Abstract
Background
Persistent E. coli asymptomatic bacteriuria (ASB) is common among persons with diabetes mellitus, but duration of colonization and re-colonization rates are unknown. We estimated duration of colonization and re-colonization among successively isolated E. coli from asymptomatic diabetic women and compared the virulence profiles to uropathogenic and commensal E. coli.
Methods
105 women with diabetes were enrolled in a randomized controlled clinical trial for treatment of ASB in Manitoba, Canada and followed at least every three months for up to three years. We analyzed 517 isolates from 70 women with repeated E. coli ASB for genetic similarity using ERIC-PCR. Unique strains were screened for uropathogenic virulence characteristics using dot blot hybridization, and compared to different collections of E. coli isolates.
Results
On average, there were differences between women assigned to treatment for ASB, those only treated for symptomatic infections and untreated women in: a) follow-up time with bacteriuria (29%, 31% and 66%, p=<0.001), b) duration of bacteriuria (2.2, 2.5 and 3.7 months, p=0.04) and c) carriage of unique isolates (2.4, 2.8 and 4 months, p=0.03). Women assigned to antibiotic treatment usually had recurrent infection (76%), 64% of the time with a genetically new E. coli strain. Virulence characteristics of these isolates were comparable to those of fecal isolates from healthy women.
Conclusions
Treatment may reduce the overall proportion of time infected in the long-term and carriage of a unique strain, but most treatment regimens were followed by subsequent re-colonization. Infecting strains did not have virulence factors characteristic of uropathogenic E. coli.
Keywords: urinary tract infection, diabetes, virulence, colonization, ERIC-PCR typing
INTRODUCTION
Urinary tract infections (UTI) occur in women with diabetes mellitus more frequently than in women without diabetes, are more severe, with pyelonephritis occurring at a five-fold higher rate, and often result in complications that are otherwise rare, such as emphysematous cystitis, and fungal infections [1, 2]. Asymptomatic bacteriuria (ASB) occurs three times more often among women with diabetes than among otherwise healthy women; ASB is associated with an increased risk of symptomatic infection but is not causative [2–5]. The presence of ASB in diabetic women is not associated with a faster decline in renal function [6], or greater risk of diabetic complications or mortality[4]. The most common infecting organism in asymptomatic bacteriuric women with diabetes is Escherichia coli; other organisms include Klebsiella spp., Enterococcus spp. and Group B Streptococcus (S. agalactiae) [2, 7]. Uropathogenic E. coli (UPEC) have a variety of virulence traits that enable them to successfully invade the normally sterile urinary tract [8, 9]. These include a number of adhesins, iron sequestration systems, and toxins which distinguish them from normal bowel flora E. coli [8].
Risk factors for ASB in diabetic women include sexual intercourse, degree of metabolic control, duration of diabetes, presence of diabetic complications, and insulin use [10–12]. In one study of symptomatic UTI among 589 women with diabetes, sexual intercourse in the preceding week was the most significant risk factor for women with Type I diabetes, whereas ASB was most significant for women with Type II diabetes [10]. Stringent control of blood glucose decreases risk of complications such as neuropathy and nephropathy, but a direct effect on bacteriuria has not been observed [1]. Neuropathy however, may affect underlying bladder dysfunction and thus contribute indirectly to a predisposition for UTI [1].
Although persistent E. coli ASB is more common in diabetics than in non-diabetics, it is unknown whether the bacteriuria is caused by the same E. coli strain, or what the effect of treatment is on the carriage of genetically similar or different E. coli strains. We conducted the present study to characterize urinary E. coli isolated from diabetic women in Manitoba, Canada, to determine if successive isolates from the same individual were genetically similar, and whether infecting organisms have a distribution of virulence genes similar to that of uropathogenic E. coli or to normal bowel flora E. coli.
METHODS
Patient Population
Diabetic women over the age of 16 were identified from 1991–1997 through ambulatory endocrinology clinics in Manitoba, Canada. 105 asymptomatic women with bacteriuria in two consecutive urine samples, obtained within a two week period, were enrolled into a prospective, randomized trial of antimicrobial or no antimicrobial treatment for ASB. Subsequently, urine specimens were obtained at least every 3 months, or more frequently after treatment or if symptoms occurred. Women were followed up to a maximum of 36 months. The original study was undertaken to determine whether there were any benefits with screening for and treatment of, ASB in diabetic women.
