Abstract
Robotic lower limb exoskeletons have been built for augmenting human performance, assisting with disabilities, studying human physiology, and re-training motor deficiencies. At the University of Michigan Human Neuromechanics Laboratory, we have built pneumatically-powered lower limb exoskeletons for the last two purposes. Most of our prior research has focused on ankle joint exoskeletons because of the large contribution from plantar flexors to the mechanical work performed during gait. One way we control the exoskeletons is with proportional myoelectric control, effectively increasing the strength of the wearer with a physiological mode of control. Healthy human subjects quickly adapt to walking with the robotic ankle exoskeletons, reducing their overall energy expenditure. Individuals with incomplete spinal cord injury have demonstrated rapid modification of muscle recruitment patterns with practice walking with the ankle exoskeletons. Evidence suggests that proportional myoelectric control may have distinct advantages over other types of control for robotic exoskeletons in basic science and rehabilitation.
I. Introduction
Engineering teams around the world are currently developing many sophisticated robotic lower limb exoskeletons. Arguably, the most advanced and most internationally visible are Berkeley Bionics’ BLEEX, Cyberdyne’s HAL, and Raytheon Sarcos’ exoskeleton [1].
There are four main purposes for robotic exoskeletons in development: augmenting human performance, assisting with disabilities, studying human physiology, or re-training motor deficiencies. Augmenting human performance refers to exoskeletons that can give neurologically intact, healthy humans capabilities above and beyond what they currently have. This has usually been focused on increasing strength and/or enhancing endurance. Assisting with disabilities refers to exoskeletons that allow individuals with physical disabilities the ability to perform like a non-disabled individual. The goal is for the exoskeleton to provide benefits only when it is being worn. Studying human physiology refers to basic science experiments aimed at providing new insight into the biomechanics, neural control, and/or energetic cost of human movement. This is a relatively unexplored purpose but holds considerable potential for improving our understanding of how the human body works. Re-training motor deficiencies refers to the need to rehabilitate individuals with spinal cord injury, stroke, or other neurological disabilities. The goal is to have an individual use the exoskeleton during training so that the individual can then perform better without the exoskeleton later. Most exoskeleton developers have targeted the first two purposes as they hold the greatest potential for creating commercially viable products.
In the University of Michigan Human Neuromechanics Laboratory, we have focused on building simple lower limb exoskeletons to provide insight into human locomotion physiology and to be used as possible motor training aids after neurological injury [2–14]. Because these goals rely on having human subjects in a laboratory or clinic setting, it removes many challenging obstacles to creating functional robotic exoskeletons. First, our exoskeletons do not have to be self-contained. We can locate the power source and computer processor off the user as it does not have to be fully portable. This greatly reduces the weight of the material donned by the user. Second, because of the reduced material on the user, we can more easily make custom exoskeletons for each subject. This ensures a comfortable fit and good physical connection for transmitting mechanical forces to the user. Third, there are far fewer safety concerns in a laboratory or clinic compared to in the real world. Testing and use of the exoskeletons can be carefully constrained so that the possibility of incidents leading to injury is reduced.
In the following sections, we describe our exoskeleton hardware and controllers, summarize key experimental results on locomotor adaptation in neurologically intact subjects, and present example data from a subject with incomplete spinal cord injury undergoing motor re-training with an ankle exoskeleton.
II. Design
A. Artificial pneumatic muscles
We have primarily used artificial pneumatic muscles (sometimes called McKibben muscles) to actuate our exoskeletons. The pneumatic muscles were constructed using an expandable internal bladder (e.g. surgical tubing) surrounded by a braided polyester shell (e.g. wire sleeving). We inserted plastic pneumatic fittings inside the ends of the surgical tubing and steel hose clamps around the bladder and shell onto the outside of the pneumatic fittings. When the internal bladder is pressurized with air, it expands and produces tension due to the braided shell. The biggest advantages of the artificial pneumatic muscles are that they are very lightweight and can produce high power outputs. The biggest disadvantage of the artificial pneumatic muscles is that they are highly underdamped. This would make them poor choices for position control tasks, but not for human locomotion. The lower limbs have inherent damping about the joints and fine position control is not a priority for the lower limbs during locomotion.
