In this chapter, we take stock of the impact of systemic lupus erythematosus on the health-related quality of life (HRQOL) and employment of persons with this condition. Of course, far more than impaired health status can affect an individual’s quality of life. The term health-related quality of life is used to connote the decrement in an individual’s quality of life specifically attributable to a decrease in health status. In the chapter, we present evidence on employment because it plays a crucial role in determining the quality of life of the majority of Americans who are in the normal working ages. However, we also present evidence with respect to other domains of activity since most of us work to live but many of us don’t live to work.
Conceptualizing the impact of SLE on HRQOL is far more difficult than for rheumatoid arthritis (RA), let alone osteoarthritis (OA) or other non-systemic musculoskeletal conditions. In RA, as opposed to OA, one has to take into account the impact of profound fatigue beyond the obvious impact of symmetrical joint involvement and joint destruction. Symptoms like fatigue that are invisible to the observer may lead others to discount the impacts of the condition. The disconnect between what others perceive and what the person with RA perceives may be a source of psychological disturbance. Also, the uncertainty associated with an uneven course of illness can also take a toll on the individual, at the very least because it makes planning for the future difficult. In SLE, some of the same issues arise, but may be amplified because of the range of manifestations that may occur, adding complexity to invisibility of some symptoms and uncertainty of course.
Thus, measuring the impact of SLE on HRQOL may be a daunting challenge. However, it is nevertheless a propitious time to take stock of the impact of SLE on HRQOL. There is good evidence that improved treatment for SLE has resulted in decreased mortality associated with the condition, turning a condition frequently fatal into one in which concern about quantity of life has segued into a concern about its quality.
Reflecting decreased mortality, the literature on the impact of SLE on quality of life and employment has grown substantially in recent years. For example, a comprehensive literature review on employment and SLE 1 searched for articles on this topic from 1950 forward, but the earliest found was from 1994 and only another eight were published before the end of the 1990s. Since 2000, 18 more have appeared, with 11 of these published after 2005.
However, the most important reason to take stock of the impact of SLE on quality of life and employment is that, for the first time in memory, we are on the cusp of new treatments, particularly as the biological era in rheumatology expands to encompass SLE. By estimating the impact of SLE on quality on life now, we will be able to judge the impact of these new treatments as they diffuse into practice in the years to come.
We begin by providing a framework for discussing HRQOL in general so that when we review some of the literature on HRQOL on SLE, the reader can see how groups of studies address the different elements in the framework. Often, literature reviews encompass studies across the elements without clarifying how these studies relate to the elements. The framework outlined incorporates integrative activities such as employment as the end result of a process that begins with the onset of SLE (or at an even earlier stage, the risk factors for onset), permitting us to see the studies of employment in the same framework of the remainder of the HRQOL literature.
We pay special attention to the impact of SLE on employment among all of the integrative activities because SLE typically is diagnosed early in the career of those with the condition, perhaps limiting their ability to establish careers and, even if this is not the case, preventing them from gaining the kind of traction in work that normally occurs in the absence of a severe chronic disease.
Health Related Quality of Life
Throughout the course of their disease, individuals with SLE face considerable physical, psychological and social challenges. As long-term survival in SLE has become commonplace, outcome measures that move beyond mortality or medical morbidity to capture the patient’s perspective have become a critical aspect of appraising outcomes. Instruments that measure HRQOL attempt to characterize this subjective experience of illness. On many fronts, rheumatologists have pioneered the field of HRQOL measurement, dispelling notions that HRQOL is somehow not as important or valid as other traditionally used clinical end points. In fact, a growing body of literature demonstrates that HRQOL is a useful and valid end point for incorporation into clinical research and practice, and should be used alongside physician assessments and laboratory studies.
In SLE, considerable research has accumulated regarding HRQOL. We begin this section by defining the theoretical concepts underlying HRQOL and then, using a well-defined model, discuss what is known regarding HRQOL in SLE. We end the section with a brief discussion of the tools available to measure HRQOL in SLE and recommend a general approach to selecting a measure for use in clinical practice or research studies.
Defining HRQOL
Although there is no universally agreed upon definition of HRQOL, the last several decades of scientific research suggest that it should be viewed as a multi-dimensional construct. This approach ensures that health status and quality of life are examined distinctly, with quality of life representing a more global view of the patients’ social and psychological environment that may influence the response to illness.
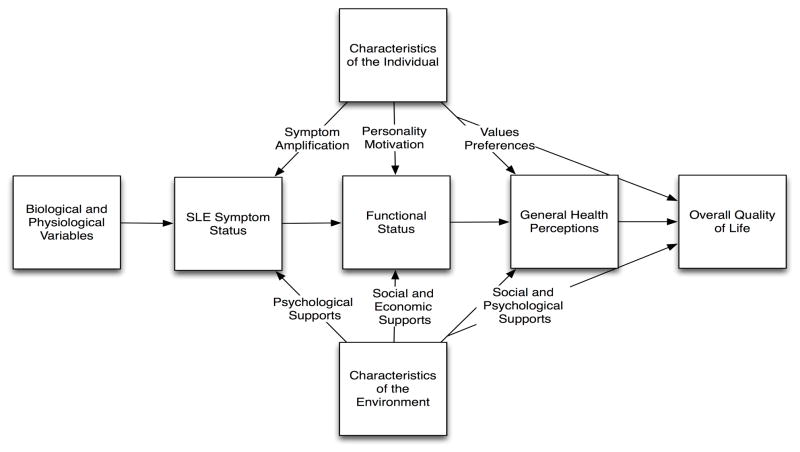
In 1995, Wilson and Cleary put forth a now classic model that emphasizes the multidimensional inputs to HRQOL (Figure 1)2. Although over a decade old, the model is still useful in thinking about the relationships among the conceptual areas represented. The model begins with biological and physiological variables, which in the case of SLE might reflect factors such as an individual patient’s genetic predisposition to disease, autoantibody production, and organ manifestations. Second, the model contains symptom status, which relates the patient’s perception of their symptoms. In SLE, this would include the physical, cognitive and emotional symptoms experienced by the patient. Third, functional status is assessed, which incorporates physical, psychological and social functioning. These preceding domains are related to the fourth concept, general health perceptions, which entail a subjective synthesis of preceding factors in the model. Finally, the last domain is overall quality of life, a global concept that may incorporate notions such as life satisfaction and overall ratings of quality of life. The model’s structure implies causal relationships between these content areas, with the dominant direction of causation proceeding from left to right.1
Figure 1.
Relationships among measures of patient outcome in a health-related quality of life conceptual model. Adapted from Wilson and Cle
In the next section, we will break down the components of the Wilson and Cleary model, highlighting examples of research in SLE that has examined the relationships between the relevant domains.
Biological and physiological variables and symptom status
The relationship between biological and physiological variables and symptoms (the patient’s subjective physical, emotional or cognitive state) is perhaps the most familiar concept to physicians and others involved in the clinical care of SLE. However, even this relationship proves surprisingly complex. Providers who care for patients with SLE intuitively realize this; in some patients, perceived symptoms correlate well with physician and laboratory assessments of disease activity. However, very frequently, patients experience symptoms in the absence of detectable disease activity or have no symptoms in the face of obvious disease activity.
Although a patient’s subjective report of symptoms correlates globally with formal disease activity measurements made by a physician, the correlation is only modest. This discrepancy is perhaps best illustrated by examining the growing literature examining the validity of self-report measures of disease activity, such as the Systemic Lupus Activity Questionnaire (SLAQ)6. For many items on the SLAQ, patient reports of symptoms correlate only weakly with physician assessments. For example, for skin disease the correlation coefficient comparing patient reported symptoms to physician assessments was 0.34, for arthralgia/arthritis 0.50, and for myalgia/myositis 0.27 6. In a validation study for the SLE Symptom Checklist (SSC), physician assessments of disease activity (as measured by the SLE Disease Activity Index (SLEDAI) or the physician global assessment) correlated only weakly to patient symptoms 7. Similarly, several studies have documented significant differences between patient and physician assessments of disease activity 8–10. For example, in the LUMINA cohort, 58% of patients had a significant disagreement regarding their disease activity compared to their physicians’ assessment 10.
Given that known biological and physiological parameters seem to only modestly affect individual perceptions of symptoms in SLE, what other factors are playing a role? As depicted in Figure 1, a variety of things are postulated to influence the perception of symptoms. In SLE, a few studies have attempted to further investigate these influences. Adams et al. found a relationship among psychological factors (stress, depression, anxiety, and anger) and SLE symptoms in a small study of 41 patients, particularly among a group whom they termed “stress responders” 11. Similarly, in a study that followed 23 patients with SLE prospectively every 2 weeks for up to 40 weeks, Ward et al. demonstrated that changes in a depression and anxiety scores were correlated with simultaneous changes in patients’ global assessments of their SLE activity 12. Other studies have also found that assessment of disease status by patients is influenced by their psychological well-being 8, 9.
Beyond understanding the multidimensional inputs into HRQOL, these findings have broader implications for patient care and medical care utilization. Addressing the symptoms that patients experience requires a comprehensive approach that reaches beyond overt biological and physiological parameters. Similarly, in trying to understand the cost impacts associated with SLE and why these differ significantly among patient groups, factors beyond assessments of disease activity must be considered.
Functional status
The next central area depicted in the Wilson and Cleary model is functional status. Functional status can be thought of broadly as a patient’s ability to perform a variety of activities, and encompasses not only physical function, but also social, role and psychological function. In Figure 1, symptoms are one important influence on functional status, but a variety of other inputs are often present. Again, for clinicians, this may be intuitive; two patients with similar SLE symptoms may have vastly different functioning. Social support, levels of helplessness, illness-related behaviors, environment, and access to medical care are just some of factors that may influence functional outcomes.
Decrements in functional status in SLE have been well documented. All domains of function appear to be influenced by the disease, although some appear more affected than others. Reductions in physical function in SLE are substantial compared to individuals with other chronic medical conditions (hypertension, diabetes, depression, myocardial infarction) and the general population 13–16, although appear less severe than in rheumatoid arthritis. In the LUMINA cohort, Alarcon et al. demonstrated that a variety of factors influence physical functioning in SLE beyond disease activity: lower socioeconomic status assessed at baseline predicted poorer physical functioning, as did higher degrees of helplessness, abnormal illness-related behaviors, and lower social support 17. Similarly, other studies have demonstrated that poor social support was associated with lower functional status 14, 18.
Social functioning, which is defined by normative behaviors in social situations, is also severely affected by SLE compared to the general population and to those with other chronic medical conditions; impairments in SLE are similar to individuals with depression 13–14, 16. In addition to higher disease activity, lower socioeconomic status, higher levels of helplessness, abnormal illness-related behaviors and poorer social support all predict lower social functioning17.
Using a novel measure set, valued life activities (VLAs; which are a wide range of life activities deemed to be important by the individual), that moves beyond the basic functional status items examined in the studies above, Katz et al. have demonstrated significant impairments in SLE. Discretionary VLAs, such as leisure activities, social activities, and hobbies were more severely affected by SLE than obligatory VLAs, such as basic self-care, driving a car or using transit 19. Although disease-related factors played a role, additional factors such as low educational attainment or cognitive impairment also influenced VLA impairment.
Reductions in psychological functioning in SLE are also substantial. Understanding the factors contributing to poor psychological function in SLE is complex, given that the disease itself has neuropsychiatric manifestations with direct effects on mood (e.g. cerebrovascular accidents, cortical inflammation, and seizures). Studies evaluating the relationship between disease activity and psychological functioning are mixed, and comparisons are difficult because findings seem to depend on the disease activity measure that was assessed. For example, several studies using the SLE Activity Index (SLEDAI) found no significant relationship with the psychological functioning domain of the SF-36 15, 20–21, although a study using the Mexican version of the SLEDAI did find a relationship 22. Most, but not all, studies that have used the British Isles Lupus Activity Score (BILAG) or the Systemic Lupus Activity Measure (SLAM) seem to demonstrate some relationship between disease activity and psychological functioning 23–24. In the LUMINA cohort, even after disease activity is accounted for, lower socioeconomic status, higher degrees of helplessness, abnormal illness related behaviors and poorer social support appear to have a role in psychological functioning 17.
The Wilson and Cleary model has directionality, implying that biological and physiological parameters are among the factors that lead to symptoms, and symptoms are among the factors that lead to decrements in functional status. Although the predominant causal relationships therefore run from left to right in the model, there may be instances where reverse relationships also exist (for example, depression leading to altered biological or physiological variables). Painting a more accurate picture regarding the multidimensional inputs into functional status will require further research; however, the growing literature cited above supports the view that a broad-based, multidisciplinary approach is required to characterize and understand functional impairments in SLE.
General Health Perceptions and Overall Quality of Life
As patients subjectively respond to the previous factors discussed in the model (symptoms, functional status, individual characteristics, and the environment), the more global concept of general health perceptions emerges. One of the fascinating aspects of subjective assessments of general health relate to their powerful predictive value. Numerous studies have demonstrated that self-rated health is a predictor of mortality, even when specific health status indicators and other relevant covariates that are known to predict mortality are taken into account25.
In SLE, studies have demonstrated that a significant proportion of patients rate their general health as poor. For example, in a study using three large observational cohorts, individuals with SLE were more likely to rate their health as poor (47%) compared to individuals with RA (37%) or COPD (40%) 26. Whether or not these ratings are associated with mortality in SLE as they are in a variety of other chronic health conditions requires further investigation, although preliminary data from one small study in Brazil found that self-rated health was among the predictors of mortality in a group of 63 patients 27.
When taken together, the studies discussed above illustrate the validity of the Wilson and Cleary model, and suggest that it provides a useful framework for thinking broadly about the concept of HRQOL in SLE.
Measuring HRQOL in SLE
In the section above, we have attempted to illustrate the multidimensional inputs into the concept of HRQOL. With this framework in mind, how do we go about assessing HRQOL in patients? A number of measures have been developed over the last several decades that attempt to measure HRQOL in SLE. However, as illustrated in Table 1, commonly used instruments cover a variety of different domains. Although there are several types of measures that fall under the general rubric of HRQOL, we will focus on two main categories below: generic HRQOL measures and SLE-specific measures. Other measures, such as utility-based measures (which incorporate preferences and are commonly used for economic evaluations), individualized measures (which allow patients to weigh the importance of items in their own life), and dimension-specific measures (which focus on a single area of HRQOL such as fatigue or depression) are not discussed here 28.
Table 1.
Examples of health related quality of life measures used in SLE.
| SLE-specific Measures | # Items | Domains | Scores derived | Item Responses | Score range | Administration | Time to complete |
|---|---|---|---|---|---|---|---|
| Lupus Quality of Life (L-QoL) 46 | 25 | Overall impact of SLE and its treatment on patient | Count of symptoms | Yes/No | 0–25 | Self-completed | <5 minutes |
| SLE Symptom Checklist (SSC) 7 | 38 | Checklist of disease and treatment related physical symptoms | Count of symptoms | Yes/No, followed by 4-point response for Yes responses | 0–38 | Self-completed | <10 minutes |
| SLE Quality of Life (SLEQoL) 30 | 40 | 6 (physical functioning, activities, symptoms, treatment, mood, self-image) | Summary score | 7-point response | 40–280 | Self-completed | Not reported |
| Lupus Quality of Life (LupusQoL)39 | 34 | 8 (physical health, emotional health, body image, pain, planning, fatigue, intimate relationships, and burden to others) | Subscale scores for the 8 domains | 5-point response | Scores are standardized to range 0 to 100 | Self-completed | <10 minutes |
| Generic Instruments | |||||||
| Medical Outcomes Study Short Form (SF- 36) 47 | 36 | 8 (physical function, physical role function, vitality, bodily pain, mental health, emotional role function, social function, general health perceptions) | Subscale scores for the 8 domains. 2 summary scores (Physical and Mental Component Scores) | Mixture of 3, 5 and 6-point response scales. | Scores are standardized to range 0 to 100. | Self-completed to interviewer- administered | 10–15 minutes |
| Quality of Life Scale (QOLs) 48 | 16 | 5 (material and physical well-being, relationships, social/community/civic activities, personal development and fulfillment, recreation) | Total score | 7-point scale | 16–112 | Self-administered or interview- administered | 5 minutes |
| Euopean Quality of Life Scale (EuroQoL) 49 | 5 | 5 (mobility, self-care, usual activities, pain/discomfort, anxiety/depression) and VAS for overall general health | 3 scores: a profile (five- digit descriptor indicating extent of problems in each domain), a population preference- weighted index, and VAS. | 3-level response and a VAS | Profile score: five-digit descriptor (lists scores ranging from 1 to 3 for all five dimensions, e.g. 33333) Index score: −0.11 to 1 |
Self-completed | 2 minutes |
| Sickness Impact Profile (SIP) 50 | 136 | 2 domains, 12 categories (sleep and rest, eating, work, home management, recreation and pastimes, ambulation, mobility, body care and movement, social interaction, alertness behavior, emotional behavior, communication) | 12 category scores, 2 domain scores and a total score | Respondents check items that describe them on a given day; items weighted to reflect the relative severity of each statement. | 0–100 | Self-completed or interviewer administered | 20–30 minutes |
| WHOQoL-Bref 51 | 26 | 4 (physical health, psychological health, social relationships, environment) and overall quality of life and health | 4 domain scores, raw scores can then be transformed to 0–100 scale | 5-level response | 0–100 | Self-completed or interview- administered | 10 minutes |
Generic instruments
A variety of generic measures are available, and several have been validated in SLE (Tables 1 and 2). Generic HRQOL measures generally include a variety of domains. For example, the most commonly used generic HRQOL instrument in SLE, the Medical Outcomes Study Short-Form 36 (SF-36), incorporates physical functioning, role limitations due to physical problems, bodily pain, general health, social functioning, mental health, role limitations due to emotional problems, and vitality.
Table 2.