Women randomized to treatment received antimicrobial therapy for initial ASB, any subsequent ASB identified on 3 monthly screening, and any symptomatic infections. Women randomized to no treatment received treatment only for symptomatic urinary infection. All women received antimicrobials as ordered by their physicians for other indications. The trial design and patient population have been described in detail elsewhere [3].
We provide here further observations on E. coli isolates from a subgroup of 70 women who had at least two positive E. coli cultures during the study period. All E. coli isolates (517) from asymptomatic and symptomatic women in both study arms were typed for genetic similarity using ERIC–PCR. Genetically unique strains (238) from each individual were then analyzed for known UTI-associated virulence genes. We weighted data analyses for the length of follow-up time and interval between visits by assuming that ASB extended up to the midpoint between visits on either side of each sample. We compared means using the student’s t test and ANOVA, and used chi-square tests to compare the proportions of virulence genes between diabetic women and isolates from otherwise healthy women. This study was approved by the University of Manitoba Conjoint Ethics Committee for Human subjects.
Definitions
Asymptomatic E. coli bacteriuria was defined as urine specimens with an E. coli culture of ≥ 105 CFU/ml colony forming units/milliliter of urine (cfu/ml) and no symptoms referral to the genitourinary tract. When symptoms consistent with infection were present, a urine specimen yielding an E. coli culture of ≥103 cfu/ml was sufficient to diagnose urinary infection.
Recurrent infection was defined as a urine specimen growing E. coli after a subject received antimicrobial therapy (either for ASB, symptomatic UTI or other indications) or following spontaneous resolution of ASB. Recurrent infection was considered relapse when the strain of E. coli isolated following antimicrobial therapy was similar to the pre-therapy strain by ERIC-PCR typing, and reinfection when a new strain was isolated. Infection was considered persistent when additional urine specimens yielded E. coli cultures of ≥105 cfu/ml among subjects who did not receive any antimicrobials for the duration of their follow-up period.
Molecular Typing
ERIC-PCR
Enterobacterial repetitive intergenic consensus (ERIC) sequences are highly conserved sequences found in intergenic regions of the genome in Enterobacteriaceae, but whose chromosomal location differs between species [13]. They are small in length (approximately 126 base pairs), and contain a central core inverted repeat. Although the function of these sequences is not fully known, amplification of these sequences by PCR allows clear distinction between different bacterial species and strains which contain these elements [13].
Briefly, cultures were grown overnight, lysed and the crude DNA lysate used for PCR under the following conditions: 94°C for 2 minutes, followed by 35 cycles of: 94°C for 30 seconds, 60°C for 1 minute, and 72°C for 4.5 minutes, with a 1 minute final extension at 72°C, using the ERIC primer: AAGTAAGTGACTGGGGTGAGCG in a thermal cycler (DNA Engine PTC-200, MJ Research, Inc.). UPEC sequenced strain E. coli CFT073 was used as a positive control. Samples were resolved on a 2% agarose gel (Figure 1).
Figure 1.