The mechanical force properties of the pneumatic muscles have been quantified in detail [12, 15–17]. In isometric benchtop tests, the pneumatic muscles demonstrate a linear force-length relationship with their maximum force and length at resting length. Unlike human muscle, their force is virtually independent of velocity. The amount of force it takes to stretch the pneumatic muscle eccentrically is almost identical to the amount of force they produce when shortening. This characteristic increases safety of the pneumatic muscles for human interaction because it does not take relatively large forces to drive them backwards. Like human muscle, the maximum force of the pneumatic muscles is directly proportional to the cross sectional area. Increasing the size of the muscle or putting muscles in parallel increases the total force. Also like human muscle, the maximum contraction distance of a pneumatic muscle is directly proportional to the length of the muscle. Pneumatic muscles shorten approximately one-third of their resting length at maximum contraction.
Depending on the hardware and setup, artificial pneumatic muscles can also have activation dynamics very similar to human muscle. As used in the UM Human Neuromechanics Laboroatory, pneumatic muscles have a force bandwidth of 2.4 Hz [12]. This is similar to the force bandwidth of human muscles: 2.2 Hz [18]. The UM pneumatic muscles also have similar twitch mechanics and electromechanical delay as human muscle. The UM pneumatic muscles have a time to peak tension of 92 ms and a half relaxation time of 96 ms. Respective values for the human triceps surae are 101 ms and 94 ms [19] and for human tibialis anterior are 99 ms and 87 ms [20]. When activated using proportional myoelectric control, the electromechanical delay between muscle activity onset and initial rise in UM pneumatic muscle tension is 56 ms. This is comparable to the electromechanical delay values for human soleus and gastrocnemius (27 ms and 35 ms, respectively) [21]. In summary, most mechanical properties of the UM pneumatic muscles are reasonably similar to mechanical properties of human muscle with the exception of the force-velocity relationship.
B. Ankle exoskeleton
We concentrated on the ankle joint with our first design [2, 4] because the ankle is the joint with the most positive mechanical work performed during stance in human walking [22]. The second generation ankle exoskeleton had a bivalve design with plastic buckles for ease of donning and doffing (Figure 1). The shank section was made from carbon fiber for high stiffness and strength and the foot section was made from polypropylene for flexibility and comfort. A steel hinge joint between the shank and foot sections allowed ankle dorsiflexion and plantar flexion. Steel brackets were attached to the posterior shank and foot for connecting one or more artificial pneumatic muscles to provide plantar flexor torque [12]. The ankle exoskeleton was able to provide about 60 Nm or 57% of peak plantar flexor torque during human walking [12],
Figure 1.
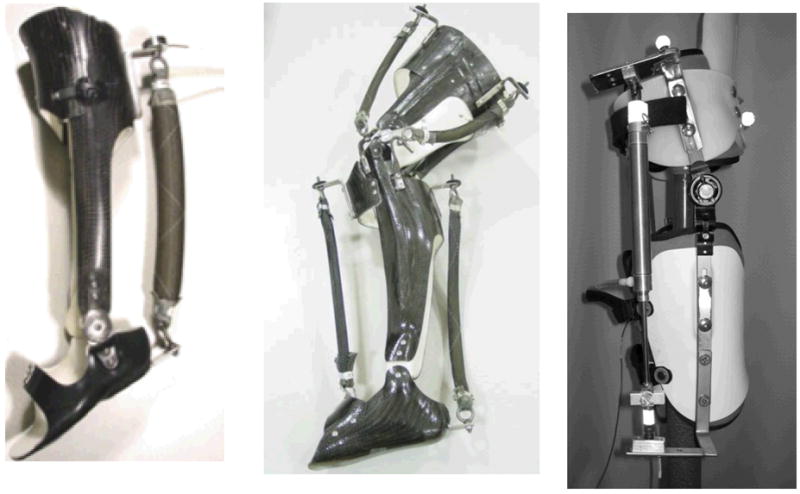
University of Michigan Human Neuromechanics Laboratory exoskeletons. Above left: ankle exoskeleton with artificial pneumatic muscle providing plantar flexor torque. Above middle: knee and ankle exoskeleton with artificial pneumatic muscles providing extensor and flexor torques. Above right: hip exoskeleton with a pneumatic cyclinder providing extensor and flexor torques.
C. Dorsiflexor assist exoskeleton
To study robotic assistance to dorsiflexion only, we designed a lighter weight exoskeleton composed of just a shank cuff and a foot section without a hinge joint [14]. Because peak dorsiflexor torque during gait is substantially less than peak plantar flexor torque, we were able to use polypropylene for both shank and foot sections. A steel bracket was attached to the anterior portion of the shank section and a steel hook to the dorsum of the foot section for connecting the pneumatic muscle. When activated, the pneumatic muscle provided approximately 20 Nm peak dorsiflexor torque during human walking (~130% of the peak biological net muscle torque).
D. Knee and ankle exoskeleton
We designed and tested a combined ankle and knee exoskeleton as well (Figure 1) [23]. It was essentially an extension of the ankle exoskeleton with the addition of a bivalve carbon fiber thigh section and two steel hinge joints at the knee [9]. The exoskeleton was equipped with artificial pneumatic muscles to provide ankle plantar flexor and dorsiflexor torque, and knee flexor and extensor torque. The exoskeleton produced approximately 42%–46% of the peak ankle plantar flexor moment, 83%–129% of the peak dorsiflexor moment, 22%–33% of the peak knee flexor moment, and 15%–33% of the peak extensor moment [23].
E. Hip exoskeleton
Recently, we have begun testing a hip exoskeleton design that can provide both hip flexor and hip extensor torque (Figure 1). We modified a prefabricated adjustable hip abduction brace (OPTEC, Inc, Lawrenceville, GA) to accommodate steel brackets and a pneumatic cylinder (Bimba Manufacturing, Monee, IL). The main benefit of using a modified prefabricated orthosis is that we can significantly reduce production time and cost. The hip exoskeleton consisted of a bivalve thigh cuff and a pelvic band, which we expanded to include a polypropylene lumbosacral support. The two sections are connected with an ATM™ joint which allows both flexion and extension, and abduction and adduction. The switch from a pneumatic muscle to a pneumatic cylinder was chosen for the hip because the artificial pneumatic muscles have a smaller range of active force production due to their force-length properties. The pneumatic cylinder, in contrast, can produce the same amount of force through the actuator’s range of motion. This is preferred to provide active torque generation through the full range of hip motion. The hip exoskeleton provides about 40 Nm or 43% of the peak hip flexion torque during walking.
III. Controllers
A. Hand held controls
One simple method for controlling air pressure in the pneumatic muscles is a hand held pushbutton controller [13]. When the pushbutton plunger was fully depressed, a control signal (10 V) was sent to the pressure regulator for maximal air pressure to the pneumatic muscle. When the pushbutton plunger is not depressed at all, no control signal (0 V) was generated and no air pressure was supplied to the artificial muscle. The controller was programmed to exhibit linear behavior proportional to the displacement of the plunger. This control scheme allowed volitional control over the pneumatic muscle by the user, rather than having a computer algorithm determine when and how much to activate the robotic exoskeleton torque. Our original study with this control mode examined gait training in individuals with incomplete spinal cord injury [13]. While this control mode had the advantage that it did not require a detectable muscle activation pattern in the plantar flexors, it had the disadvantage that it required higher level cognition. Our results indicated that the majority of the incomplete spinal cord injury subjects we tested could not readily learn to push the handheld buttons with reliable timing for adequate gait training [13].
B. Footswitch control
A second control scheme we have tested is a footswitch for simple state control [10]. With this control scheme, the pressure in the pneumatic muscle was controlled in a bang-bang mode based on the signal from a footswitch (B & L Engineering, Santa Ana, CA) placed in the subject’s shoe. When the footswitch signal indicated that the foot was on the ground, a control signal (10 V) was sent to the pressure regulator for maximal air pressure. When the footswitch signal was below threshold, no control signal (0 V) was sent and no air pressure was produced in the artificial muscle. Because the activation dynamics of the artificial pneumatic muscle included a force bandwidth of about 2.4 Hz [12], the actual joint torque produced by the ankle exoskeleton had a normal physiological rise and fall during stance in spite of the bang-bang control [10].
C. Proportional myoelectric control
Proportional myoelectric control could be considered a much more physiologic method for controlling robotic exoskeletons than the previous two methods presented above. In this control scheme, the air pressure delivered to the artificial pneumatic muscle was directly proportional to the electromyographic (EMG) signal from a biological muscle [2]. The EMG signal was high-pass filtered to remove movement artifact, full-wave rectified, and then low-pass filtered to smooth the signal (Figure 2). A threshold was then used to eliminate background electrical noise. Above that threshold, the control signal was directly proportional to the EMG signal up to a saturation level. When the EMG signal was below the threshold, no control signal (0 V) was generated. When the EMG signal was above the saturation level, a maximal control signal (10 V) was generated for maximal air pressure. Between the threshold and the saturation level, the control signal was directly proportional to the processed EMG signal amplitude. This method provides a direct link between the user’s nervous system and the exoskeleton torque, albeit a nonlinear one. Typically, the control muscle has been one with the same biomechanical function as the artificial muscle. For example, soleus muscle EMG was used to control plantar flexor torque from the ankle exoskeleton while tibialis anterior EMG was used to control dorsiflexor torque from the ankle exoskeleton [2, 4].
Figure 2.
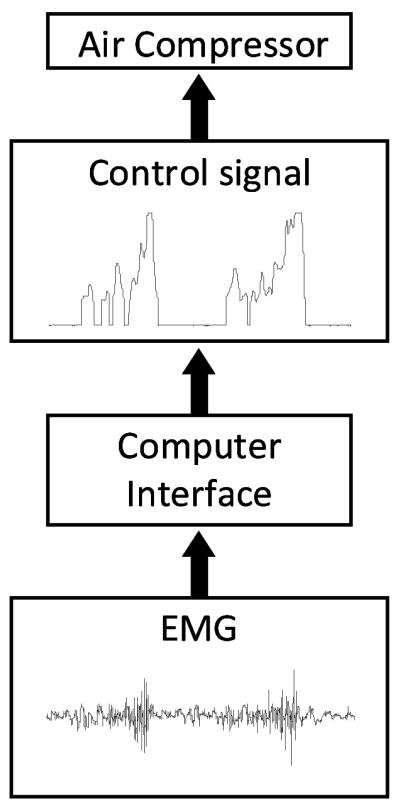
Schematic of the proportional myoelectric controller. Surface electromyography electrodes recorded muscle activation signals that were processed with high-pass filtering, rectification, and then low-pass filtering to yield a control signal for the air pressure regulator.
D. Proportional myoelectric control of antagonistic artificial muscles
When two artificial pneumatic muscles are used in opposition (e.g. one muscle providing ankle dorsiflexion torque and one muscle providing ankle plantar flexion torque), a simple modification can be made to the control strategy to facilitate exoskeleton use. Proportional myoelectric control allows for co-activation of the artificial muscles. When we have implemented simultaneous control of an artificial plantar flexor with soleus EMG and control of an artificial dorsiflexor with tibialis anterior EMG, subjects walked with significant co-activation [4]. The co-activation was eliminated by adding a control rule that inhibited artificial dorsiflexor activation when the soleus EMG exceeded a threshold. As a result, subjects found it was much easier to walk with a normal gait pattern compared to when there was substantial co-activation [4]. Use of this flexor-inhibition rule also made it much easier to walk with a combined knee and ankle exoskeleton with both artificial flexors and extensors at both joints [9]
IV. Locomotor Adaptation
A. Adaptation to robotic plantar flexor assistance
Humans can adapt their lower limb muscle activation patterns to control robotic plantar flexor assistance provided by the ankle exoskeleton in approximately forty-five minutes of walking [6]. When the artificial muscle was first turned on, the subjects demonstrated an immediate and substantial change in walking pattern. Subjects walked with increased plantar flexion so that they had no heel strike during stance. After practice walking with the ankle exoskeleton, subjects learned to substantially decrease soleus activation. The reduction in soleus activation decreased plantar flexor torque provided by the exoskeleton. This combined reduction in both biological and robotic plantar flexor torque allowed the subjects to walk with more normal kinematics. Following a second day of training for 30 minutes with the exoskeleton powered, subjects’ knee and hip kinematics returned to normal, and the ankle kinematics were close to normal [6].
B. Adaptation depends on the exoskeleton mechanics and not the biological control signal
When soleus EMG was used to control the robotic ankle exoskeleton, both the biological control muscle and the artificial pneumatic muscle had the same function. That is, both were uniarticular plantar flexor muscles. Subjects wearing the powered exoskeleton adapted by reducing soleus muscle activity preferentially over other biarticular extensor muscles (i.e. medial and lateral gastrocnemius) [6]. However, it is unclear if the reduction in activity was because soleus EMG was the control signal or because the artificial muscle was substituting for the mechanical function of the soleus muscle. In order to test this difference, Kinnaird and Ferris had subjects walk with the robotic ankle exoskeleton under the control of the medial gastrocnemius muscle [8]. The medial gastrocnemius is an ankle plantar flexor like the soleus, but also crosses the knee joint. Following two 30-minute training sessions, subjects reduced soleus muscle activity by 27% and only reduced medial gastrocnemius muscle activity by 12%. This preferential reduction in the biomechanical synergist to the exoskeleton indicates that the nervous system responded primarily to the mechanical assistance provided by the exoskeleton [8].
