Abstract
The antimalarial sesquiterpene, artemisinin, is in short supply; demand is not being met, and the role of artemisinin in the plant is not well established. Prior work showed that addition of dimethyl sulfoxide (DMSO) to seedlings increased artemisinin in their shoots and this study further investigated that serendipitous observation. When in vitro-cultured Artemisia annua rooted shoots were fed different amounts of DMSO (0–2.0% v/v), artemisinin levels doubled and showed biphasic optima at 0.25 and 2.0% DMSO. Both artemisinin and its precursor, dihydroartemisinic acid, increased with the former continuing 7 days after DMSO treatment. There was no stimulation of artemisinin production in DMSO-treated unrooted shoots. The first gene in the artemisinin biosynthetic pathway, amorphadiene synthase, showed no increase in transcript level in response to DMSO compared to controls. In contrast, the second gene in the pathway, CYP71AV1, did respond to DMSO but at a level of transcripts inverse to artemisinin levels. When rooted shoots were stained for the reactive oxygen species (ROS), H2O2, ROS increased with increasing DMSO concentration; unrooted shoots produced no ROS in response to DMSO. Both the increases in DMSO-induced ROS response and corresponding artemisinin levels were inhibited by addition of vitamin C. Together these data show that at least in response to DMSO, artemisinin production and ROS increase and that when ROS is reduced, so also is artemisinin suggesting that ROS may play a role in artemisinin production in A. annua.
Keywords: DMSO, Elicitation, Artemisinin, ROS
Introduction
“Why does the plant make artemisinin?” This frequently asked teleological question usually elicits an explanation suggesting defense against pathogens. Indeed artemisinin (Fig. 1), a potent antimalarial therapeutic, has considerable activity against many pathogens including viruses (Efferth 2007; Romero et al. 2005), parasites other than Plasmodium sp. (Jones-Brando et al. 2006; Utzinger et al. 2001; Merali and Meshnick 1991), and even cancer tumor lines (de Vries and Dien 1996; Nam et al. 2007; Singh and Lai 2004). However, its role in the plant, Artemisia annua, is rather circumspect. Here, we present evidence suggesting that it may be acting to some degree as a sink for reactive oxygen species (ROS).
Fig. 1.
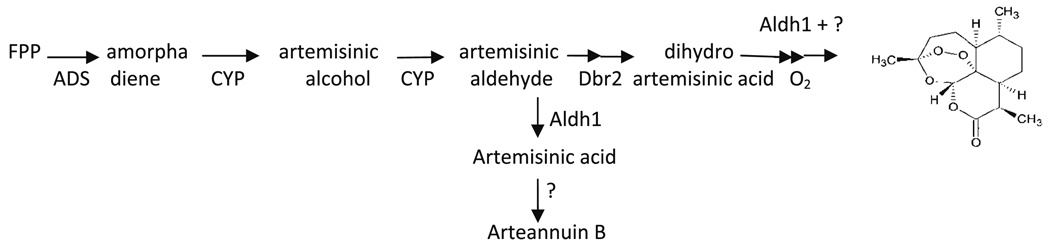
Artemisinin structure and simplified biosynthetic pathway. ADS amorpha-4,11-diene synthase, Aldh1 aldehyde dehydrogenase, CYP P450 CYP71AV, Dbr2 double bond reductase
Although artemisinin and its derivatives are the drugs of choice to treat malaria, supply is desperately short and there has been considerable effort to increase production not only in planta, but also by heterologous means (reviewed by Arsenault et al. 2008). Towards that goal we recently reported that addition of dimethyl sulfoxide, DMSO, to the roots of young axenic seedlings significantly increased artemisinin production in the shoots of A. annua (Towler and Weathers 2007).
Artemisinin is produced in shoots, sequestered to trichomes, and often reported at its highest level in association with flowering (Arsenault et al. 2008; Duke and Paul 1993; Ferreira and Janick 1995). However, despite the absence of artemisinin and its precursors (e.g. artemisinic acid) in the roots of A. annua (Ferreira and Janick 1996), this organ plays a key role in production of the terpene. Indeed, it has been shown, and we confirm here, that A. annua rooted shoots produce significantly more artemisinin than shoots lacking roots (Ferreira and Janick 1996). It is not known, however, how the roots influence production of artemisinin in the shoots.