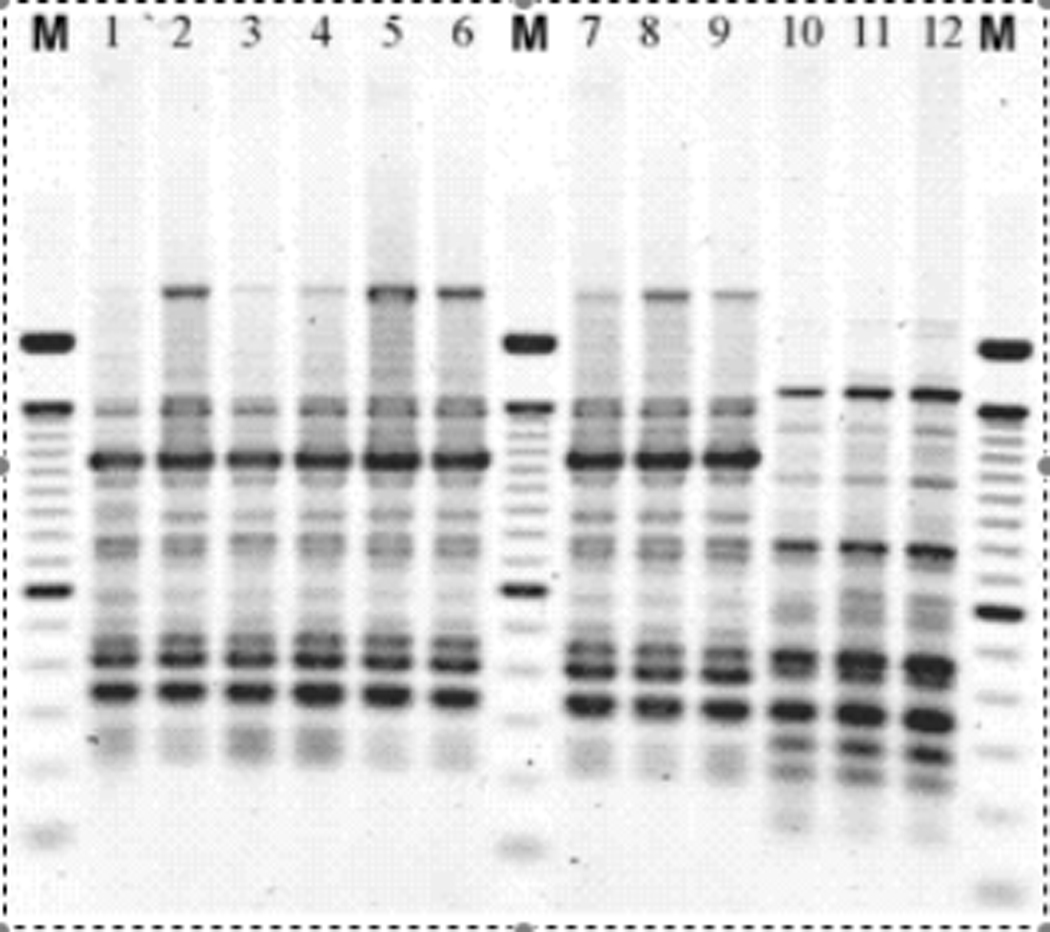
Agarose gel of ERIC-PCR patterns from E. coli. Lanes marked M contain a molecular weight marker. Lanes 1-9 are isolates from one individual. Lanes 10–12 are from a different woman.
The presence of a same size band of a similar intensity, identified using BioNumerics software (Applied Maths Inc., Austin, Texas), was used to compare isolates and create a dendogram using the unweighted pair group method with arithmetic averages (Figure 2). Strains were considered identical if they had 90% or greater similarity. Laboratory analyses were completed before clinical linkages between isolates were considered.
Figure 2.
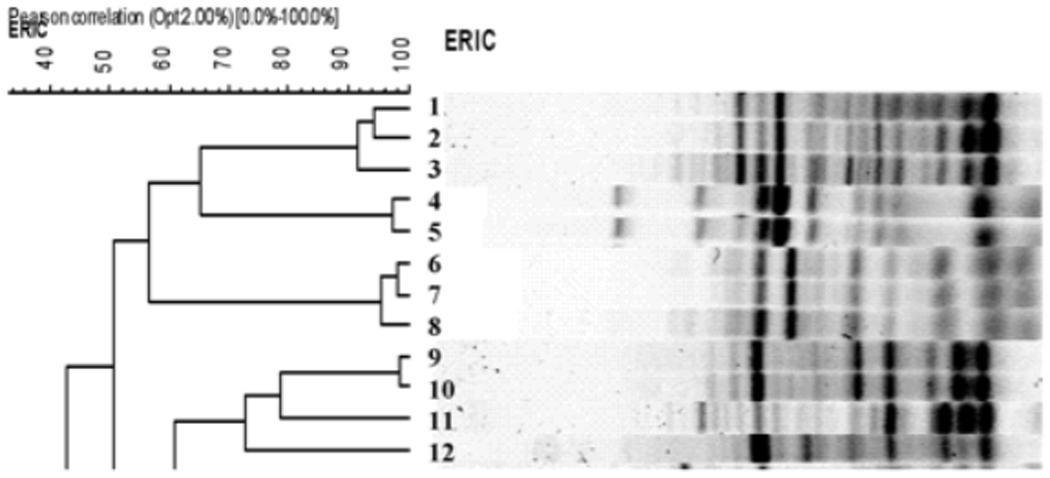
Dendogram showing grouping of isolates according to similarity in banding pattern of samples from 5 women using the unweighted pair group method with arithmetic averages (UPGMA) of BioNumerics software. Lanes 1–3 are samples from person 1, lane 4 from person 2, lane 5 from person 3, lanes 6, 7, 8 from person 4 and lanes 9–12 from the fifth person. ERIC: enterobacterial repetitive intergenic consensus sequence.
Dot Blot Hybridization
Briefly, dot blot hybridization involves fixing crude genomic DNA from each isolate on a nylon membrane using a Bio-Dot Microfiltration apparatus (Bio-Rad Laboratories, Hercules, CA). Fixed DNA were hybridized with a fluorescent labeled probe, the presence of which was detected using a fluorescein based system using the Amersham prime labeling and ECF detection system (Amersham, Arlington Heights, IL). A STORM 860 Phosphor Imager (Molecular Dynamics, Sunnyvale, CA) was used to scan the membranes to capture signal intensity of the image. The image was then analyzed using ImageQuant 5.2 software (Molecular Dynamics, Sunnyvale, CA). The signal of each isolate on the blot was expressed as a percentage of its respective positive control for each probe, after correcting for background signal [14]. Each isolate was tested in duplicate on independent membranes and any discrepancy found in a particular isolate was retested using either another dot blot or southern blot hybridization.
We tested 238 unique E. coli isolates from 70 women for the presence of the following virulence genes associated with UTI using dot blot hybridization: the P pili family (papF), its subclasses papGJ96, papGAD, prsGJ96, S fimbriae (sfa), the Dr family of adhesins drb, cytotoxic necrotizing factor 1 (cnf1), hemolysin (hly), aerobactin (iucD), Group II capsule (kpsMT) and outer membrane protease T (ompT). Virulence genes in the isolates from diabetic women were compared to previously published distributions among isolates collected from otherwise healthy Michigan women with a first UTI and recurring UTI, and periurethral and fecal isolates from healthy Michigan women [15].
RESULTS
The median age of the 70 women with E. coli ASB included in this analysis was 54 years (interquartile range (IQR): 43–64 years). Twelve women (17%) had bladder neuropathy, and 11/70 (16%) had prior genitourinary surgery. Overall, women had ASB for 36% of their follow-up time, with an average duration of bacteriuria of 2.6 months, and carried a unique single strain an average of 2.8 months (range: 0.6–13). Seventeen of the 68 (25%) women followed for six months or longer remained continuously colonized with a single strain for at least a six month period.
Of the 70 women, 36 were originally randomized to receive treatment and 34 to no treatment. Among the 34 women originally assigned to no treatment, 22 received treatment for symptomatic urinary infection or other indications at least once during the study period, and are referred to as women who received “symptomatic” treatment. Twelve women received no treatment for symptomatic UTI or other infections for the duration of their follow-up. Three of these 12 women had spontaneous resolution of E. coli ASB; one of whom was subsequently reinfected.
Women with either bladder neuropathy, prior genitourinary surgery, or both (N=20), had bacteriuria for 43% of their follow-up time compared to 26% for women without those conditions, although the difference was not statistically significant (p=0.1). They also did not carry a single strain for longer (2.5 months vs. 2.4 months, p=0.9), or have a different mean duration of E. coli bacteriuria than women without these conditions (2.2 months and 2.0 months; p=0.8).
There were no statistically significant differences in mean follow-up time between women who received treatment for ASB, symptomatic treatment only or no treatment (Table 1). However, treatment groups varied significantly in the mean proportion of follow-up time with E. coli bacteriuria (p<0.001), the mean duration of bacteriuria (p=0.04), and the mean length of carriage of a single strain (p=0.03) (Table 1). Specifically, women in the treatment group had bacteriuria for a lower proportion of their follow-up time, had shorter durations of bacteriuria and carried a single unique isolate for less time as compared to women who did not receive treatment. Women who received symptomatic treatment had a statistically lower proportion of their follow-up time with bacteriuria, but were not statistically different from women with no treatment in their duration of bacteriuria and length of carriage of a unique isolate.
Table 1.
E. coli bacteriuria, strain carriage, and recurrent infection among diabetic women with asymptomatic bacteriuria by treatment received.
| Treatment* N=36 |
Symptomatic treatment* N=22 |
No treatment* N=12 |
p-value | |
|---|---|---|---|---|
| Mean person-months of follow-up (IQR) | 26 (18–36 ) | 29 (20–36) | 27 (17–36) | 0.74 |
| Mean proportion of follow-up time with bacteriuria (IQR) |
0.29 (0.09–0.40) | 0.31 (0.16–0.40) | 0.66 (0.46–0.91) | <0.001 |
| Mean duration of bacteriuria in months (IQR) | 2.2 (1.6–2.9) | 2.5 (1.8–3.1) | 3.7 (1.31–5.4) | 0.04 |
| Mean length of carriage of a single strain in months (IQR) |
2.4 (1.6–2.8) | 2.8 (2.1–3.2) | 4 (1.6–6.6) | 0.03 |
| Mean number of times received treatment (IQR) |
3.2 (1–4) | 2.0 (1–3) | -- | 0.05 |
| Mean proportion of treatment courses followed by recurrent E. coli (IQR) |
0.76 (0.67–1) | 0.65 (0.5–1) | -- | 0.18 |
| Mean proportion of recurrence caused by reinfection**(IQR) |
0.64 (0.25–1) | 0.43 (0–1) | -- | 0.16 |
“Treatment” indicates women originally randomized to treatment for asymptomatic bacteriuria (ASB). “Symptomatic treatment” indicates women originally randomized to no treatment for ASB, but who received antimicrobials for symptomatic urinary infections or other indications over the course of their follow-up. “No treatment” indicates women originally randomized to no treatment for ASB and who did not receive any antimicrobials for the duration of their follow-up.
Reinfection is defined as recurrent E. coli that is genetically different from the pre-treatment strain.
Abbreviations: N=number of women, IQR = inter-quartile range.
On average, women assigned to treatment for ASB, received more antimicrobial courses than women who received only symptomatic treatment (Table 1). Among the 57 treated women with complete data, the majority of treatment regimens among women in the treatment group (76%) were followed by recurrent E. coli bacteriuria, most (64%) with a new strain of E. coli. Women who received treatment only for symptomatic infections also had frequent recurrent bacteriuria (65%). However, most (57%) were relapses with a strain genetically identical to the previous infecting strain. The differences between the two groups were not statistically significant, possibly due to small sample size.
The frequency of uropathogenic virulence characteristics among isolates causing ASB in diabetic women were not statistically different from the frequency found among fecal E. coli in healthy women (Table 2), except for cytotoxic necrotizing factor 1, which was higher. Three virulence factors, pff, kpsMT and ompT were found in significantly lower frequency than that seen in fecal strains.
Table 2.
Comparison of the frequency of E. coli virulence genes seen in asymptomatic bacteriuric (ASB) diabetic women with E. coli isolates from various other collections.
| Percentage of E. coli strains harboring the gene |
||||||
|---|---|---|---|---|---|---|
| Virulence Factor (Gene name ) |
ASB Diabetic women, Age >16 yrs (n=238) |
Fecal (no UTI) Age 18–39 yrs (n=269) |
First UTI Age 18–39 yrs (n=237) |
Recurring UTI Age 18–39 yrs (n=27) |
UTI Age 40–65 yrs (n=87) |
Periurethral Age 18–39 yrs (n=53) |
| Dr family of adhesins (drb) |
9.7 | 5.6 | 15.2 | 7.4 | 10.3 | 3.8 |
| P-pili family (pff) |
24.1 | 34.2 | 49.8 | 59.3 | 57.5 | 34.0 |
| Class I, P-pili (papGJ96) |
0.0 | 0.0 | 2.1 | 3.7 | 2.3 | 0.0 |
| Class II, P-pili (papGAD) |
18.5 | 23.1 | 27.0 | 37.0 | 31.0 | 11.3 |
| Class III, P-pili (prsGJ96) |
11.1 | 8.6 | 21.1 | 29.6 | 24.1 | 20.8 |
| S fimbriae (sfa) |
18.5 | 12.6 | 27.9 | 44.4 | 41.4 | 24.5 |
| Cytotoxic necrotizing factor 1 (cnf 1) |
18.3 | 10.0 | 26.6 | 40.7 | 29.9 | 24.5 |
| Hemolysin (hly) |
18.3 | 14.9 | 37.6 | 48.2 | 44.8 | 26.4 |
| Aerobactin (aer) |
40.6 | 41.3 | 46.0 | 40.7 | 37.9 | 26.4 |
| Outer membrane protease T (ompT) |
31.4 | 67.7 | 83.1 | 85.2 | 88.5 | 75.5 |
| Capsule, Group II (kpsMT) |
38.4 | 63.6 | 81.9 | 74.1 | 75.9 | 73.6 |
DISCUSSION
This study demonstrates that untreated diabetic women with ASB may carry a genetically unique E. coli strain for up to 13 months, whereas treated women had more frequent acquisition of new strains. Women who received treatment for ASB had bacteriuria for a shorter duration and carried a single strain of E. coli for a shorter period of time as compared to women who did not receive treatment. However, treatment was followed by recurrent infections for the majority of women, usually with a new strain of E. coli. The ASB-causing E. coli from diabetic women did not have virulence characteristics typical of UTI-causing strains.