C. Adaptation to robotic dorsiflexor assistance
A recent study on dorsiflexor adaptation from our laboratory suggests that not all muscles reduce muscle activity with robotic assistance. Kao and Ferris studied adaptation of neurologically intact subjects to a flexion assist exoskeleton under tibialis anterior control [14]. During gait, the tibialis anterior muscle has two main activity bursts; one at heel strike to slow the progression of the foot to the ground, and a second at toe-off to assist with foot clearance during swing. Once adapted to walking with the powered dorsiflexion assist exoskeleton, subjects decreased the tibialis anterior activation during the first burst at heel strike by ~28%, but did not modify muscle activity during swing. Instead, subjects walked with significantly greater ankle dorsiflexion during swing. This difference in adaptation of the two bursts may indicate fundamental differences in the neural control of the tibialis anterior bursts. The additional flexor torque at heel strike resulted in increased ankle dorsiflexion and increased knee flexion. These significant changes in gait kinematics during stance may be a sufficient adaptation stimulus to modify muscle recruitment. In contrast, there is likely no penalty for exaggerated dorsiflexion during swing. As a result, the nervous system may not have a strong enough stimulus with added dorsiflexion during swing to modify muscle recruitment in the tibialis anterior.
D. The control method matters
Does the adaptation depend on the type of controller used? Cain et al. examined this question by comparing subjects training with either footswitch based control or proportional myoelectric control [10]. Both groups of subjects achieved steady state walking by the end of two 30-minute training sessions, but there were significant differences in the gait patterns chosen by the two groups. The group using proportional myoelectric control reduced soleus muscle activity and negative exoskeleton work more than the group using footswitch control. These differences in adaptation allowed the proportional myoelectric group to walk with ankle kinematics much closer to baseline than the footswitch control group [10]. Thus, the type of controller used in a robotic lower limb exoskeleton can have a substantial effect on the physical performance independent of the robotic hardware.
V. Motor Re-Training after Neurological Injury
We propose that proportional myoelectric control may provide a powerful stimulus to an impaired nervous system to alter its muscle recruitment patterns. Essentially, it gives the patient’s nervous system control over the magnitude and timing of robotic gait assistance rather than giving it to a computer algorithm. Engineers have used proportional myoelectric control in powered upper limb prostheses for decades [24–27]. However, it has not been extensively used as a means to control robotic devices for therapeutic purposes. There has been some success with electromyography-triggered electrical stimulation [28–32] and electromyography-triggered mechanical assistance [33], but neither approach provides a continuous relationship between muscle activation amplitude and intervention magnitude.
The rationale for proportional myoelectric control as a rehabilitative approach is grounded in the neural deficits underlying motor disorders. Neurological motor disorders often result in: 1) reduced volitional muscle activation amplitude, 2) impaired proprioception, and 3) disordered muscle coordination (e.g. inappropriately timed muscle activation). We propose that reduced volitional muscle activation and impaired proprioception contribute to disordered muscle coordination because the combination results in poor resolution for the nervous system to learn how muscle recruitment affects joint motion. Thus, the impaired nervous system has difficulty learning the proper muscle coordination strategy because of a poor signal-to-noise ratio identifying proper neuromechanical control [34].
The premise that amplifying the relationship between muscle activation and proprioceptive feedback can improve muscle coordination is supported by studies using error augmentation interventions. Increased movement error produced by mechanical perturbation [34, 35] or visuomotor distortion [36–38] help overcome poor resolution in relating efferent motor signals to resulting afferent feedback by amplifying afferent feedback. We suggest that robotic devices using proportional myoelectric control can increase the consequences of muscle activation, amplifying afferent feedback in a more physiologic manner. A similar rationale has been proposed for how brain-machine interfaces might enhance neurorehabilitation [39, 40]. Our robotic exoskeleton with proportional myoelectric control could be considered a brain-machine interface with a more distal connection to the nervous system. It augments muscle strength via a synergistic artificial pneumatic muscle under nervous system control. It is possible that a robotic device interfaced with the nervous system in this manner could improve muscle coordination by enhancing motor adaptation within the impaired nervous system.