Artemisinin is a sesquiterpene lactone that contains five oxygen molecules and is rather unique in its structure (Fig. 1). Although sesquiterpenes are in the end synthesized in the cytosol, we have shown through inhibitor studies, and others have verified with isotopic labeling, that this sesquiterpene uses 5-carbon prenyl building blocks originating from both the cytosolic and the plastidic terpene pathways (Towler and Weathers 2007; Schramek et al. 2007). Once farnesyl diphosphate is formed, it becomes committed to the artemisinin pathway when it undergoes cyclization via amorphadiene synthase (ADS) to form amorpha-4,11-diene (Fig. 1). Amorpha-4,11-diene is then converted in planta via two additional steps catalyzed by a cytochrome P450, CYP71AV1 (CYP), to artemisinic aldehyde. Subsequent steps to artemisinin have only recently had their enzymes isolated and mainly lead to dihydroartemisinic acid (Teoh et al. 2006, 2009; Zhang et al. 2008), with an alternative route leading instead to artemisinic acid (AA) and arteannuin B (AB) (Brown and Sy 2004, 2007). The prevalence of one pathway versus the other seems to determine various chemotypes of the plant (Fig. 1; Wallaart et al. 2000). The final step from dihydroartemisinic acid (DHAA) to artemisinin (AN) appears to involve a non-enzymatic photo-oxidative step to add the final three oxygen atoms to the molecule (Fig. 1; Wallaart et al. 1999; Sy and Brown 2002).
In plants DMSO has been reported in different species to both stimulate and inhibit protoplast division (Hahne and Hoffmann 1984; Carswell et al. 1989), stimulate cation uptake in bean (Bajaj et al. 1970; Schmid 1968), and elicit sesquiterpene production in Tessaria absinthioides cells (Kurina-Sanz et al. 2000), which can be synergistically increased in the presence of CuSO4 (Hernandez et al. 2005). In contrast, Bozom et al. (1998) showed DMSO decreased alkaloid accumulation in Catharanthus roseus. In animal cells, DMSO has been shown to have a myriad of activities including as an anti-inflammatory agent, a ROS scavenger, a modulator of cytokine activation, and a histone deacetylase inhibitor (Marks and Breslow 2007; also see review by Santos et al. 2003). DMSO is also reported to affect transcription (Chen and Zhang 2005), however, the mechanism(s) of action of DMSO in cells is not clear (Chen and Zhang 2005).
DMSO is a compound produced by marine and possibly other microbial species (Simó et al. 2000; Chen et al. 2000). Of particular interest here is that DMSO can act as both an oxidizing and a reducing agent, where it is reduced to dimethyl sulfide or oxidized to dimethyl sulfone, respectively; DMSO can also associate with unshared electron pairs of oxygen in alcohols (Kharasch and Thyagarajan 1983). Indeed DMSO can act as a “radical trap” functioning as an intermediate in radical transfer, especially of hydroxyl radicals, and at modest concentrations it appears to promote peroxidation (Kharasch and Thyagarajan 1983). Here, we describe results that show DMSO, reactive oxygen, and vitamin C affect the production of artemisinin in A. annua.
Materials and methods
Clone and culture conditions
Two grams fresh weight (FW) per flask of 14- to 21-day-old, 1- to 2-cm-tall shoots from cultures of A. annua [a Chinese (CH) variety, line PEG01, seeds a gift of CZ Liu] maintained on shooting medium, pH 5.8 [MS medium (Murashige and Skoog 1962) Phytotechnology Laboratories, cat # M404] containing 30 g L−1 d-sucrose (Phyto-technology cat # S391), 2.5 µM benzylaminopurine HCl (Sigma cat # B5920), 0.25 µM naphthaleneacetic acid (Sigma cat # N0640) and 5 g L−1 Agargel (Sigma cat # A3301)] were inoculated into 250-mL Erlenmeyer flasks containing 15 mL liquid rooting medium (1/2 MS medium with 20 g L−1 d-sucrose, pH 5.8) and placed on an orbital shaker at 90 rpm, at 24 µmol m−2 s−1 cool white fluorescent light on a 16:8 h photoperiod for 14 days at about 25°C. Roots usually appeared about 8 days after transfer to rooting medium.
DMSO additions to cultures
The effect of DMSO concentration and exposure time was tested using rooted shoot cultures. To measure the optimum concentration of DMSO on 14-day rooted cultures, the old liquid rooting medium was poured out, plantlets were washed three times with sterile distilled water, and 15 mL of freshly prepared 1/2 MS liquid rooting media was added with increasing concentrations of DMSO (0% control, 0.1, 0.25, 0.5, 1.0 and 2.0% v/v) fed to the roots of the A. annua plantlets. Flasks were placed again on an orbital shaker at 90 rpm for another 7 days under the same growth conditions. Plantlets were harvested after 7 days in DMSO (21 days from shoot inoculation) and FW of shoots and roots were taken. Dry weight (DW) was measured after tissues were dried in an oven at 60°C for 3 days. