM versus adenosylcobalamin dependent radical mediated rearrangements in enzymology.
Scheme 3.
Scheme 5.
In addition to the deamination of its biological substrate 15, DesII can also catalyze the two-electron oxidation of the non-physiological substrate TDP-d-quinovose to TDP-3-keto-6-deoxy-d-glucose (18 → 19, Scheme 3).33 This dehydrogenation activity makes the chemistry of DesII catalysis unique, because the enzyme can act as either a redox neutral deaminase or an oxidative alcohol dehydrogenase depending on the nature of the substrate. This raises questions as to the overall redox chemistry in the DesII-catalyzed reaction. In light of the precedence set by B12-dependent enzymes as well as the radical SAM isomerases LAM and SPL (Scheme 2A), regeneration of SAM following the deamination of 15 is an attractive mechanism. However, this hypothesis is tempered by the observation of [5'-2H]-5'-deoxyadenosine ([5'-2H]-14) formation in the deuterium transfer experiments with TDP-[3-2H]-4-amino-4,6-dideoxy-d-glucose ([3-2H]-15), and the net oxidative transformation of 18 catalyzed by DesII.33 As an alternative, the deamination reaction may in fact be a redox reaction as well, driven by the reduction of SAM and consequently requires a continuous input of reducing equivalents.
To address these questions, we determined the stoichiometry of SAM consumption versus the conversion of the TDP-sugar substrates in both the deamination and dehydrogenation reactions. The effect of both a non-biological as well as a biological reducing source is also studied due to reports that the degree to which SAM is consumed may vary with the reducing system employed.44–48 In addition, the ability of DesII to regenerate the reduced [4Fe-4S]1+ cluster during each catalytic cycle in the absence of an external source of reducing equivalents is also investigated. These results are summarized herein, and the mechanistic implications of DesII catalysis are discussed. Overall, this study emphasizes the underappreciated redox capability of SAM in enzyme catalysis (Scheme 2D).1,49 The general methods reported in this paper should be applicable for determining the stoichiometry of many other radical SAM enzymes.
EXPERIMENTAL PROCEDURES
Materials
The C-terminal His6-tagged DesII from Streptomyces venzuelae was expressed using Escherichia coli BL21 Star (DE3) cells transformed with a pET24b(+) expression plasmid, which contains the desII gene, and purified aerobically by Ni-NTA chromatography and FPLC as previously described.33 Isolated enzyme was judged to be >90% pure based on SDS-PAGE. Following purification, the enzyme was dialyzed into a storage buffer containing 50 mM KPi (pH 7.5, KOH) and 15% glycerol, concentrated to approximately 5 mg/mL, flash frozen, and stored at −80 °C until use. The enzymes DesI from S. venezuelae, FLD and FNR from E. coli, and RfbB from Salmonella typhi were similarly expressed and purified according to published procedures.33,50–52 Unless otherwise specified, all chemical reagents were purchased from commercial sources and used without further purification. TDP-d-quinovose (18),33,53 TDP-d-glucose (20)54 and 5'-deoxyadenosine (14)55 were prepared according to reported procedures. Commercial sodium dithionite is known to be contaminated with sodium bisulfite, sodium thiosulfate and sodium sulfide among other decomposition products.56,57 Therefore, dithionite concentrations are reported based on absorbance measurements of standards at 315 nm using an extinction coefficient of 6900 M−1cm−1 at this wavelength.58 Unless otherwise specified, all reactions were performed in a Coy anaerobic glovebox under an atmosphere of > 98% N2 and ~1.5% H2 with < 1 ppm O2. Anaerobic water was prepared by first boiling deionized H2O for 1 h and immediately transferring the boiling water to the glovebox where it was stirred open to the atmosphere for at least 48 h. Anaerobic buffer, 100 mM Tris·HCl (pH 8.0), was prepared by one freeze-thaw vacuum cycle on an anaerobic Schlenck line under an argon atmosphere and then transferred to the glovebox where it was stirred open to the atmosphere for at least 48 h. Anaerobic water was then added to the buffer to compensate for any loss of volume.
HPLC Conditions
Unless otherwise specified, all analytical HPLC experiments were performed using the same method. This involved a two-solvent system of A, H2O, and B, 1.5 M ammonium acetate in H2O. An analytical 4 × 250 mm Dionex CarboPac PA1 anion exchange column was employed with a 4 × 50 mm guard column. The flow rate was 1 mL/min and the detector was set to 267 nm. The column was eluted with a two-phase linear gradient, 2.5% to 15% B in 20 min then 15% to 20% B in 20 min, followed by a return to 2.5% B in 5 min.
Reconstitution of the DesII [4Fe-4S] Cluster
One day before reconstitution, approximately 5 mg of DesII in a volume of roughly 1 mL of storage buffer was thawed and dialyzed overnight against 1 L of 100 mM Tris·HCl buffer (pH 8.0, 10% glycerol) at 4 °C under an argon atmosphere. The buffer was first deaerated via 2 – 3 cycles of argon purge (5 min) / vacuum (20 min) immediately prior to dialysis. Following buffer exchange, the dialysis system was taken into the glovebox, where the argon atmosphere was removed just before cycling in the airlock. Once in the glovebox, the contents of the dialysis bag were transferred to a 2 mL conical vial chilled to approximately 10 °C, and the solution was diluted to 2 mL with anaerobic 100 mM Tris·HCl buffer (pH 8.0). The solution was kept cold (10 °C) and stirred for 2 h with the cap off to fully remove any remaining oxygen. This time period was judged sufficient based on preliminary measurements of deoxygenation using photoreduced methylene blue.59 Following deaeration, the solution was incubated for 15 min in 5 mM DTT by adding 100 µL of 100 mM DTT. The solution was then incubated for 5 min with 1.5 mM SAM by adding 32 µL 100 mM SAM. A 40 mM solution of Fe(NH4)2(SO4)2 in anaerobic water was then added over 10 min to a final concentration of 1 mM. Finally, a 40 mM solution of Na2S in 100 mM Tris·HCl (pH 8.0) was added over 20 min to a final concentration of 1 mM. The resulting reconstitution mixture, which had the appearance of black coffee, was kept cold (10 °C) and stirred for 3 h with the vial open to the anaerobic atmosphere, then capped and stirred for an additional 2 h. The mixture was loaded directly onto a 50 mL Sephadex-G25 column preequilibrated with 100 mM Tris·HCl (pH 8.0, 1 mM DTT), and eluted with the same buffer at room temperature. Colored fractions were collected and assayed with Bradford reagent to ensure the presence of protein. The enzyme generally eluted in 4 – 5 mL, and these were concentrated to 2 mL using YM10 centrifugal filters in the glovebox at room temperature. The final enzyme concentration was typically 1 to 2 mg/mL (18 – 36 µM) for a recovery of ~ 60%. The solutions have a brown to olive-green color and were stored at approximately 10 °C in the glovebox. Enzyme reconstituted in this manner has similar properties in terms of iron-sulfur content as that previously reported33 and a specific activity of 0.2 ± 0.1 U/mg (± standard deviation), where 1 U of enzyme converts 1 µmole of 15 to 16 per minute in the presence of 0.5 mM 15, 0.5 mM SAM and 0.4 mM Na2S2O4 at pH 8.0 and 25 °C.
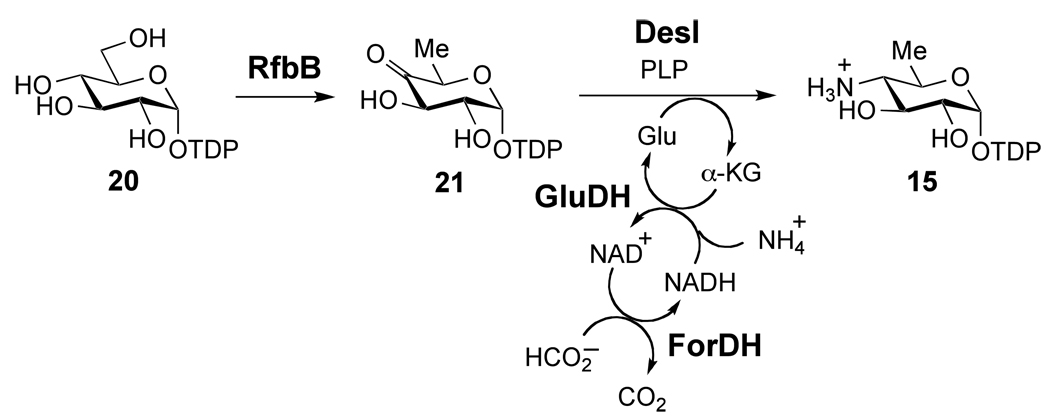
Synthesis of TDP-4-amino-4,6-dideoxy-d-glucose (15)
The DesII substrate 15 was synthesized with nearly quantitative conversion by coupling the transamination of TDP-4-keto-6-deoxy-d-glucose (21) by DesI with the oxidation of formate by formate dehydrogenase (ForDH), as shown in Scheme 4. This coupled reaction drives the transamination equilibrium to completion and thus, improves the conversion yield from approximately 20% to nearly quantitative. A 10 mL solution containing 10 mM TDP-d-glucose (20), 0.3 mM NAD+, 50 mM KPi (pH 7.5, KOH) and ~ 5 mg RfbB was incubated for 1 h at 37 °C. The above reaction mixture was then made 10 mM in glutamate (monosodium salt), 50 mM in NH4Cl, 50 mM in sodium formate, 1 mM in pyridoxal-5'-phosphate, and ~ 3 mg DesI, 100 U glutamate dehydrogenase (GluDH) and ~ 10 U ForDH were added. The reaction was monitored by HPLC and was typically complete (> 95% conversion) within 5 – 10 h in the dark at 37 °C. The protein was removed using YM10 centrifugal filters, and the product purified by FPLC using a MonoQ 16/10 column. A flow rate of 3 mL/min was used with the following gradient: 100% H2O isocratic for 3.3 min, 0 to 350 mM NH4HCO3 in 26.6 min, 350 to 500 mM NH4HCO3 in 6.7 min, 500 mM NH4HCO3 isocratic for 13.3 min. The product typically elutes at 25 min in approximately 5 – 10 mL. The eluant was acidified to pH 6 – 7 by purging with CO2 prior to freezing and lyophilization. Recovery was greater than 50% and the final ratio of ammonium salts to product was approximately 5 to 1 based on the assay with GluDH. The ESI-MS of the isolated product gives clear signals at 547.8 (positive ion mode) and 546.2 (negative ion mode) consistent with the M + H+ and (M − H+)− ions, respectively, as the neutral monoisotopic mass of 15 is 547. 1H NMR spectra were obtained in unbuffered D2O and are consistent with previous reports.33,50
Scheme 4.
Stoichiometry of SAM Consumption Versus the Turnover of TDP-4-amino-4,6-dideoxy-d-glucose (15) and TDP-d-quinovose (18)
Stoichiometry measurements of SAM consumption versus 15 or 18 conversion during turnover by DesII were made using the analytical HPLC methodology described above. Reactions were performed in a volume of 1 mL containing 100 mM Tris·HCl (pH 8.0), 2 mM DTT, 0.25 – 0.5 mM SAM, and 0.25 – 0.5 mM of TDP-sugar substrate. In the case of TDP-d-quinovose (18), initial SAM and substrate concentrations up to 5 mM were also included. As a source of reducing equivalents, the reactions contained either 0.2 – 0.4 mM Na2S2O4 or 0.33 mM NADPH, 20 µM FLD and 70 µM FNR, depending on whether dithionite or the FLD/FNR/NADPH reducing system was being evaluated. Prior to adding DesII, a 100-µL aliquot was removed and filtered using YM10 centrifugal filters and frozen until assay by HPLC. This aliquot was used to determine the initial ratio of TDP-sugar substrate to SAM in the reaction mixture (see Results). DesII was then added to a concentration of 4 µM. During the reaction, 100-µL aliquots were removed at appropriate times and filtered using YM10 centrifugal filters to remove the protein. The filtrate of each sample was frozen until analysis by HPLC. Chromatograms were used to determine the stoichiometry as well as to monitor the reaction. Sluggish reactions were accelerated by adding additional reductant or DesII, which did not affect the measurement (see Results for justification). Due to the relative instability of the products and SAM, reactions were run no longer than 2 – 3 h. Reactions typically reached over 60% conversion in the limiting reactant during the period of the experiment, which increased to > 90% on longer incubations with additional Na2S2O4. Reactions using the FLD/FNR/NADPH reducing system were typically slower, and lower fractions of turnover were achieved in the time period of the stoichiometry experiments. In these cases only experiments that achieved at least 20% conversion of the TDP-sugar substrate were included in the analysis.
Requirements for a Constant Source of Reducing Equivalents
In the case of TDP-4-amino-4,6-dideoxy-d-glucose (15), a 100 µL solution of 30 µM reconstituted DesII was made 0.4 mM in Na2S2O4 by adding 10 µL 4 mM freshly dissolved Na2S2O4. The solution was incubated for 30 min at room temperature and then 15 h at 10 °C. Following the reduction, 10 µL of the reduced DesII was added to 90 µL of 100 mM Tris·HCl (pH 8.0) containing 2 mM DTT, 0.5 mM SAM and 0.5 mM of 15. As a control for DesII inactivation during the 15-h incubation with Na2S2O4, a second aliquot of 10 µL of the reduced DesII was added to 90 µL of the same solution that also included 0.4 mM fresh Na2S2O4. Following the addition of the reduced enzyme, 50-µL aliquots were filtered to remove protein at 5 and 85 min, and frozen prior to analysis by analytical HPLC. This experiment was performed twice on two separate days.
An analogous experiment was performed with TDP-d-quinovose (18) using the same preparation of DesII following the 15 h reduction with Na2S2O4. In this experiment a control was also included to ensure that all of the Na2S2O4 had either been consumed in the reduction of DesII or decomposed during the overnight incubation. This control involved filtration of the reduced DesII enzyme following the 15 h incubation with Na2S2O4 using YM10 centrifugal filters. The previously described reaction system where 15 is replaced with 18 was then treated with 10 µL of 30 µM non-reduced DesII and 10 µL of the filtrate so as to be in proportions equal to those used in the experimental sample. Following addition of the non-reduced enzyme and filtrate, 50-µL aliquots were filtered to remove protein at 5 and 85 min, and frozen prior to analysis by analytical HPLC. In tandem with substrate 15, these experiments with substrate 18 were performed twice on two separate days.
RESULTS
Stoichiometry of SAM Consumption Versus TDP-4-amino-4,6-dideoxy-d-glucose (15) Conversion
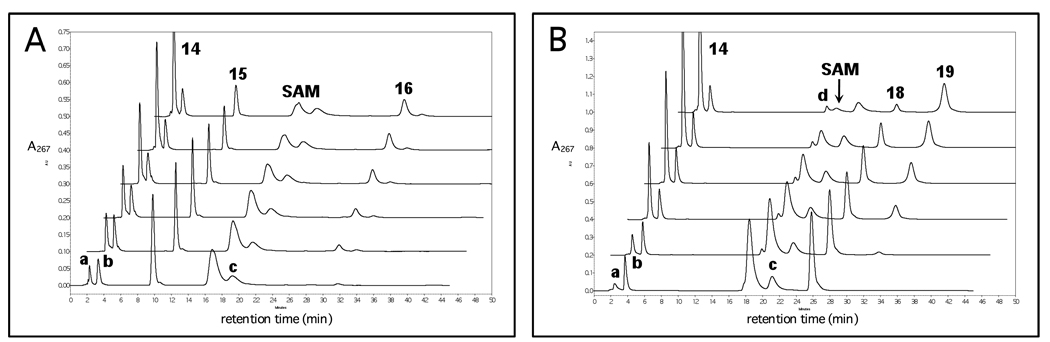
Stoichiometry measurements were made by comparing the relative peak integrations of the corresponding signals from the analytical ion exchange HPLC chromatograms. A set of representative HPLC chromatograms with Na2S2O4 as the reducing agent is shown in Figure 1A. Approximate retention times are as follows: 5'-deoxyadenosine (14) at 2.3 min, 15 at 10.0 min, SAM at 17.0 min and TDP-3-keto-4,6-dideoxyglucose (DesII product, 16) at 30.2 min. Additional peaks observed at 2.5 (peak a), 3.0 (peak b) and 19.5 min (peak c) are due to contaminants in the commercial SAM. Chromatograms from experiments using FLD/FNR/NADPH as the reducing system are identical to those shown in Figure 1A with the addition of a broad, poorly defined peak at approximately 36 min corresponding to NADP+. NADPH elutes at a much higher ionic strength than that employed in the HPLC program and thus is not observed. As can be seen from the chromatograms in Figure 1A, the peaks from the contaminants (except for the one at 2.5 min, see below) neither overlap significantly with those from the reactants and products nor change during the experimental time period. Control reactions were run to > 99% conversion in both SAM and 15 demonstrating no significant contaminants underlying these peaks.
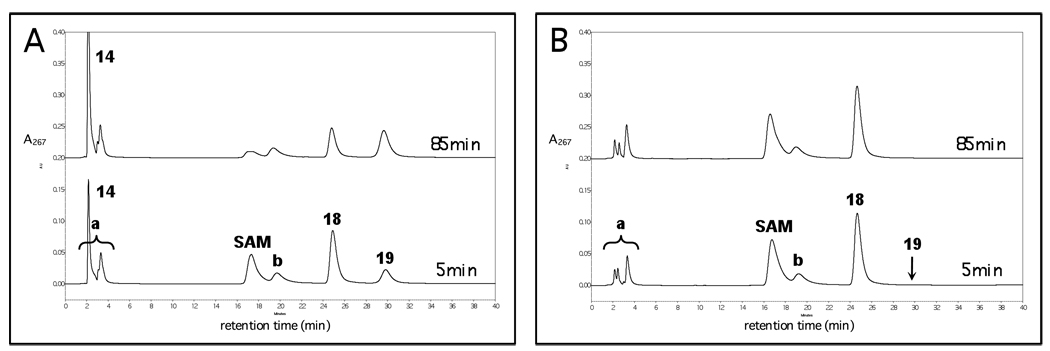
Figure 1.
Representative HPLC chromatograms for stoichiometry experiments with Na2S2O4 as the reductant. (A) Deamination of TDP-4-amino-4,6-dideoxy-d-glucose (15). (B) Dehydrogenation of TDP-d-quinovose (18). The bottom chromatogram in both (A) and (B) corresponds to the reaction before adding DesII. Subsequent traces are at increasing incubation times not exceeding 90 and 130 min, respectively. Peaks 14, 16 and 19 correspond to reaction products 5'-deoxyadenosine, TDP-3-keto-4,6-dideoxy-d-glucose and TDP-3-keto-6-deoxy-d-glucose, respectively. Peaks a, b and c are contaminants in the commercial SAM. Peak d is a contaminant in the TDP-d-quinovose (18) and corresponds to deoxythymidine monophosphate. Peaks a and 14 overlap, and the correction for fSAM is described in the Results and further detailed in the Supporting Information.
Stoichiometry measurements were performed by monitoring changes in fraction of reaction for 15, fsub, versus that for SAM, fSAM. These are each determined per chromatogram by dividing the area of the product peak by the sum of areas for the product and corresponding reactant peaks. By doing so, the measurements are insensitive to small variations in the HPLC injection volumes as well as adjustments to the reaction system, such as the addition of additional enzyme or reductant, so long as no additional SAM or 15 is added.
For each experiment an initial chromatogram was obtained for the reaction system containing all components except DesII, which was added last to initiate the reactions. This chromatogram is used to determine two correction factors for each experiment. The first, denoted γ, indicates the initial relative amounts of 15 versus SAM in the reaction,
| (1) |
where and are the peak integrations for 15 and SAM, respectively, in the initial chromatogram, and is the ratio of extinction coefficients for SAM and 15 at 267 nm. The ratio is found to be 1.525 based on diode-array UV-vis spectra from adenosine and thymidine and using literature values of M−1cm−1 and M−1cm−1, respectively, to normalize the concentrations. The second correction factor, denoted κ, is used to remove the contribution from a small contaminating peak (peak a) that coelutes with 5'-deoxyadenosine (14) and is determined according to
| (2) |
where is the area of this contaminating peak in the initial chromatogram. Assuming the relative intensity of this peak versus the sum of those from SAM and 5'-deoxyadenosine does not change over the course of the experiment, then the true fraction of reaction for SAM, fSAM, can be calculated from the observed value, , as
| (3) |
A detailed explanation and derivation of this correction is provided in the Supporting Information.
To determine the stoichiometry, x, of SAM versus 15,
| (4) |
the fraction of reaction fSAM is plotted against that for fsub, as shown in Figure 2A and B for representative experiments with each of the two reducing systems. These plots cannot be fit using standard least squares linear regression, because there is error in both fSAM and fsub. This can be accounted for by assuming equal variance in both variates and determining the slope, β, and ordinate intercept, α, of the fitted line according to
| (5) |
| (6) |
where and mSAM are the sample variance and mean for the measured values of fSAM, and msub are the corresponding values for fsub, and sSAM,sub is the sample covariance of fSAM and fsub.60,61 The slope of the fitted line in any given experiment is not equal to the stoichiometry, x, because fractions of reaction are being considered rather than absolute concentrations and the initial concentrations of SAM and 15 are not precisely equal in any given experiment. The stoichiometry is instead given by the ratio
| (7) |
for which a detailed derivation and explanation is provided in the Supporting Information.
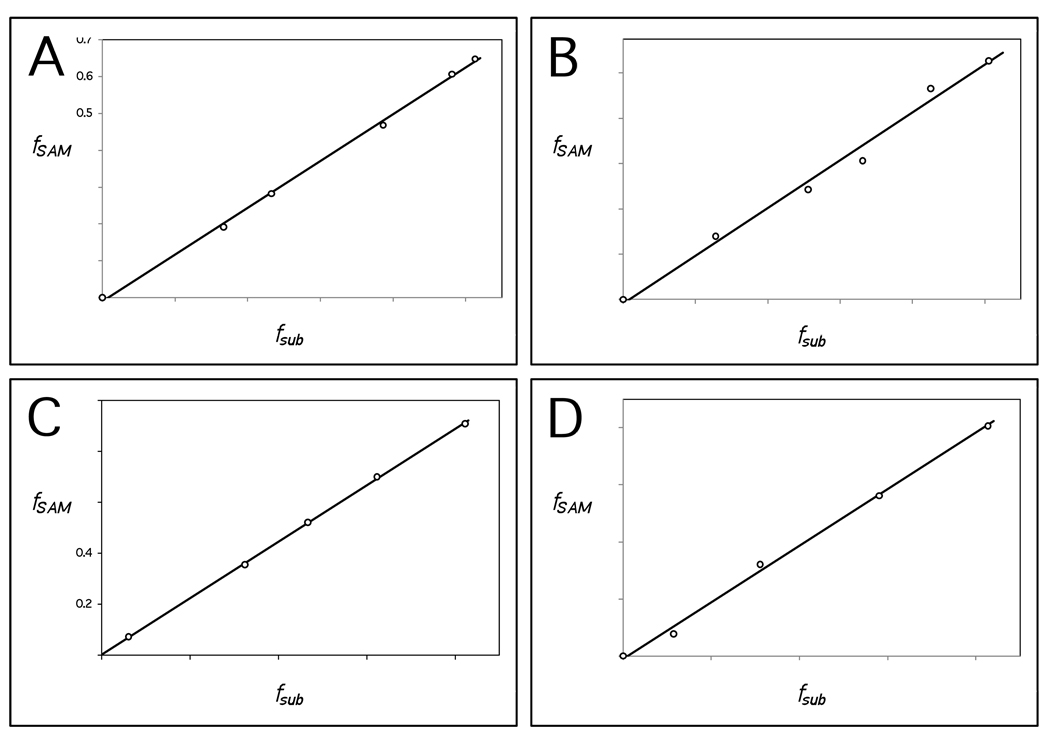
Figure 2.
Representative fits of the fraction of reaction in terms of SAM, fSAM, versus TDP-4-amino-4,6-dideoxy-d-glucose (15) or TDP-d-quinovose (18), fsub. Trend lines are fit under the assumption of equal variance in both fSAM and fsub. The stoichiometry is equal to the slope divided by the correction factor, γ, for the given experiment. (A) Substrate 15 with Na2S2O4 (γ = 1.23). (B) Substrate 15 with FLD/FNR/NADPH (γ = 1.05). (C) Substrate 18 with Na2S2O4 (γ = 1.16). (D) Substrate 18 with FLD/FNR/NADPH (γ = 2.04).
Three measurements of the stoichiometry with 15 were performed for each reducing system. Variances and errors are determined based on the overall samples of three measurements each. For the dithionite reducing system, the mean value of x is 0.96 with a standard deviation of 0.08 and a standard error of 0.05. For the FLD/FNR/NADPH reducing system, the mean value of x is 1.05 with a standard deviation of 0.08 and a standard error of 0.05. These stoichiometries are not significantly different based on a two-tailed Student's t-test using a 95% confidence interval.
Stoichiometry of SAM Consumption Versus TDP-d-quinovose (18) Conversion
Stoichiometry measurements of SAM versus 18 were made in a fashion analogous to that of SAM versus 15. A set of representative HPLC chromatograms using Na2S2O4 as the reducing system is shown in Figure 1B. These chromatograms are similar to those observed for the reaction with 15 except that peaks at 10.0 and 30.2 min are replaced with peaks at 26.0 and 31.5 min, which correspond to substrate 18 and the TDP-3-keto-6-deoxy-d-glucose (19) product, respectively. A small peak (peak d) eluted just prior to SAM, which corresponds to deoxythymidine monophosphate (TMP, present as a contaminant in 18), is well resolved, separable and less than 5% relative to the initial area of the SAM peak. As in the case of 15, control reactions were run to > 99% conversion of 18, demonstrating no significant contaminants underlying the peak corresponding to 18.
While five measurements of the stoichiometry were performed for the Na2S2O4 experiment only a single measurement was performed using FLD/FNR/NADPH as the reducing system. Fits of fSAM versus fsub for 18 were obtained as described above for 15, and examples using both reducing systems are shown in Figures 2C and D. Variances and errors are determined for the Na2S2O4 reducing system based on the overall sample of five measurements. For the dithionite reducing system the mean value of x is 1.01 with a standard deviation of 0.11 and a standard error of 0.05. For the single measurement with the FLD/FNR/NADPH reducing system, the observed value of x is 0.97. The stoichiometries between substrates 15 and 18 using Na2S2O4 as the reducing system are not significantly different based on a two-tailed Student's t-test using a 95% confidence interval.
Additional Controls for Uncoupled Turnover of SAM
Control reactions were also performed to check for the ability of DesII to consume SAM in the absence of 15 or 18. Three reactions were run anaerobically at room temperature (~ 25 °C) in the glovebox in 100 mM Tris·HCl (pH 8.0), 10% glycerol, 2 mM DTT and 0.2 mM freshly prepared Na2S2O4. The first reaction included approximately 5 mM 18 and SAM as well as 12 µM DesII to ensure the enzyme was active. The second reaction included 5 mM SAM and 12 µM DesII but no TDP-sugar substrate. Aliquots of this reaction were assayed by HPLC at 0.5, 7 and 48 h (data not shown). Based on peak integrations, 60% of the SAM was decomposed by 48 h, indicative of a half-life of approximately 35 h. This is compatible with reported non-enzymatic decomposition of SAM to form homoserine lactone and 5'-methylthioadenosine by one pathway as well adenine and S-ribosylmethionine by another, where the combined half-life at pH 7.5 and the elevated temperature of 37 °C is estimated to be ~17 h.62 At the retention time of 5'-deoxyadenosine (14), a small increase in the size of the contaminating peak was observed; however, this is found to account for only ~8% of the decrease in the SAM peak. In contrast, there was a significant increase in a peak with retention time similar to adenine at ~3 min. The increase in this peak accounts for over 95% of the decrease in the SAM peak, assuming similar extinction coefficients at 267 nm. In the final control approximately 5 mM SAM was incubated in the absence of both DesII and TDP-sugar substrate. In this case it was also found that the estimated half-life of SAM was approximately 35 h, there was marginal increase in the peak at the retention time of 5'-deoxyadenosine (14) and the majority of SAM decomposition was accounted for by an increasing peak with a retention time similar to that of adenine. Therefore, no significant differences were observed compared to the reaction in the presence of DesII.
Requirements for a Constant External Source of Reducing Equivalents
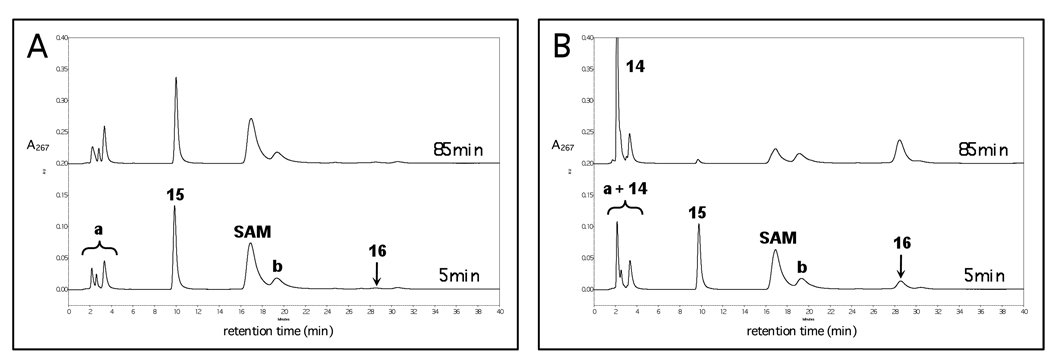
Reconstituted DesII that had been incubated overnight with an excess of Na2S2O4 is unable to catalyze appreciable turnover of either 15 or SAM. This is shown in Figure 3A where < 2% conversion of 15 is observed after 85 min incubation with the reduced DesII in the absence of any fresh reductant. However, if fresh Na2S2O4 was added in a concentration equal to that at the beginning of the overnight incubation, turnover of both SAM and 15 was observed. This is shown in Figure 3B where 23% and 94% conversion of 15 are noted at 5 and 85 min, respectively. This indicates that DesII is still active and the [4Fe-4S] cluster remains intact following the overnight incubation with the strong reductant.
Figure 3.
HPLC experiments demonstrating a requirement for a constant source of reducing equivalents in the deamination of TDP-4-amino-4,6-dideoxy-d-glucose (15) to TDP-3-keto-4,6-dideoxy-d-glucose (16) by DesII. (A) Incubation of reduced DesII with 15 at 5 and 85 min demonstrating less than 2% turnover at each time point. (B) Incubation of reduced DesII with 15 in the presence of fresh Na2S2O4 at 5 and 85 min demonstrating 23% and 94% turnover in terms of 15, respectively. Peak 14 corresponds to 5'-deoxyadenosine generated as SAM is consumed. Peaks a and b correspond to contaminants in the commercial SAM. See text for details and Figure 4 for a comparison with the dehydrogenation reaction.
In contrast, when 18 was incubated with the reconstituted and reduced DesII in the absence of any additional fresh Na2S2O4, turnover of both 18 as well as SAM was observed. As shown in Figure 4A, conversions of 26% and 55% in terms of 18 were observed at 5 and 85 min, respectively, the reaction being limited by a substoichiometric amount of SAM. As shown in Figure 4B, when the deproteinized filtrate from the reduced DesII was incubated with 18 along with DesII that had not been treated overnight with Na2S2O4, less than 1% conversion of both SAM and 18 was observed at 85 min.
Figure 4.
HPLC experiments demonstrating that a constant external source of reducing equivalents is not required for the dehydrogenation of TDP-d-quinovose (18) to TDP-3-keto-6-deoxy-d-glucose (19) by DesII. (A) Incubation of reduced DesII with 18 at 5 and 85 min demonstrating 26% and 55% reaction in terms of 18, respectively (SAM is substoichiometric versus 18 in this experiment). (B) Incubation of nonreduced DesII with 18 in the presence of deproteinized reduced DesII filtrate at 5 and 85 min demonstrating less than 1% reaction at each time point. Peak 14 corresponds to 5'-deoxyadenosine generated as SAM is consumed. Peaks a and b correspond to contaminants in the commercial SAM. See text for details and Figure 3 for a comparison with the deamination reaction.
DISCUSSION
In its biological context, DesII catalyzes the deamination of 15 to 16 in the biosynthesis of TDP-d-desosamine. 30,52 As there is no net redox change in the biologically relevant transformation of 15 to 16, one would expect SAM to be regenerated with each catalytic cycle as has been observed with LAM5,10,11 and SPL.12,13 Therefore, the observation that one equivalent of SAM is reduced to l-methionine (3) and 5'-deoxyadenosine (14) for each equivalent of 15 deaminated is unusual both with respect to other radical SAM mutases and lyases as well as the B12-dependent lyase EAL and the dioldehydratases.
A recognized aspect of radical SAM enzymology is the uncoupling of homolytic cleavage of SAM from formation of the substrate radical.44 This phenomenon is characterized by the one-electron reduction of SAM by the reduced [4Fe-4S]1+ cluster to methionine (3) and a 5'-deoxyadenosyl radical (4) that is further reduced to 14 by hydrogen atom transfer or proton coupled electron transfer from a source other than the nominal substrate. In the case of biotin synthase, reductive cleavage of SAM by [4Fe-4S]1+ has been noted in the absence of the dethiobiotin substrate (5).47 Thus, stoichiometry of SAM consumption versus biotin (6) production has been reported to range from 2.1 to 3.1.14,26,46,48 The stoichiometries of turnover for biotin synthase26 and lipoyl synthase27 are also known to increase beyond 2.0 with increasing of fraction of reaction. Similar observations have been made with SPL63,64 and anaerobic ribonucleotide reductase,65 where in the latter system the uncoupled reaction is noted to require the presence of DTT.
In the case of DesII, all fits are subjectively linear with both substrates as shown in Figure 2. Had there been a significant uncoupling side reaction, one would expect dependence of the slope on fsub leading to an upward curvature. Even with reactions reaching over 75% conversion in 15, no apparent curvature is seen, indicating that uncoupling remains insignificant and SAM consumption is not facilitated by the presence of product at the concentrations and time scale considered. Likewise, only a marginal increase in 5'-deoxyadenosine (14) is observed in the control reaction where DesII is incubated with Na2S2O4, DTT and SAM in the absence of TDP-sugar substrate, and a similar observation is also noted in the absence of DesII. In these controls, the majority of SAM consumption is best attributed to the known non-enzymatic decomposition to adenosine and S-ribosylmethionine, and/or to homoserine lactone and 5'-methylthioadenosine.62,66 Most importantly, deuterium incorporation into 5'-deoxyadenosine (14) has been observed in the DesII reaction with TDP-[3-2H]-4-amino-4,6-dideoxy-d-glucose ([3-2H]-15),33 presenting a direct link between SAM reduction and the chemical step of substrate turnover. Together these results argue against the observed stoichiometry in the deamination reaction being simply a result of an uncoupling process.
Further support is provided by comparison with the DesII catalyzed dehydrogenation of 18. As this reaction involves an overall two-electron oxidation of the TDP-sugar substrate, a stoichiometry of 1 is expected for the consumption of SAM versus 18. Indeed, the observed stoichiometry is 1.01 ± 0.05, and there is no subjective evidence of curvature in the plots of fSAM versus fsub as seen in Figures 2C and D. This is consistent with the results from the deamination reaction, arguing against uncoupling as the cause of SAM depletion. The one-to-one consumption of SAM per equivalent of 18 dehydrogenated by DesII is well within the expected behavior of the oxidizing radical SAM enzymes such as BtrN,22 HemN,28 anSMEs,29 and the sulfur insertion enzymes (see Scheme 2).16,17,25–27
While the occurrence of uncoupled reduction of SAM in the presence of 15 seems unlikely, this does not necessarily rule out an abortive process leading to the net consumption of SAM. A scenario can be envisioned where in the deamination of 15 in vivo, SAM is regenerated with each catalytic cycle analogous to LAM and SPL. However, rapid, premature reduction of the [4Fe-4S]2+ cluster may take place in vitro with the strong dithionite reductant. In this situation, reduction of [4Fe-4S]2+ by dithionite may occur after initial formation of the 5'-deoxyadenosyl radical (4) but before hydrogen atom abstraction from 5'-deoxyadenosine (14) by the product radical. While the 5'-deoxyadenosyl radical (4) (and the one-electron reduced SAM (2)) can still be regenerated at the end of the catalytic cycle, regeneration of SAM via one-electron reduction of the already reduced [4Fe-4S]1+ cluster by 2 would not be possible. The nascent 2 and/or 4 radical may thus be quenched via electron transfer from dithionite via the iron-sulfur center or hydrogen atom transfer from an external source such as DTT. The net result would be an artificial one-to-one stoichiometry.
In order to test for an abortive process due to dithionite, the biological FLD/FNR/NADPH reducing system was considered. This system is also of interest as it has been suggested that, in the reaction catalyzed by biotin synthase, changes in the reducing system employed may lead to slight variations in the observed stoichiometry.67 However, a one-to-one consumption of SAM by DesII during deamination of 15 is again observed when FLD/FNR/NADPH was used as the source of reducing equivalents. A similar result is also obtained with the dehydrogenation of 18, though only one trial was attempted once it was discovered that an external source of reductant is not required with this substrate (see below). These results indicate that in a more biologically relevant context the DesII enzyme clearly facilitates deamination of 15 by the net reduction of SAM with a stoichiometry not significantly different from that with dithionite.
The above findings revealed that, contrary to expectations, SAM is clearly not regenerated with each deamination reaction. In this situation, a continuous supply of external reducing equivalents is required for the net reduction of SAM to complete the catalytic cycle, because deamination of 15 is redox neutral. In contrast, the dehydrogenation of 18 should have no such requirement, since the reducing equivalents would come from the substrate itself as it is oxidized. Such a mechanism has been proposed for the radical SAM dehydrogenase BtrN (see Scheme 2C).22 If correct, this hypothesis implies that in the absence of any external reducing source DesII should only be active in vitro for the dehydrogenation reaction so long as DesII is primed to the [4Fe-4S]1+ state prior to introducing substrate.
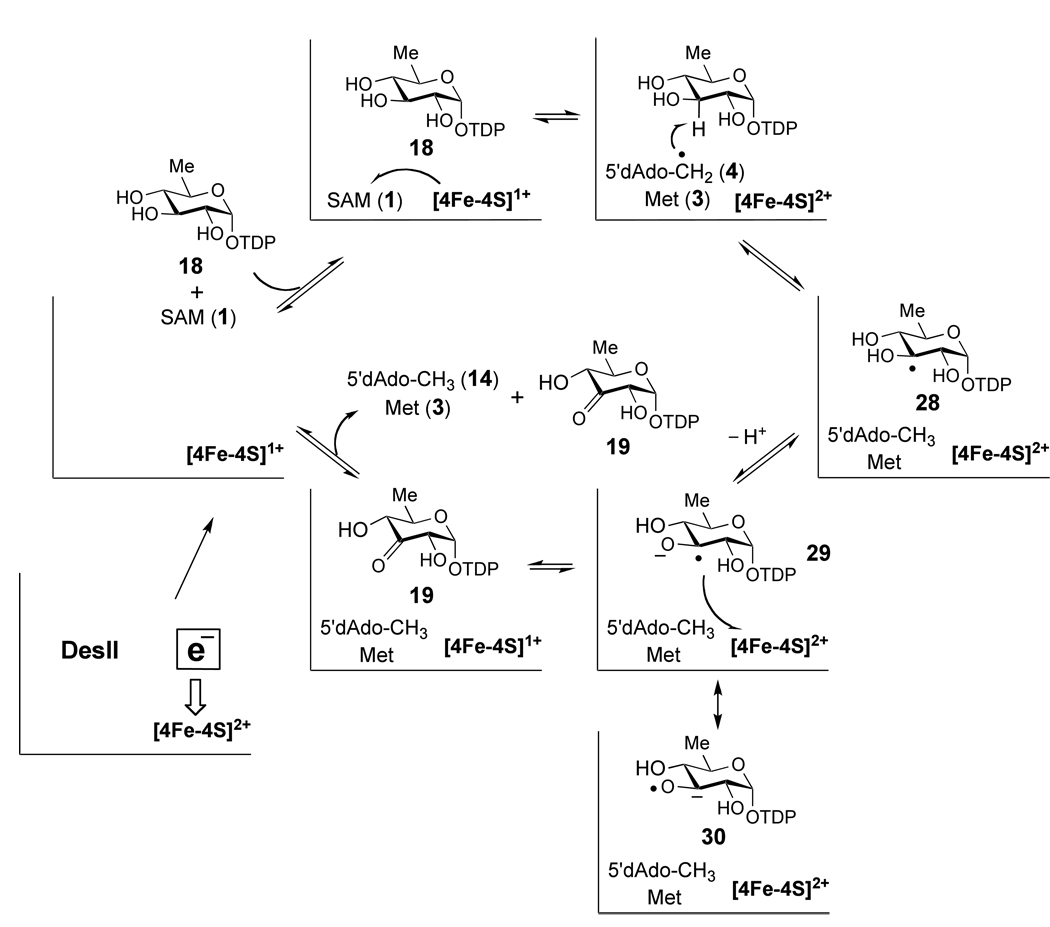
To test this hypothesis, reconstituted DesII in the [4Fe-4S]2+ state was reduced by overnight incubation with Na2S2O4, during which time DesII was fully reduced and excess dithionite decomposed via disproportionation.56 Upon subsequent incubation with substrate and SAM, significant turnover was observed only in the dehydrogenation of 18 and not in the deamination of 15 (Figure 4A and Figure 3A, respectively). These two experiments likewise control for one another ensuring that the reductant was exhausted, the cluster was not destroyed, and DesII remained in the reduced [4Fe-4S]1+ state. Our data also showed that incubation of 18 with DesII in the oxidized [4Fe-4S]2+ state mixed with the deproteinized filtrate from the overnight reduction (in which dithionite had already decomposed) failed to react (Figure 4B), whereas incubation of 15 with the reduced DesII and freshly prepared dithionite demonstrated clear turnover (Figure 3B). Taken together, these results indicate that an external reducing source is required in the deamination of 15, implying that consumption of SAM is not an artifact of the ultimate source of reducing equivalents. Furthermore, the results with 18 are consistent with either direct or indirect reduction of the oxidized [4Fe-4S]2+ cluster by the product radical (29/30 in Scheme 7) in the formation of 19.
Scheme 7.
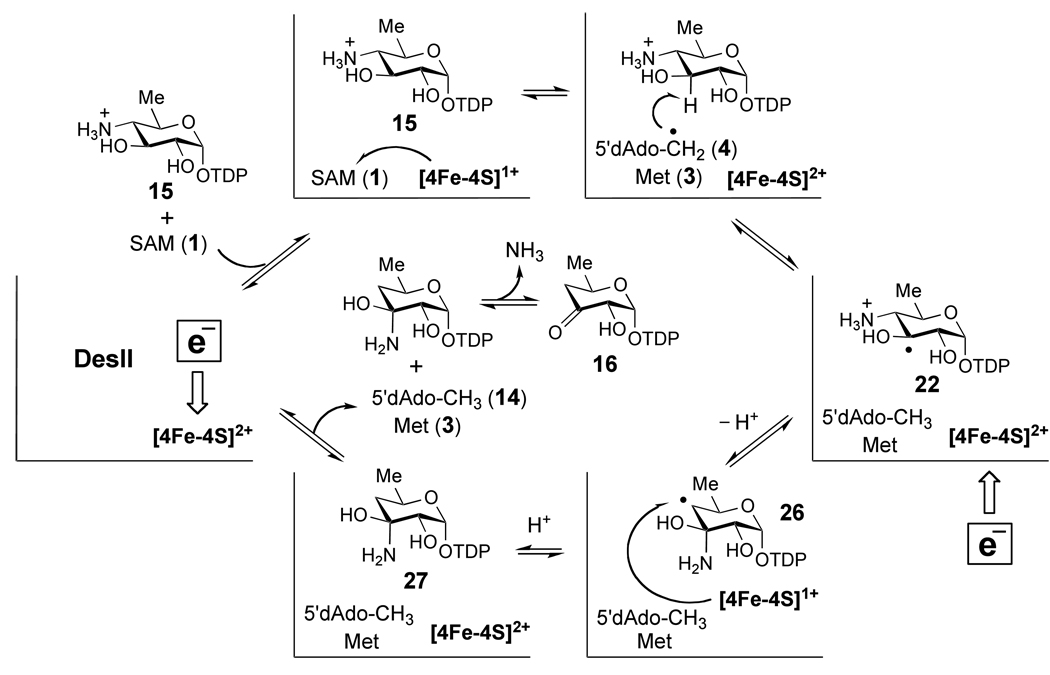
Based on these observations, DesII may represent a unique example of a radical SAM enzyme with dual redox capability.68 The enzyme is able to catalyze both a two-electron oxidation of 18 and a redox neutral deamination of 15, while requiring SAM as a two-electron oxidant in both instances. Mechanistic models for these two modes of DesII catalysis are proposed in Scheme 5 – Scheme 7. During deamination, formation of the ternary complex between reduced DesII, SAM and 15 facilitates one-electron reduction of SAM by the [4Fe-4S]1+ cluster triggering homolysis of the C5'-S bond as shown in Scheme 1. The 5'-deoxyadenosyl radical (4) then abstracts the C-3 hydrogen atom from 15 completing the two-electron reduction of SAM (see Scheme 2D) in a manner directly coupled to the production of the substrate radical 22. In the mechanism of Scheme 5, l-methionine (3) and 5'-deoxyadenosine (14) are the by-products, and their dissociation may occur at any subsequent point in the catalytic cycle.
The fate of the substrate radical is uncertain at this point. However, as shown in Scheme 5 one possibility is deprotonation of the C-3 hydroxyl group facilitated by the C-3 radical (22 → 23), consistent with the ability of a carbon-centered radical to decrease the pKa of an adjacent hydroxyl moiety by 4 – 5 units.69 The resulting ketyl radical (23/24) could then expel ammonia by an E1cb-type elimination to provide the enol/α-keto radical 25. As an alternative to the E1cb-type elimination from a ketyl radical, direct, via a 1,2-shift of the amino group, or indirect, via dissociation/reassociation of ammonia, migration of the amino group may take place to form 26 as shown in Scheme 6. These latter possibilities are similar to the current hypotheses regarding B12-dependent mutases, which are implicated by computational investigations.70
Scheme 6.
In any case, the radical intermediate (25 or 26) is one-electron more oxidized than the substrate and product, such that a one-electron reduction is required to complete the catalytic cycle. In Scheme 5 and Scheme 6, this is proposed to take place via the [4Fe-4S]1+ cluster, consistent with the ability of the cluster to oxidize the product radical in the dehydrogenation of 18 (see Scheme 7). The point at which the [4Fe-4S]2+ cluster is reduced back to the 1+ state in Scheme 5 and Scheme 6 is uncertain and could conceivably occur at any point following the reductive homolysis of SAM. Protonation of the resulting enolate concomitantly or in a stepwise manner with regards to electron transfer then completes the catalytic cycle of the ketyl radical mechanism (Scheme 5). In the case of the amino-migration type mechanism (Scheme 6), the net transfer of a hydrogen atom would yield the carbinolamine 27 that subsequently eliminates ammonia to afford the ketone product, 16. Reduction of the iron-sulfur cluster then completes the catalytic cycle.