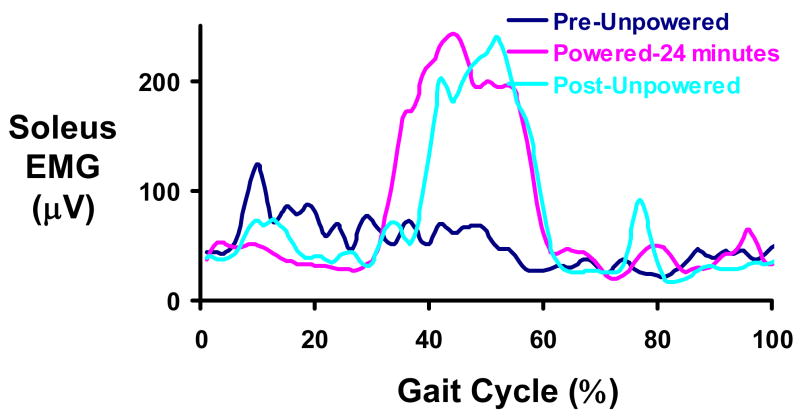
As an initial test of this idea, we have tested two subjects with incomplete spinal cord injury as they walk with the robotic ankle exoskeleton under proportional myoelectric control. Example data from one of the subjects is shown in Figure 3. After just 24 minutes of walking with a robotic ankle exoskeleton providing plantar flexor assistance, the subject had greatly altered the recruitment pattern of his soleus to achieve a strong pushoff at the end of stance. Unlike neurologically intact subjects that are provided too much torque from the combined biological and artificial muscles, the incomplete spinal injured subject was able to generate closer to normal plantar flexor torque with the ankle exoskeleton. The result was that the spinal cord injured subject increased muscle activity at pushoff to take advantage of the exoskeleton plantar flexion assistance. This improved recruitment pattern remained after the exoskeleton power was turned off.
Figure 3.
Mean soleus EMG patterns for a subject with incomplete spinal cord injury. The ASIA-D subject completed 24 minutes of walking with the robotic ankle exoskeleton providing plantar flexor assistance under proportional myoelectric control of the soleus. Prior to training with the exoskeleton powered (Pre-Unpowered, dark blue), the soleus EMG profile is abnormal in that it does not have an increase at the end of stance (40–65% of the gait cycle). After 24 minutes of walking with the powered exoskeleton (Powered-24 minutes, pink), the subject recruited soleus primarily in late stance. When the exoskeleton was turned off (Post-Unpowered, light blue), the soleus recruitment pattern remained.
VI. Conclusion
Mounting evidence suggests that proportional myoelectric control of robotic lower limb exoskeletons can be used to study locomotor adaptation in a novel manner. The control method is physiologically based and presents a perturbation very different from mechanical force fields. Preliminary data from our laboratory also suggest that proportional myoelectric control may also enhance motor re-training in neurologically impaired subjects because it augments the movement errors related to inappropriate muscle activation patterns.
Acknowledgments
The authors thank members of the Human Neuromechanics Laboratory for assistance in collecting and analyzing data. We also thank Jake Godak and Anne Manier for help with designing and fabricating the exoskeleton shells.
This work was supported in part by the National Institutes of Health (NS45486, NS062119, & HD055010), National Science Foundation (BES-0347479), and the Christopher Reeve Paralysis Foundation.
Contributor Information
Daniel P. Ferris, Email: ferrisdp@umich.edu, School of Kinesiology, University of Michigan, Ann Arbor, MI 48109-2013 USA (phone: 734-647-6878; fax: 734-647-2808;)
Cara L. Lewis, Email: caralew@umich.edu, School of Kinesiology, University of Michigan, Ann Arbor, MI 48109-2214 USA
References
- 1.Mone G. Building the real Iron Man. Popular Science. 2008 April; [Google Scholar]
- 2.Ferris DP, Czerniecki JM, Hannaford B. An ankle-foot orthosis powered by artificial pneumatic muscles. Journal of Applied Biomechanics. 2005 MAY;21:189–197. doi: 10.1123/jab.21.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferris DP, Bohra ZA, Lukos JR, Kinnaird CR. Neuromechanical adaptation to hopping with an elastic ankle-foot orthosis. Journal of Applied Physiology. 2006 Jan;100:163–70. doi: 10.1152/japplphysiol.00821.2005. [DOI] [PubMed] [Google Scholar]
- 4.Ferris DP, Gordon KE, Sawicki GS, Peethambaran A. An improved powered ankle-foot orthosis using proportional myoelectric control. Gait and Posture. 2006 Jun;23:425–8. doi: 10.1016/j.gaitpost.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Ferris DP, Sawicki GS, Daley MA. A physiologist’s perspective on robotic exoskeletons for human locomotion. International Journal of Humanoid Robotics. 2007;4:507–528. doi: 10.1142/S0219843607001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon KE, Ferris DP. Learning to walk with a robotic ankle exoskeleton. Journal of Biomechanics. 2007;40:2636–44. doi: 10.1016/j.jbiomech.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Sawicki GS, Ferris DP. Mechanics and energetics of level walking with powered ankle exoskeletons. J Exp Biol. 2008 May;211:1402–13. doi: 10.1242/jeb.009241. [DOI] [PubMed] [Google Scholar]