Women in the treatment group received antimicrobial therapy an average of three times, but some received treatment up to 15 times over the course of the trial either for ASB, symptomatic infections or other indications. The high proportion of recurrent infections indicates that repeated treatment does not resolve asymptomatic bladder infection over the long-term for the majority of diabetic women who have frequent E. coli ASB. These findings are consistent with the results of the clinical trial from which patients in this analysis were selected, showing a much higher frequency of recurrent ASB in women who received treatment for ASB [3].
Whereas treated women had shorter time with bacteriuria but frequent reinfections, untreated, diabetic, asymptomatic bacteriuric women carried a single strain for longer periods of time with eventual clearance in a minority of patients. In a study of otherwise healthy women with ASB, less than 1% of women had ASB lasting for longer than 2 consecutive monthly cultures, 26% percent were colonized with the same strain, and persistent infection with the same strain over time was uncommon [16]. In contrast, our data from diabetic women with ASB showed long-term carriage of the same strain of E. coli over time (25% of women carried the same strain for at least a six-month period), and whether they received treatment or not, the majority had recurrent asymptomatic infections, symptomatic infections, or both. Interestingly, diabetic, asymptomatic bacteriuric women with conditions predisposing them to UTI (bladder neuropathy or prior genitourinary surgery), did not differ from ASB diabetic women without those conditions in the proportion of time that they were infected, the length of carriage of a single strain, or the average duration of a bacteriuric episode. The reason for this is unclear and may be a result of the small number of women in these groups, but warrants further research.
In uncomplicated UTI, infecting E. coli have a number of virulence factors that assist in their colonization of the urinary tract including a variety of adhesins, iron sequestration systems, and toxins [8]. Most of the published literature on ASB causing E. coli indicates that these strains are less virulent [17–19]. Recent molecular studies demonstrate that some ASB causing E. coli strains are non-virulent commensal strains, whereas others were originally virulent strains which have evolved to commensalism [20, 21]. We show that virulence characteristics of isolates from diabetic women with ASB were not different from those seen in fecal isolates. This low prevalence of virulence characteristics is consistent with previous reports among otherwise healthy individuals [22] and among diabetic women with ASB as compared to diabetic women with symptomatic UTI [23]. Only cytotoxic necrotizing factor 1 was found more frequently than in fecal E. coli, the presence of which has been associated with a decline in renal function in diabetic women [23]. Moreover, three virulence genes, pff, ompT and kpsMT, occurred at a significantly lower frequency than observed in our collection of fecal E. coli from healthy young women. These data combined with the strain carriage patterns that were observed, indicate that virulence characteristics typically found in UPEC are uncommon among isolates that infect the urinary tract in diabetic women with ASB. Thus, normal bowel inhabitants which do not invade the urinary tract under normal circumstances, may be capable of doing so in diabetic women, and can persist for long periods of time.
There are several limitations in this analysis. The ERIC-PCR technique has a degree of variability in the banding pattern intensity seen in strains. By running a variable control in each run, and by running large batches of isolates together, we attempted to minimize this variation. Most isolates from an individual were run in the same batch. Additionally, individuals may carry multiple E. coli strains, but the strain collection techniques only allowed for the collection of the predominant, morphologically distinct strain for genetic analysis. This could have resulted in an underestimation of strain turnover. Molecular typing and dot blot hybridization may not account for minor mutations arising in isolates over the long term. However, the small proportion of isolates in which this could have occurred is unlikely to have influenced the current analyses.
Our analyses of diabetic women with long-term ASB show that a diverse group of E. coli strains are capable of long-term urinary colonization in diabetic women. Recurrent infections were common after treatment, frequently with a new E. coli strain. The proportion of strains with UTI virulence characteristics was not significantly different from that seen in fecal strains from healthy women, indicating that in a predisposed host, additional bacterial aids for initiating infection are not a necessity.
ACKNOWLEDGEMENTS
The authors would like to thank Patricia Tallman and Joanna Brunka for the isolation and maintenance of E. coli strains. This work was supported by an award from the National Institutes of Health (RO1 DK55496 to CFM), and in Canada, National Health Research and Development Program number 6607-1618-502. The authors declare that they do not have any conflicts of interest.
Funding sources: This work was supported by an award from the National Institutes of Health (RO1 DK55496 to CFM), and in Canada, National Health Research and Development Program number 6607-1618-502.
Footnotes
We estimated duration of colonization and re-colonization among successively isolated E. coli from asymptomatic diabetic women and compared the virulence profiles to uropathogenic and commensal E. coli. Virulence factors of infecting strains were typical of commensal, not uropathogenic isolates.
REFERENCES
- 1.Stapleton A. Urinary tract infections in patients with diabetes. Am J Med. 2002;113 Suppl 1A:80S–84S. doi: 10.1016/s0002-9343(02)01062-8. [DOI] [PubMed] [Google Scholar]
- 2.Zhanel GG, Nicolle LE, Harding GK. Prevalence of asymptomatic bacteriuria and associated host factors in women with diabetes mellitus. The Manitoba Diabetic Urinary Infection Study Group. Clin Infect Dis. 1995;21(2):316–322. doi: 10.1093/clinids/21.2.316. [DOI] [PubMed] [Google Scholar]
- 3.Harding GK, Zhanel GG, Nicolle LE, Cheang M. Antimicrobial treatment in diabetic women with asymptomatic bacteriuria. N Engl J Med. 2002;347(20):1576–1583. doi: 10.1056/NEJMoa021042. [DOI] [PubMed] [Google Scholar]
- 4.Geerlings SE, Stolk RP, Camps MJ, et al. Consequences of asymptomatic bacteriuria in women with diabetes mellitus. Arch Intern Med. 2001 Jun 11;161(11):1421–1427. doi: 10.1001/archinte.161.11.1421. [DOI] [PubMed] [Google Scholar]
- 5.Ribera MC, Pascual R, Orozco D, Perez Barba C, Pedrera V, Gil V. Incidence and risk factors associated with urinary tract infection in diabetic patients with and without asymptomatic bacteriuria. Eur J Clin Microbiol Infect Dis. 2006 Jun;25(6):389–393. doi: 10.1007/s10096-006-0148-5. [DOI] [PubMed] [Google Scholar]
- 6.Meiland R, Geerlings SE, Stolk RP, Netten PM, Schneeberger PM, Hoepelman AI. Asymptomatic bacteriuria in women with diabetes mellitus: effect on renal function after 6 years of follow-up. Arch Intern Med. 2006 Nov 13;166(20):2222–2227. doi: 10.1001/archinte.166.20.2222. [DOI] [PubMed] [Google Scholar]
- 7.Ronald A, Ludwig E. Urinary tract infections in adults with diabetes. Int J Antimicrob Agents. 2001;17(4):287–292. doi: 10.1016/s0924-8579(00)00356-3. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Foxman B. Molecular epidemiology of Escherichia coli mediated urinary tract infections. Front Biosci. 2003;8:e235–e244. doi: 10.2741/1007. [DOI] [PubMed] [Google Scholar]
- 9.Johnson J. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991;4:80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geerlings SE, Stolk RP, Camps MJ, Netten PM, Collet TJ, Hoepelman AI. Risk factors for symptomatic urinary tract infection in women with diabetes. Diabetes Care. 2000;23(12):1737–1741. doi: 10.2337/diacare.23.12.1737. [DOI] [PubMed] [Google Scholar]