Psychometric properties of commonly used HRQoL instruments in SLE.
| SLE-specific Measures | Construct validity | Internal consistency | Test-retest reliability | Floor and ceiling effects | Responsiveness |
|---|---|---|---|---|---|
| L-QoL 46 | ✓ | ✓ | ✓ | ||
| SSC 30, 52 | ✓ | ✓ | ✓ | ✓ | |
| SLEQoL 7, 52 | ✓ | ✓ | ✓ | ✓ | ✓ |
| LupusQoL 39, 53, 54 | ✓ | ✓ | ✓ | ✓ | |
| Generic Instruments | |||||
| SF-36 29, 30, 36, 55 | ✓ | ✓ | ✓ | ✓ | ✓ |
| QOLs 56 | ✓ | ✓ | ✓ | ||
| EuroQol 57, 58 | ✓ | - | ✓ | ✓ | ✓ |
| SIP 59 | ✓ | ✓ | ✓ | ||
| WHOQoL-Bref 60 | ✓ |
Checkmarks indicate that at least one published study has examined that psychometric property in patients specifically with SLE.
L-QoL=Lupus Quality of Life, SSC=SLE-symptom checklist, SLEQoL=SLE Quality of Life, LupusQoL=Lupus Quality of Life, SF-36=Medical Outcomes Study Short-Form 36, SIP=Sickness Impact Profile.
Generic instruments have significant advantages, but also notable disadvantages. A benefit is that they allow comparison of the HRQOL in one condition to other related conditions or to population norms, something that has been useful in documenting that SLE has similar or worse HRQOL decrements compared to other severe chronic conditions 13. In addition, many generic instruments have undergone validation testing, and may be available in different languages. The major drawback to generic instruments in SLE is that they may not capture symptoms or issues that are specific to the disease, and therefore, may have reduced sensitivity to detect meaningful changes over time. For example, there is some literature to suggest that the SF-36 is insufficiently sensitive to change in longitudinal studies 29–30 and may lack domains that are particularly relevant to a population with SLE, such as fatigue or sleep 31. In contrast, results from recent clinical trials show that the instrument may respond to change over the short-term 32–33 -- findings that emphasize the need to carefully examine the psychometric properties of an instrument before employing it in different demographic groups, regions or settings.
Generic instruments have been used for quite some time in observational studies of SLE, but the addition of these measures routinely to clinical trials is a relatively new development. Many recent studies, including trials investigating treatment with dehydroepiandrosterone 34, mycophenolate mofetil versus oral cyclophosphamide 35, abetimus sodium 36, and belimumab 33 have included HRQOL measures (and all used the SF-36 in addition to other measures). These trials have demonstrated that generic HRQOL measures may demonstrate responses to treatment that are not necessarily captured with traditional disease activity and damage assessments. As the use of these instruments increases, further information about their psychometric properties and how to interpret improvements or decrements in scores related to specific therapies will likely be forthcoming. Therefore, several groups have recommended the use of HRQOL measures as routine endpoints in SLE studies moving forward 37–38.
SLE-specific instruments
To date, four SLE-specific HRQOL instruments are available, although additional measures are in development (Table 1). As opposed to generic instruments, these measures were designed to measure HRQOL among individuals with SLE, and therefore focus on the specific challenges and issues important to patients with the disease. Some were developed with structured input from patients regarding how the disease has affected their lives. For example, McElhone et al. performed 30 face-to-face, recorded interviews with patients as the first step in developing items for the LupusQoL 39. Instruments such as this one therefore are likely to capture the concepts relevant to individuals with SLE more accurately. However, because notions of HRQOL can vary significantly among persons from different demographic groups or from different countries, further validation work is needed before application to settings where the instruments have not been tested.
As illustrated in Table 2, preliminary validation work has been done for some of these instruments in defined populations, although further work is needed. Such measures will likely have a place in SLE studies moving forward, although as mentioned above, their use precludes comparing HRQOL across conditions or in the general population.
Choosing a HRQOL measure in SLE
In the previous two sections, we have outlined a conceptual overview of HRQOL in SLE and briefly discussed available instruments and their characteristics. In this section, we will summarize the relevant issues in actually selecting a HRQOL instrument for use in clinical practice or research.
Below, we list four questions to consider when selecting an instrument:
Are the domains covered by the instrument relevant to the question or use at hand? As illustrated in Table 1, available HRQOL instruments in SLE cover a variety of different domains. Given the complexity and multiple inputs to HRQOL (Figure 1), available measures are unlikely to capture all relevant concepts. Increasingly, SLE researchers are using several instruments concomitantly (generic instruments and disease-specific instruments) in clinical trials and population-based studies.
Have sufficient validation studies been performed to assure that the instrument is psychometrically sound (valid, reliable and responsive)? As illustrated in Table 2, HRQOL instruments currently used in SLE have undergone varying degrees of testing. Even when such testing has been performed, it is important to remember that validation of HRQOL is always a work-in-progress. A single validation study in a particular demographic group or region does not always seamlessly apply to other populations. Generally, the more validation studies available demonstrating similar psychometric properties, the more likely that the instrument will behave similarly in future applications.
Are there floor and ceiling effects that are relevant? A ceiling effect is when individuals with the best score may still have substantial HRQOL impairment that is not captured by the instrument. Alternatively, a floor effect is when patients with the worst score may deteriorate further (Table 2). In some cases, this lack of variability can seriously compromise the utility of the measurement.
What resources are available to assess HRQOL? All HRQOL instruments are subjective – that is, in attempting to capture the patient’s perspective, they must be reported by the patient him or herself. Several methods are available to achieve this objective. Commonly used methods include in-person interviews, telephone interviews and self-completed questionnaires. Therefore, choosing an instrument entails careful assessment of resources (in-person interviews are most expensive, self-completed questionnaires less expensive, and telephone interview methods somewhere in between) and yield (in-person and telephone interview-based methods generally have higher response rates while self-completed questionnaires have lower response rates). Attention to whether the instrument has been validated using the chosen administration route is also important (Table 1).
Employment
Although, as indicated in the introduction, work may not be central to the lives of all persons of working age, it is often the portal to activities that are central. For example, it may provide the resources to travel or partake in hobbies. It is also crucial to the accumulation of assets that can provide for an adequate standard of living in retirement. In the HRQOL scheme developed by Wilson and Cleary, employment would be captured by the functional status domain. Because of the integrative set of skills necessary to sustain employment, it would be categorized in the subdomain of social role participation.
The literature on work loss associated with chronic disease in general emanates disproportionately from medical researchers rather than labor market analysts. The latter tend to be more precise in defining employment in a manner consistent with national unemployment statistics, with the consequence that not all the studies use the same terms to estimate the employment rate and to provide consistent inclusion and exclusion criteria 1. However, the level of precision may not matter in SLE since the impact of this condition on employment is so great.
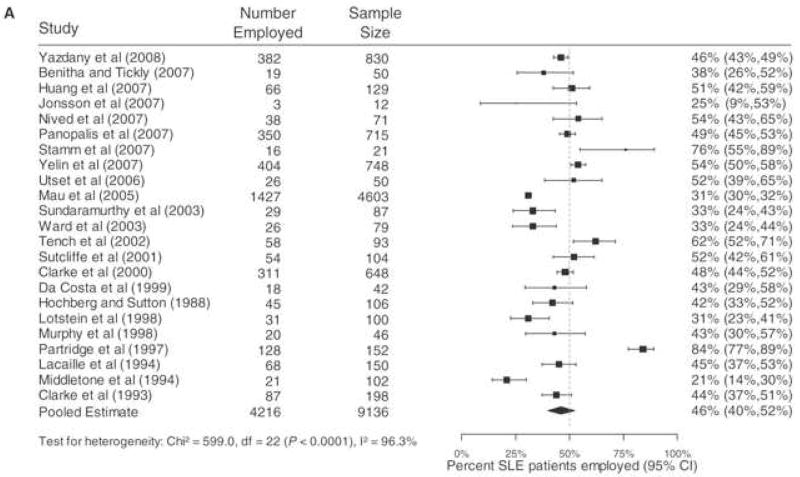
Figure 2, from the review article by Baker and Pope 1, summarizes the employment results from 23 studies. In all of the studies, the average age of the persons with SLE was between 34 and 47, usually the age range at which employment rates peak because almost all people have completed their educations. The late forties are the ages in which most of us have achieved seniority in jobs but have not yet been subjected to age-related job displacement. It is therefore telling that, on average, only 46 percent of persons with SLE reported being employed. The largest study 40 was from Germany, and reported one of the lowest employment rates. The next four largest studies used similar methods and reported employment rates of between 46 and 54% 41–44, consistent with the overall results. The overall results, disproportionately affected by the other large studies, indicate that just under half of working age adults with SLE are employed.
Figure 2.
Meta-analysis of percentage of SLE Patients employed. Adapted from Baker and Pope.1
How does that compare with employment rates among people without SLE? In the U.S. as a whole, in 2007, fewer than 80 percent of persons 45–54 were employed. In SLE, of course, the majority of those affected are women. In 2007, approximately 74 percent of women these ages were employed. Thus, the employment rate of persons with SLE is 38 percent lower than the rate among women 45 to 54, and 43 percent lower than all people these ages.
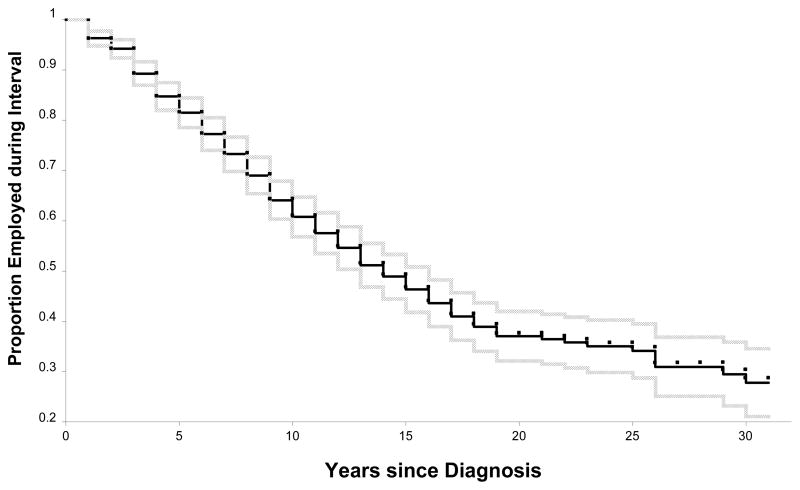
Overall employment rates mask the volatility of employment among persons with SLE. In two studies, we have estimated the frequency with which transitions between being employed and not employed occur.2 In the first study 44, we used retrospective data among those with SLE to estimate transitions in employment status between diagnosis of SLE and the study year, an average of slightly more than twelve years later. At diagnosis, 74 percent of the persons with SLE had been employed, but as of the study year, only 55 percent were employed. Accordingly, there was a substantial decline in the percentage employed. Figure 3, reprinted from that study, shows the percentage employed by the number of years since diagnosis among those employed at that time. By five years after diagnosis, 15 percent had stopped working; by ten, fifteen, and twenty years, just over a third, just over a half, and just under two-thirds had stopped working.
Figure 3.
Proportion employed (95% confidence interval), by year since diagnosis, among persons with systemic lupus erythematosus under age 65 who were employed at diagnosis. Adapted from Yelin, et al.44
Overall, among those employed at diagnosis, 41 percent had stopped working by the study year, an average of about 13 years after diagnosis. However, among the 26 percent not employed at diagnosis, 40 percent started working. Thus, despite the overall decline in the percentage working, there was substantial movement into employment as well as out of it.
In the second study 45, we tracked transitions in employment prospectively from the baseline year of a longitudinal cohort and compared the frequency of such transitions to those of a matched sample nationally. Interestingly, rates of work loss did not differ between persons with SLE and the matched sample until age 55. Presumably this is because in the U.S. labor market, transitions out of work are the norm. However, rates of work entry were lower among persons with SLE under age 55, suggesting that when they lose jobs, they are less likely to re-enter the labor market than their peers. Nor are they able to accommodate decreased ability to work by reduction in hours. Among all persons with SLE ever employed, annual work hours declined by about a third between the year of diagnosis and the study year, but such hours only declined by one percent among those continuously employed 44.
Thus, both because work entry once work loss occurs is less common than among their peers and because reduction in work hours is relatively uncommon, helping persons with SLE retain employment is crucial to their welfare.