- 8.Kinnaird CR, Ferris DP. Medial gastrocnemius myoelectric control of a robotic ankle exoskeleton. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2009;17:31–7. doi: 10.1109/TNSRE.2008.2008285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawicki GS, Ferris DP. A pneumatically powered knee-ankle-foot orthosis (KAFO) with myoelectric activation and inhibition. Journal of Neuroengineering and Rehabilitation. 2009 doi: 10.1186/1743-0003-6-23. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cain SM, Gordon KE, Ferris DP. Locomotor adaptation to a powered ankle-foot orthosis depends on control method. Journal of Neuroengineering and Rehabilitation. 2007;4:48. doi: 10.1186/1743-0003-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawicki GS, Lewis CL, Ferris DP. It pays to have a spring in your step. Exercise and Sport Science Reviews. 2009 doi: 10.1097/JES.0b013e31819c2df6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon KE, Sawicki GS, Ferris DP. Mechanical performance of artificial pneumatic muscles to power an ankle-foot orthosis. Journal of Biomechanics. 2006 Jul 12;39:1832–41. doi: 10.1016/j.jbiomech.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Sawicki GS, Domingo A, Ferris DP. The effects of powered ankle-foot orthoses on joint kinematics and muscle activation during walking in individuals with incomplete spinal cord injury. Journal of Neuroengineering and Rehabilitation. 2006;3:3. doi: 10.1186/1743-0003-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kao PC, Ferris DP. Motor adaptation during dorsiflexion-assisted walking with a powered orthosis. Gait Posture. 2009 Feb;29:230–6. doi: 10.1016/j.gaitpost.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klute GK, Hannaford B. Fatigue characteristics of McKibben artificial muscle actuators. IEEE/RSJ International Conference on Intelligent Robots and Systems; Victoria, BC, Canada. 1998. pp. 1776–1782. [Google Scholar]
- 16.Klute GK, Czerniecki JM, Hannaford B. McKibben artificial muscles: pneumatic actuators with biomechanical intelligence. IEEE/ASME International Conference on Advanced Intelligent Mechatronics; Atlanta, GA. 1999. pp. 221–226. [Google Scholar]
- 17.Klute GK, Hannaford B. Accounting for elastic energy storage in McKibben artificial muscle actuators. Journal of Dynamic Systems, Measurement and Control. 2000;122:386–388. [Google Scholar]
- 18.Aaron SL, Stein RB. Comparison of an EMG-controlled prosthesis and the normal human biceps brachii muscle. American Journal of Physical Medicine. 1976 Feb;55:1–14. [PubMed] [Google Scholar]
- 19.Rice CL, Cunningham DA, Taylor AW, Paterson DH. Comparison of the histochemical and contractile properties of human triceps surae. European Journal of Applied Physiology and Occupational Physiology. 1988;58:165–170. doi: 10.1007/BF00636621. [DOI] [PubMed] [Google Scholar]
- 20.Connelly DM, Rice CL, Roos MR, Vandervoort AA. Motor unit firing rates and contractile properties in tibialis anterior of young and old men. Journal of Applied Physiology. 1999 Aug;87:843–852. doi: 10.1152/jappl.1999.87.2.843. [DOI] [PubMed] [Google Scholar]
- 21.Komi PV, Salonen M, Jarvinen M, Kokko O. In vivo registration of achilles tendon forces in man 1. methodological development. International Journal of Sports Medicine. 1987 Mar;8:3–8. doi: 10.1055/s-2008-1025697. [DOI] [PubMed] [Google Scholar]
- 22.Gitter A, Czerniecki JM, DeGroot DM. Biomechanical analysis of the influence of prosthetic feet on below-knee amputee walking. American Journal of Physical Medicine and Rehabilitation. 1991;70:142–8. doi: 10.1097/00002060-199106000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Sawicki GS, Ferris DP. A pneumatically powered knee-ankle-foot orthosis (KAFO) with myoelectric activation and inhibition. Journal of Neuroengineering and Rehabilitation. 2009 doi: 10.1186/1743-0003-6-23. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker PA, Scott RN. Myoelectric control of prostheses. Critical Reviews in Biomedical Engineering. 1986;13:283–310. [PubMed] [Google Scholar]