In the dehydrogenation of 18, generation of the substrate radical (28) is proposed to take place via an analogous process. However, rather than eliminating water in place of ammonia, the anionic substrate radical 29/30 directly or indirectly transfers an electron back to the oxidized [4Fe-4S]2+ cluster, completing the net two-electron oxidation of 18 and regenerating the reduced form of the enzyme. This mechanism likely involves the deprotonation of the C-3 hydroxyl group of the neutral substrate radical 28, because the redox potential of a RR'C•–O− radical is significantly reduced compared to the corresponding RR'C•–OH radical.69 This process may also be facilitated by the inability of the enzyme to stabilize a hydroxyl versus an amino leaving group at C-4 due to the typical 10- to 15-unit difference in pKa between a secondary hydroxonium and ammonium cation. This is in contrast with deamination of 15 where elimination of ammonia from 24 to form the more stable enol/α-keto radical 25 proceeds more rapidly than internal electron transfer to reduce the cluster. Therefore, the combination of a poor leaving group and strong reductant would leave the ketyl radical 29/30 either proceeding with dehydrogenation or reversing back to substrate and SAM, a phenomenon consistent with deuterium transfer experiments with both DesII and BtrN.22,33
These results raise the question as to why in a biological context the deamination of 15 would be coupled to the consumption SAM. A possible explanation comes from the observation that the immediate precursors to the deamination reaction, such as 15, 20 and 21, are also precursors to a wide variety of modified monosaccharides.71 Because the net two-electron reduction of SAM is expected to be a thermodynamically favorable process, which is consistent with the observation of virtually quantitative dehydrogenation of 18, coupling deamination of 15 with reduction of SAM would lead the DesII catalyzed deamination reaction to function as a first-committed, flux-controlling step in the biosynthesis of TDP-d-desosamine (17). Investigation of these and other questions pertaining to the mechanism of DesII are currently a focus of efforts in our laboratory.
Supplementary Material
ACKNOWLEDGEMENT
This work was supported by a grant provided by the National Institutes of Health (GM035906) and a fellowship award (F32AI082906) from the National Institute of Allergy and Infectious Diseases to M.W.R. The authors would like to thank Steven O. Mansoorabadi and Wei-chen Chang for helpful comments on the manuscript.
Abbreviations
- 5'dAdo
5'-deoxyadenosine
- anSME
anaerobic sulfatase maturating enzyme
- DTT
dithiothreitol
- EAL
ethanolamine ammonia lyase
- FLD
flavodoxin
- FNR
flavodoxin NADP+ oxidoreductase
- ForDH
formate dehydrogenase
- FPLC
fast protein liquid chromatography
- Glu
glutamate
- GluDH
glutamate dehydrogenase
- HemN
oxygen-independent coproporphyrinogen III oxidase
- HPLC
high performance liquid chromatography
- α-KG
α-ketoglutarate
- KPi
potassium phosphate
- LAM
lysine 2,3-aminomutase
- LCP
lipoyl carrier protein
- Met
l-methionine
- NAD+
nicotinamide adenine dinucleotide (oxidized form)
- NADH
nicotinamide adenine dinucleotide (reduced form)
- NADP+
nicotinamide adenine dinucleotide phosphate (oxidized form)
- NADPH
nicotinamide adenine dinucleotide phosphate (reduced form)
- PLP
pyridoxal-5'-phosphate
- SAM
S-adenosyl-l-methionine
- SPL
spore photoproduct lyase
- TDP
deoxythymidine diphosphate
- TMP
deoxythymidine monophosphate
- TpT
thymidine dinucleoside monophosphate.
Footnotes
Supporting Information Available: Further detail regarding the analytical theory behind the stoichiometry measurements. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Frey PA, Hegeman AD, Ruzicka FJ. Crit. Rev. Biochem. Mol. Biol. 2008;43:63–88. doi: 10.1080/10409230701829169. [DOI] [PubMed] [Google Scholar]
- 2.Frey PA, Magnusson OT. Chem. Rev. 2003;103:2129–2148. doi: 10.1021/cr020422m. [DOI] [PubMed] [Google Scholar]
- 3.Sofia HJ, Chen G, Hetzler BH, Reyes-Spindola JF, Miller NE. Nuc. Acids Res. 2001;29:1097–1106. doi: 10.1093/nar/29.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magnusson OT, Reed GH, Frey PA. J. Am. Chem. Soc. 1999;121:9764–9765. [Google Scholar]
- 5.Moss M, Frey PA. J. Biol. Chem. 1987;262:14859–14862. [PubMed] [Google Scholar]
- 6.Magnusson OT, Reed GH, Frey PA. Biochemistry. 2001;40:7773–7782. doi: 10.1021/bi0104569. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee R. Chem. Rev. 2003;103:2083–2094. doi: 10.1021/cr0204395. [DOI] [PubMed] [Google Scholar]
- 8.Banerjee R, Ragsdale SW. Annu. Rev. Biochem. 2003;72:209–247. doi: 10.1146/annurev.biochem.72.121801.161828. [DOI] [PubMed] [Google Scholar]
- 9.Toraya T. Chem. Rev. 2003;103:2095–2127. doi: 10.1021/cr020428b. [DOI] [PubMed] [Google Scholar]
- 10.Baraniak J, Moss ML, Frey PA. J. Biol. Chem. 1989;264:1357–1360. [PubMed] [Google Scholar]
- 11.Chirpich TP, Zappia V, Costilow RN, Barker HA. J. Biol. Chem. 1970;245:1778–1789. [PubMed] [Google Scholar]
- 12.Buis JM, Cheek J, Kalliri E, Broderick JB. J. Biol. Chem. 2006;281:25994–26003. doi: 10.1074/jbc.M603931200. [DOI] [PubMed] [Google Scholar]
- 13.Cheek J, Broderick JB. J. Am. Chem. Soc. 2002;124:2860–2861. doi: 10.1021/ja017784g. [DOI] [PubMed] [Google Scholar]
- 14.Guianvarc'h D, Florentin D, Tse Sum Bui B, Nunzi F, Marquet A. Biochem. Biophys. Res. Commun. 1997;236:402–406. doi: 10.1006/bbrc.1997.6952. [DOI] [PubMed] [Google Scholar]
- 15.Ifuku O, Kishimoto J, Haze S-i, Yanagi M, Fukushima S. Biosci. Biotech. Biochem. 1992;56:1780–1785. doi: 10.1271/bbb.56.1780. [DOI] [PubMed] [Google Scholar]
- 16.Marquet A, Tse Sum Bui B, Smith AG, Warren MJ. Nat. Prod. Rep. 2007;24:1027–1040. doi: 10.1039/b703109m. [DOI] [PubMed] [Google Scholar]
- 17.Booker SJ, Cicchillo RM, Grove TL. Curr. Opin. Chem. Biol. 2007;11:543–552. doi: 10.1016/j.cbpa.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller JR, Busby RW, Jordan SW, Cheek J, Henshaw TF, Ashley GW, Broderick JB, John E, Cronan J, Marletta MA. Biochemistry. 2000;39:15166–15178. doi: 10.1021/bi002060n. [DOI] [PubMed] [Google Scholar]
- 19.Zhao X, Miller JR, Jiang Y, Marletta MA, Cronan JE. Chem. Biol. 2003;10:1293–1302. doi: 10.1016/j.chembiol.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Layer G, Moser J, Heinz DW, Jahn D, Schubert W-D. EMBO J. 2003;22:6214–6224. doi: 10.1093/emboj/cdg598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Layer G, Verfürth K, Mahlitz E, Jahn D. J. Biol. Chem. 2002;277:34136–34142. doi: 10.1074/jbc.M205247200. [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama K, Numakura M, Kudo F, Ohmori D, Eguchi T. J. Am. Chem. Soc. 2007;129:15147–15155. doi: 10.1021/ja072481t. [DOI] [PubMed] [Google Scholar]
- 23.Benjdia A, Leprince J, Guillot A, Vaudry H, Rabot S, Berteau O. J. Am. Chem. Soc. 2007;129:3462–3463. doi: 10.1021/ja067175e. [DOI] [PubMed] [Google Scholar]
- 24.Benjdia A, Subramanian S, Leprince J, Vaudry H, Johnson MK, Berteau O. J. Biol. Chem. 2008;283:17815–17826. doi: 10.1074/jbc.M710074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Escalettes F, Florentin D, Tse Sum Bui B, Lesage D, Marquet A. J. Am. Chem. Soc. 1999;121:3571–3578. [Google Scholar]
- 26.Shaw NM, Birch OM, Tinschert A, Venetz V, Dietrich R, Savoy L-A. Biochem. J. 1998;330:1079–1085. doi: 10.1042/bj3301079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cicchillo RM, Iwig DF, Jones AD, Nesbitt NM, Baleanu-Gogonea C, Souder MG, Tu L, Booker SJ. Biochemistry. 2004;43:6378–6386. doi: 10.1021/bi049528x. [DOI] [PubMed] [Google Scholar]
- 28.Layer G, Grage K, Teschner T, Schünemann V, Breckau D, Masoumi A, Jahn M, Heathcote P, Trautwein AX, Jahn D. J. Biol. Chem. 2005;280:29038–29046. doi: 10.1074/jbc.M501275200. [DOI] [PubMed] [Google Scholar]
- 29.Benjdia A, Leprince J, Sandström C, Vaudry H, Berteau O. J. Am. Chem. Soc. 2009;131:8348–8349. doi: 10.1021/ja901571p. [DOI] [PubMed] [Google Scholar]
- 30.Szu P-h, He X, Zhao L, Liu H-w. Angew. Chem. Int. Ed. 2005;44:6742–6746. doi: 10.1002/anie.200501998. [DOI] [PubMed] [Google Scholar]
- 31.Thorson JS, Hosted TJ, Jr., Jiang J, Biggins JB, Ahlert J. Curr. Org. Chem. 2001;5:139–167. [Google Scholar]
- 32.Xue Y, Zhao L, Liu H-w, Sherman DH. Proc. Natl. Acad. Sci. U.S.A. 1998;95:12111–12116. doi: 10.1073/pnas.95.21.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szu P-H, Ruszczycky MW, Choi S-h, Liu H-w. J. Am. Chem. Soc. 2009;131:14030–14042. doi: 10.1021/ja903354k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Babior BM. J. Biol. Chem. 1969;244:449–456. [PubMed] [Google Scholar]
- 35.Bandarian V, Reed GH. Biochemistry. 2000;39:12069–12075. doi: 10.1021/bi001014k. [DOI] [PubMed] [Google Scholar]
- 36.Bradbeer C. J. Biol. Chem. 1965;240:4675–4681. [PubMed] [Google Scholar]
- 37.Warncke K, Canfield JM. J. Am. Chem. Soc. 2004;126:5930–5931. doi: 10.1021/ja031569d. [DOI] [PubMed] [Google Scholar]
- 38.Cockle SA, Hill HAO, Williams RJP, Davies SP, Foster MA. J. Am. Chem. Soc. 1972;94:275–277. doi: 10.1021/ja00756a050. [DOI] [PubMed] [Google Scholar]
- 39.Finlay TH, Valinsky J, Mildvan AS, Abeles RH. J. Biol. Chem. 1973;248:1285–1290. [PubMed] [Google Scholar]
- 40.Frey PA, Essenberg MK, Abeles RH. J. Biol. Chem. 1967;242:5369–5377. [PubMed] [Google Scholar]
- 41.Rétey J, Umani-Ronchi A, Seibl J, Arigoni D. Experientia. 1966;22:502–503. doi: 10.1007/BF01898652. [DOI] [PubMed] [Google Scholar]
- 42.Valinsky JE, Abeles RH, Fee JA. J. Am. Chem. Soc. 1974;96:4709–4710. doi: 10.1021/ja00821a077. [DOI] [PubMed] [Google Scholar]
- 43.Yamanishi M, Ide H, Murakami Y, Toraya T. Biochemistry. 2005;44:2113–2118. doi: 10.1021/bi0481850. [DOI] [PubMed] [Google Scholar]
- 44.Duschene KS, Veneziano SE, Silver SC, Broderick JB. Curr. Opin. Chem. Biol. 2009;13:74–83. doi: 10.1016/j.cbpa.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jarrett JT. Curr. Opin. Chem. Biol. 2003;7:174–182. doi: 10.1016/s1367-5931(03)00022-x. [DOI] [PubMed] [Google Scholar]
- 46.Lotierzo M, Raux E, Tse Sum Bui B, Goasdoue N, Libot F, Florentin D, Warren MJ, Marquet A. Biochemistry. 2006;45:12274–12281. doi: 10.1021/bi060662m. [DOI] [PubMed] [Google Scholar]
- 47.Ollagnier-de Choudens S, Sanakis Y, Hewitson KS, Roach P, Münck E, Fontecave M. J. Biol. Chem. 2002;277:13449–13454. doi: 10.1074/jbc.M111324200. [DOI] [PubMed] [Google Scholar]
- 48.Taylor AM, Farrar CE, Jarrett JT. Biochemistry. 2008;47:9309–9317. doi: 10.1021/bi801035b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang SC, Frey PA. Trends Biochem. Sci. 2007;32:101–110. doi: 10.1016/j.tibs.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Szu P-H. Ph.D. Thesis. University of Texas at Austin; 2008. [Google Scholar]
- 51.Zhao L. Ph.D. Thesis. University of Minnesota; 2000. [Google Scholar]
- 52.Zhao L, Borisova S, Yeung S-M, Liu H-w. J. Am. Chem. Soc. 2001;123:7909–7910. doi: 10.1021/ja010587x. [DOI] [PubMed] [Google Scholar]
- 53.Elling L, Rupprath C, Günther N, Römer U, Verseck S, Weingarten P, Dräger G, Kirschning A, Piepersberg W. ChemBioChem. 2005;6:1423–1430. doi: 10.1002/cbic.200500037. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi H, Liu Y-n, Liu H-w. J. Am. Chem. Soc. 2006;128:1432–1433. doi: 10.1021/ja0562144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robins MJ, Hansske F, Wnuk SF, Kanai T. Can. J. Chem. 1991;69:1468–1474. [Google Scholar]
- 56.de Carvalho LM, Schwedt G. Analytica Chimica Acta. 2001;436:293–300. [Google Scholar]
- 57.McKenna CE, Gutheil WG, Song W. Biochimica et Biophysica Acta. 1991;1075:109–117. doi: 10.1016/0304-4165(91)90082-r. [DOI] [PubMed] [Google Scholar]
- 58.Creutz C, Sutin N. Inorg. Chem. 1974;13:2041–2043. [Google Scholar]
- 59.Hamlin PA, Lambert JL. Anal. Chem. 1971;43:618–620. [Google Scholar]
- 60.Keeping ES. Introduction to statistical inference. New York, New York: Dover Publications, Inc.; 1995. [Google Scholar]
- 61.Mandel J. The statistical analysis of experimental data. New York, New York: Dover Publications, Inc.; 1984. [Google Scholar]
- 62.Iwig DF, Booker SJ. Biochemistry. 2004;43:13496–13509. doi: 10.1021/bi048693+. [DOI] [PubMed] [Google Scholar]
- 63.Pieck JC, Hennecke U, Pierik AJ, Friedel MG, Carell T. J. Biol. Chem. 2006;281:36317–36326. doi: 10.1074/jbc.M607053200. [DOI] [PubMed] [Google Scholar]
- 64.Rebeil R, Nicholson WL. Proc. Natl. Acad. Sci. U.S.A. 2001;98:9038–9043. doi: 10.1073/pnas.161278998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Padovani D, Thomas F, Trautwein AX, Mulliez E, Fontecave M. Biochemistry. 2001;40:6713–6719. doi: 10.1021/bi002936q. [DOI] [PubMed] [Google Scholar]
- 66.Hoffman JL. Biochemistry. 1986;25:4444–4449. doi: 10.1021/bi00363a041. [DOI] [PubMed] [Google Scholar]
- 67.Farrar CE, Jarrett JT. Biochemistry. 2009;48:2448–2458. doi: 10.1021/bi8022569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.The present experiments were conducted with a C-terminal His6-tagged enzyme. As the native DesII has not been characterized and no detailed structural information is presently available, the possibility that the native enzyme may behave differently cannot be excluded
- 69.Hayon E, Simic M. Acc. Chem. Res. 1974;7:114–121. [Google Scholar]
- 70.Semialjac M, Schwarz H. J. Am. Chem. Soc. 2002;124:8974–8983. doi: 10.1021/ja020101s. [DOI] [PubMed] [Google Scholar]
- 71.Thibodeaux CJ, Melancon CE, Liu H-w. Nature. 2007;446:1008–1016. doi: 10.1038/nature05814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.