In their review of the literature concerning work disability among persons with SLE, Baker and Pope 1 note that disease characteristics (higher levels of activity, longer duration, and select manifestations, particularly neurocognitive deficits); poorer physical function; demographics (age and race); lower socioeconomic status; and the nature of work (physically demanding work and jobs with high psychological demands and low levels of autonomy) all predispose to higher rates of work loss. To put these results in the context of the Wilson and Cleary model, all of the precursor domains, including biological and physiological variables, symptoms, as well as characteristics of individuals and of the environment contribute to employment outcomes.
In our prospective study of employment dynamics 45 we observed that persons with SLE who had been out of work a longer time were significantly less likely to enter new jobs, again indicating that helping persons with this condition to maintain employment may be the most effective strategy to reduce the work impacts.
Conclusion
SLE has a profound impact on HRQOL across a variety of domains, including symptoms, functional status, and general health perceptions, and results in significant reductions in employment. Current evidence supports the validity of examining HRQOL in SLE as a multidimensional construct influenced by a variety of individual characteristics, social circumstances and environmental factors. As further studies elucidate the factors that impact HRQOL, measurement tools that capture meaningful change in this important construct will likely be forthcoming and will play a valuable role in the evaluation of outcomes in SLE clinical care, observational studies and clinical trials. As these studies emerge, it will be helpful to evaluate them in the context of the model of HRQOL outlined by Wilson and Cleary so that the reader can situate the results of each study in the context of the pathway from biological and pathophysiological factors at one end of the spectrum, to integrative measures of overall quality of life at the other.
Acknowledgments
Multidisciplinary Clinical Research Center P60 AR053308, Arthritis Foundation, American College of Rheumatology Research and Education Foundation, and 5R01AR56476-7
Footnotes
The Wilson and Cleary model of HRQOL is remarkably similar to the Nagi model of work disability3–4, more recently amended by Verbrugge and Jette5 in which pathology, e.g. SLE, begets impairment, e.g. neuropsychiatric symptoms, which in turn begets functional limitations, e.g. executive function, before affecting employment. The Nagi model is used in employment or work disability research. For space reasons and because HRQOL would certainly include employment, in this chapter we focus on the Wilson and Cleary HRQOL model.
We have avoided using the term “unemployed” to connote not working because “unemployed” in the U.S. context means that one is not working but is actively looking for work. Most of those not employed are not actively looking for work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baker K, Pope J. Employment and work disability in systemic lupus erythematosus: a systematic review. Rheumatology (Oxford) 2009 Mar;48(3):281–284. doi: 10.1093/rheumatology/ken477. [DOI] [PubMed] [Google Scholar]
- 2.Wilson I, Cleary P. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. Jama. 1995 Jan 4;273(1):59–65. [PubMed] [Google Scholar]
- 3.Nagi S. An epidemiology of disability in the United States. Milbank Memorial Fund Quarterly. 1976;54:439–468. [PubMed] [Google Scholar]
- 4.Yelin E, Nevitt M, Epstein W. Toward an epidemiology of work disability. Milbank Memorial Fund Quarterly: Health and Society. 1980;58(3):386–415. [PubMed] [Google Scholar]
- 5.Verbrugge L, Jette A. The disablement process. Soc Sci Med. 1994 Jan;38(1):1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 6.Karlson EW, Daltroy LH, Rivest C, et al. Validation of a Systemic Lupus Activity Questionnaire (SLAQ) for population studies. Lupus. 2003;12(4):280–286. doi: 10.1191/0961203303lu332oa. [DOI] [PubMed] [Google Scholar]
- 7.Grootscholten C, Ligtenberg G, Derksen RH, et al. Health-related quality of life in patients with systemic lupus erythematosus: development and validation of a lupus specific symptom checklist. Qual Life Res. 2003 Sep;12(6):635–644. doi: 10.1023/a:1025176407776. [DOI] [PubMed] [Google Scholar]
- 8.Neville C, Clarke AE, Joseph L, Belisle P, Ferland D, Fortin PR. Learning from discordance in patient and physician global assessments of systemic lupus erythematosus disease activity. J Rheumatol. 2000 Mar;27(3):675–679. [PubMed] [Google Scholar]
- 9.Yen JC, Abrahamowicz M, Dobkin PL, Clarke AE, Battista RN, Fortin PR. Determinants of discordance between patients and physicians in their assessment of lupus disease activity. J Rheumatol. 2003 Sep;30(9):1967–1976. [PubMed] [Google Scholar]
- 10.Alarcon GS, McGwin G, Jr, Brooks K, et al. Systemic lupus erythematosus in three ethnic groups. XI. Sources of discrepancy in perception of disease activity: a comparison of physician and patient visual analog scale scores. Arthritis Rheum. 2002 Aug;47(4):408–413. doi: 10.1002/art.10512. [DOI] [PubMed] [Google Scholar]
- 11.Adams SG, Jr, Dammers PM, Saia TL, Brantley PJ, Gaydos GR. Stress, depression, and anxiety predict average symptom severity and daily symptom fluctuation in systemic lupus erythematosus. J Behav Med. 1994 Oct;17(5):459–477. doi: 10.1007/BF01857920. [DOI] [PubMed] [Google Scholar]
- 12.Ward MM, Marx AS, Barry NN. Psychological distress and changes in the activity of systemic lupus erythematosus. Rheumatology (Oxford) 2002 Feb;41(2):184–188. doi: 10.1093/rheumatology/41.2.184. [DOI] [PubMed] [Google Scholar]
- 13.Jolly M. How does quality of life of patients with systemic lupus erythematosus compare with that of other common chronic illnesses? J Rheumatol. 2005 Sep;32(9):1706–1708. [PubMed] [Google Scholar]
- 14.Sutcliffe N, Clarke AE, Levinton C, Frost C, Gordon C, Isenberg DA. Associates of health status in patients with systemic lupus erythematosus. J Rheumatol. 1999 Nov;26(11):2352–2356. [PubMed] [Google Scholar]
- 15.Gilboe IM, Kvien TK, Husby G. Health status in systemic lupus erythematosus compared to rheumatoid arthritis and healthy controls. J Rheumatol. 1999 Aug;26(8):1694–1700. [PubMed] [Google Scholar]
- 16.Zheng Y, Ye DQ, Pan HF, et al. Influence of social support on health-related quality of life in patients with systemic lupus erythematosus. Clin Rheumatol. 2009 Mar;28(3):265–269. doi: 10.1007/s10067-008-1033-7. [DOI] [PubMed] [Google Scholar]
- 17.Alarcon GS, McGwin G, Jr, Uribe A, et al. Systemic lupus erythematosus in a multiethnic lupus cohort (LUMINA). XVII. Predictors of self-reported health-related quality of life early in the disease course. Arthritis Rheum. 2004 Jun 15;51(3):465–474. doi: 10.1002/art.20409. [DOI] [PubMed] [Google Scholar]
- 18.Karlson EW, Daltroy LH, Lew RA, et al. The relationship of socioeconomic status, race, and modifiable risk factors to outcomes in patients with systemic lupus erythematosus. Arthritis Rheum. 1997 Jan;40(1):47–56. doi: 10.1002/art.1780400108. [DOI] [PubMed] [Google Scholar]
- 19.Katz P, Morris A, Trupin L, Yazdany J, Yelin E. Disability in valued life activities among individuals with systemic lupus erythematosus. Arth Care Res. 2008;59(4):465–473. doi: 10.1002/art.23536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gladman DD, Urowitz MB, Gough J, MacKinnon A. Fibromyalgia is a major contributor to quality of life in lupus. J Rheumatol. 1997 Nov;24(11):2145–2148. [PubMed] [Google Scholar]
- 21.Vu TV, Escalante A. A comparison of the quality of life of patients with systemic lupus erythematosus with and without endstage renal disease. J Rheumatol. 1999 Dec;26(12):2595–2601. [PubMed] [Google Scholar]
- 22.Khanna S, Pal H, Pandey RM, Handa R. The relationship between disease activity and quality of life in systemic lupus erythematosus. Rheumatology (Oxford) 2004 Sep 1;43(12):1536–1540. doi: 10.1093/rheumatology/keh376. [DOI] [PubMed] [Google Scholar]
- 23.Dobkin PL, Da Costa D, Dritsa M, et al. Quality of life in systemic lupus erythematosus patients during more and less active disease states: differential contributors to mental and physical health. Arthritis Care Res. 1999 Dec;12(6):401–410. doi: 10.1002/1529-0131(199912)12:6<401::aid-art8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 24.Saba J, Quinet RJ, Davis WE, et al. Inverse correlation of each functional status scale of the SF-36 with degree of disease activity in systemic lupus erythematosus (m-SLAM) Joint Bone Spine. 2003 Sep;70(5):348–351. doi: 10.1016/s1297-319x(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 25.Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav. 1997 Mar;38(1):21–37. [PubMed] [Google Scholar]
- 26.Katz P, Morris A, Gregorich S, et al. Valued life activity disability played a significant role in self-rated health among adults with chronic health conditions. J Clin Epidemiol. 2009 Feb;62(2):158–166. doi: 10.1016/j.jclinepi.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freire E, Bruscato A, Ciconelli R. Quality of life in systemic lupus erythematosus patients in Northeastern Brazil: Is health-related quality of life a predictor of survival for these patients? Acta Reumatol Port. 2009 Apr–Jun;34(2A):207–211. [PubMed] [Google Scholar]
- 28.Garratt A, Schmidt L, Mackintosh A, Fitzpatrick R. Quality of life measurement: bibliographic study of patient assessed health outcome measures. Bmj. 2002 Jun 15;324(7351):1417. doi: 10.1136/bmj.324.7351.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuriya B, Gladman DD, Ibanez D, Urowitz MB. Quality of life over time in patients with systemic lupus erythematosus. Arthritis Rheum. 2008 Feb 15;59(2):181–185. doi: 10.1002/art.23339. [DOI] [PubMed] [Google Scholar]
- 30.Leong KP, Kong KO, Thong BY, et al. Development and preliminary validation of a systemic lupus erythematosus-specific quality-of-life instrument (SLEQOL) Rheumatology (Oxford) 2005 Oct;44(10):1267–1276. doi: 10.1093/rheumatology/keh605. [DOI] [PubMed] [Google Scholar]
- 31.Moses N, Wiggers J, Nicholas C, Cockburn J. Prevalence and correlates of perceived unmet needs of people with systemic lupus erythematosus. Patient Educ Couns. 2005 Apr;57(1):30–38. doi: 10.1016/j.pec.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 32.Strand V, Aranow C, Cardiel MH, et al. Improvement in health-related quality of life in systemic lupus erythematosus patients enrolled in a randomized clinical trial comparing LJP 394 treatment with placebo. Lupus. 2003;12(9):677–686. doi: 10.1191/0961203303lu440oa. [DOI] [PubMed] [Google Scholar]
- 33.Wallace DJ, Stohl W, Furie RA, et al. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis Rheum. 2009 Sep 51;61(9):1168–1178. doi: 10.1002/art.24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nordmark G, Bengtsson C, Larsson A, Karlsson FA, Sturfelt G, Ronnblom L. Effects of dehydroepiandrosterone supplement on health-related quality of life in glucocorticoid treated female patients with systemic lupus erythematosus. Autoimmunity. 2005 Nov;38(7):531–540. doi: 10.1080/08916930500285550. [DOI] [PubMed] [Google Scholar]
- 35.Tse KC, Tang CS, Lio WI, Lam MF, Chan TM. Quality of life comparison between corticosteroid- and-mycofenolate mofetil and corticosteroid- and-oral cyclophosphamide in the treatment of severe lupus nephritis. Lupus. 2006;15(6):371–379. doi: 10.1191/0961203306lu2307xx. [DOI] [PubMed] [Google Scholar]
- 36.Strand V, Crawford B. Improvement in health-related quality of life in patients with SLE following sustained reductions in anti-dsDNA antibodies. Expert Rev Pharmacoecon Outcomes Res. 2005 Jun;5(3):317–326. doi: 10.1586/14737167.5.3.317. [DOI] [PubMed] [Google Scholar]
- 37.Bertsias G, Gordon C, Boumpas DT. Clinical trials in systemic lupus erythematosus (SLE): lessons from the past as we proceed to the future--the EULAR recommendations for the management of SLE and the use of end-points in clinical trials. Lupus. 2008;17(5):437–442. doi: 10.1177/0961203308090031. [DOI] [PubMed] [Google Scholar]
- 38.Gladman D, Urowitz M, Fortin P, et al. Systemic Lupus International Collaborating Clinics conference on assessment of lupus flare and quality of life measures in SLE. Systemic Lupus International Collaborating Clinics Group. J Rheumatol. 1996 Nov;23(11):1953–1955. [PubMed] [Google Scholar]
- 39.McElhone K, Abbott J, Shelmerdine J, et al. Development and validation of a disease-specific health-related quality of life measure, the LupusQol, for adults with systemic lupus erythematosus. Arthritis Rheum. 2007 Aug 15;57(6):972–979. doi: 10.1002/art.22881. [DOI] [PubMed] [Google Scholar]
- 40.Mau W, Listing J, Huscher D, Zeidler H, Zink A. Employment across chronic inflammatory rheumatic diseases and comparison with the general population. J Rheumatol. 2005 Apr;32(4):571–574. [PubMed] [Google Scholar]
- 41.Yazdany J, Yelin E, Panopalis P, Trupin L, Julian L, Katz P. Validation of systemic lupus erythematosus activity questionnaire in a large observational cohort. Arth Care Res. 2008;59(1):136–143. doi: 10.1002/art.23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panopalis P, Julian L, Yazdany J, et al. Impact of memory impairment on employment status in persons with systemic lupus erythematosus. Arth Rheum. 2007 December 15;57(8):1453–1460. doi: 10.1002/art.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clarke A, Penrod J, St Pierre Y, et al. Underestimating the value of women: Assessing the indirect costs of women with systemic lupus erythematosus. Journal of Rheumatology. 2000;27:2597–2604. [PubMed] [Google Scholar]
- 44.Yelin E, Trupin L, Katz P, et al. Work dynamics among persons with systemic lupus erythematosus. Arth Rheum. 2007 February 15;57(1):56–63. doi: 10.1002/art.22481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yelin E, Tonner C, Trupin L, et al. Work loss and work entry among persons with systemic lupus erythematosus: comparisons with a national matched sample. Arthritis Rheum. 2009 Feb 15;61(2):247–258. doi: 10.1002/art.24213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doward LC, McKenna SP, Whalley D, et al. The development of the L-QoL: a quality-of-life instrument specific to systemic lupus erythematosus. Ann Rheum Dis. 2009 Feb;68(2):196–200. doi: 10.1136/ard.2007.086009. [DOI] [PubMed] [Google Scholar]
- 47.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992 Jun;30(6):473–483. [PubMed] [Google Scholar]
- 48.Burckhardt CS, Anderson KL. The Quality of Life Scale (QOLS): reliability, validity, and utilization. Health Qual Life Outcomes. 2003;1:60. doi: 10.1186/1477-7525-1-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.EuroQol--a new facility for the measurement of health-related quality of life. The EuroQol Group. Health Policy. 1990 Dec;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 50.Bergner M, Bobbitt RA, Carter WB, Gilson BS. The Sickness Impact Profile: development and final revision of a health status measure. Med Care. 1981 Aug;19(8):787–805. doi: 10.1097/00005650-198108000-00001. [DOI] [PubMed] [Google Scholar]
- 51.The World Health Organization Quality of Life Assessment (WHOQOL): development and general psychometric properties. Soc Sci Med. 1998 Jun;46(12):1569–1585. doi: 10.1016/s0277-9536(98)00009-4. [DOI] [PubMed] [Google Scholar]
- 52.Leong KP, Kong KO, Thong BY, et al. Psychometric properties of a new systemic lupus erythematosus-specific quality-of-life instrument (SLEQOL) Ann Acad Med Singapore. 2004 Sep;33(5 Suppl):S35–37. [PubMed] [Google Scholar]
- 53.Gonzalez-Rodriguez V, Peralta-Ramirez MI, Navarrete-Navarrete N, Callejas-Rubio JL, Santos Ruiz AM, Khamashta M. Adaptation and validation of the Spanish version of a disease-specific quality of life measure in patients with systemic lupus erythematosus: The Lupus Quality of Life. Med Clin (Barc) 2009 Jul 30; doi: 10.1016/j.medcli.2009.04.045. [DOI] [PubMed] [Google Scholar]
- 54.Jolly M, Pickard AS, Wilke C, et al. Lupus specific health outcome measure for US patients: The LupusQoL- US Version(C) Ann Rheum Dis. 2009 Jan 6; doi: 10.1136/ard.2008.094763. [DOI] [PubMed] [Google Scholar]
- 55.Stoll T, Gordon C, Seifert B, et al. Consistency and validity of patient administered assessment of quality of life by the MOS SF-36; its association with disease activity and damage in patients with systemic lupus erythematosus. J Rheumatol. 1997 Aug;24(8):1608–1614. [PubMed] [Google Scholar]
- 56.Burckhardt CS, Archenholtz B, Bjelle A. Measuring the quality of life of women with rheumatoid arthritis or systemic lupus erythematosus: a Swedish version of the Quality of Life Scale (QOLS) Scand J Rheumatol. 1992;21(4):190–195. doi: 10.3109/03009749209099220. [DOI] [PubMed] [Google Scholar]
- 57.Aggarwal R, Wilke CT, Pickard AS, et al. Psychometric properties of the EuroQol-5D and Short Form-6D in patients with systemic lupus erythematosus. J Rheumatol. 2009 Jun;36(6):1209–1216. doi: 10.3899/jrheum.081022. [DOI] [PubMed] [Google Scholar]
- 58.Wang C, Mayo NE, Fortin PR. The relationship between health related quality of life and disease activity and damage in systemic lupus erythematosus. J Rheumatol. 2001 Mar;28(3):525–532. [PubMed] [Google Scholar]
- 59.Lash A. Quality of life in systemic lupus erythematosus. Appl Nurs Res. 1998 Aug;11(3):130–137. doi: 10.1016/s0897-1897(98)80132-8. [DOI] [PubMed] [Google Scholar]
- 60.Abu-Shakra M, Keren A, Livshitz I, et al. Sense of coherence and its impact on quality of life of patients with systemic lupus erythematosus. Lupus. 2006;15(1):32–37. doi: 10.1191/0961203306lu2255oa. [DOI] [PubMed] [Google Scholar]