- 25.Scott RN. Myoelectric control of prostheses. Archives of Physical Medicine and Rehabilitation. 1966 Mar;47:174–81. [PubMed] [Google Scholar]
- 26.Scott RN, Parker PA. Myoelectric prostheses: state of the art. Journal of Medical Engineering & Technology. 1988 Jul–Aug;12:143–51. doi: 10.3109/03091908809030173. [DOI] [PubMed] [Google Scholar]
- 27.Sears HH, Shaperman J. Proportional myoelectric hand control: an evaluation. American Journal of Physical Medicine and Rehabilitation. 1991 Feb;70:20–28. doi: 10.1097/00002060-199102000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Bolton DA, Cauraugh JH, Hausenblas HA. Electromyogram-triggered neuromuscular stimulation and stroke motor recovery of arm/hand functions: a meta-analysis. Journal of Neurological Sciences. 2004 Aug 30;223:121–7. doi: 10.1016/j.jns.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Cauraugh J, Light K, Kim S, Thigpen M, Behrman A. Chronic motor dysfunction after stroke: recovering wrist and finger extension by electromyography-triggered neuromuscular stimulation. Stroke. 2000 Jun;31:1360–4. doi: 10.1161/01.str.31.6.1360. [DOI] [PubMed] [Google Scholar]
- 30.Chae J, Yu D. A critical review of neuromuscular electrical stimulation for treatment of motor dysfunction in hemiplegia. Assistive Technologies. 2000;12:33–49. doi: 10.1080/10400435.2000.10132008. [DOI] [PubMed] [Google Scholar]
- 31.Chae J, Fang ZP, Walker M, Pourmehdi S, Knutson J. Intramuscular electromyographically controlled neuromuscular electrical stimulation for ankle dorsiflexion recovery in chronic hemiplegia. American Journal of Physical Medicine and Rehabilitation. 2001 Nov;80:842–7. doi: 10.1097/00002060-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Chae J, Fang ZP, Walker M, Pourmehdi S. Intramuscular electromyographically controlled neuromuscular electrical stimulation for upper limb recovery in chronic hemiplegia. American Journal of Physical Medicine and Rehabilitation. 2001 Dec;80:935–41. doi: 10.1097/00002060-200112000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Dipietro L, Ferraro M, Palazzolo JJ, Krebs HI, Volpe BT, Hogan N. Customized interactive robotic treatment for stroke: EMG-triggered therapy. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2005 Sep;13:325–34. doi: 10.1109/TNSRE.2005.850423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patton JL, Kovic M, Mussa-Ivaldi FA. Custom-designed haptic training for restoring reaching ability to individuals with poststroke hemiparesis. Journal of Rehabilitation Research and Development. 2006 Sep–Oct;43:643–56. doi: 10.1682/jrrd.2005.05.0088. [DOI] [PubMed] [Google Scholar]
- 35.Patton JL, Stoykov ME, Kovic M, Mussa-Ivaldi FA. Evaluation of robotic training forces that either enhance or reduce error in chronic hemiparetic stroke survivors. Experimental Brain Research. 2006 Jan;168:368–83. doi: 10.1007/s00221-005-0097-8. [DOI] [PubMed] [Google Scholar]
- 36.Matsuoka Y, Brewer BR, Klatzky RL. Using visual feedback distortion to alter coordinated pinching patterns for robotic rehabilitation. Journal of Neuroengineering and Rehabilitation. 2007 doi: 10.1186/1743-0003-4-17. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brewer BR, Fagan M, Klatzky RL, Matsuoka Y. Perceptual limits for a robotic rehabilitation environment using visual feedback distortion. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2005 Mar;13:1–11. doi: 10.1109/TNSRE.2005.843443. [DOI] [PubMed] [Google Scholar]
- 38.Wei Y, Bajaj P, Scheidt R, PJL Visual error augmentation for enhancing motor learning and rehabilitative relearning. International Conference on Rehabilitation Robotics; Chicago, IL. 2005. pp. 505–510. [Google Scholar]
- 39.Dobkin BH. Brain-computer interface technology as a tool to augment plasticity and outcomes for neurological rehabilitation. Journal of Physiology (London) 2006 Nov 16; doi: 10.1113/jphysiol.2006.123067. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mussa-Ivaldi FA, Miller LE. Brain-machine interfaces: computational demands and clinical needs meet basic neuroscience. Trends in Neurosciences. 2003 Jun;26:329–34. doi: 10.1016/S0166-2236(03)00121-8. [DOI] [PubMed] [Google Scholar]