- 11.Geerlings SE, Meiland R, van Lith EC, Brouwer EC, Gaastra W, Hoepelman AI. Adherence of type 1-fimbriated Escherichia coli to uroepithelial cells: more in diabetic women than in control subjects. Diabetes Care. 2002;25(8):1405–1409. doi: 10.2337/diacare.25.8.1405. [DOI] [PubMed] [Google Scholar]
- 12.Boyko EJ, Fihn SD, Scholes D, Abraham L, Monsey B. Risk of urinary tract infection and asymptomatic bacteriuria among diabetic and nondiabetic postmenopausal women. American Journal of Epidemiology. 2005;161(6):557–564. doi: 10.1093/aje/kwi078. [DOI] [PubMed] [Google Scholar]
- 13.Hulton CS, Higgins CF, Sharp PM. ERIC sequences: a novel family of repetitive elements in the genomes of Escherichia coli, Salmonella typhimurium and other enterobacteria. Mol Microbiol. 1991;5(4):825–834. doi: 10.1111/j.1365-2958.1991.tb00755.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Gillespie BW, Marrs CF, Foxman B. Optimization of a fluorescent-based phosphor imaging dot blot DNA hybridization assay to assess E. coli virulence gene profiles. J Microbiol Methods. 2001;44(3):225–233. doi: 10.1016/s0167-7012(01)00222-6. [DOI] [PubMed] [Google Scholar]
- 15.Marrs CF, Zhang L, Tallman P, et al. Variations in 10 putative uropathogen virulence genes among urinary, faecal and peri-urethral Escherichia coli. J Med Microbiol. 2002;51(2):138–142. doi: 10.1099/0022-1317-51-2-138. [DOI] [PubMed] [Google Scholar]
- 16.Hooton TM, Scholes D, Stapleton AE, et al. A prospective study of asymptomatic bacteriuria in sexually active young women. N Engl J Med. 2000 Oct 5;343(14):992–997. doi: 10.1056/NEJM200010053431402. [DOI] [PubMed] [Google Scholar]
- 17.Holden NJ, Gally DL. Switches, cross-talk and memory in Escherichia coli adherence. J Med Micro. 2004;53:585–593. doi: 10.1099/jmm.0.05491-0. [DOI] [PubMed] [Google Scholar]
- 18.Wullt B, Bergsten G, Samuelsson M, Svanborg C. The role of P fimbriae for Escherichia coli establishment and mucosal inflammation in the human urinary tract. Intnl J Antimicrob Agents. 2002;19:522–538. doi: 10.1016/s0924-8579(02)00103-6. [DOI] [PubMed] [Google Scholar]
- 19.Hull RA, Rudy DC, Donovan WH, Wieser IE, Stewart C, Darouiche RO. Virulence properties of Escherichia coli 83972, a prototype strain associated with asymptomatic bacteriuria. Infect Immun. 1999 Jan;67(1):429–432. doi: 10.1128/iai.67.1.429-432.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zdziarski J, Svanborg C, Wullt B, Hacker J, Dobrindt U. Molecular basis of commensalism in the urinary tract: low virulence or virulence attenuation? Infect Immun. 2008 Feb;76(2):695–703. doi: 10.1128/IAI.01215-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klemm P, Hancock V, Schembri MA. Mellowing out: adaptation to commensalism by Escherichia coli asymptomatic bacteriuria strain 83972. Infect Immun. 2007 Aug;75(8):3688–3695. doi: 10.1128/IAI.01730-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vranes J, Kruzic V, Sterk-Kuzmanovic N, Schonwald S. Virulence characteristics of Escherichia coli strains causing asymptomatic bacteriuria. Infection. 2003 Aug;31(4):216–220. doi: 10.1007/s15010-003-2069-x. [DOI] [PubMed] [Google Scholar]
- 23.Geerlings SE, Brouwer EC, Gaastra W, Stolk R, Diepersloot RJ, Hoepelman AI. Virulence factors of Escherichia coli isolated from urine of diabetic women with asymptomatic bacteriuria: correlation with clinical characteristics. Antonie Van Leeuwenhoek. 2001;80(2):119–127. doi: 10.1023/a:1012263304999. [DOI] [PubMed] [Google Scholar]


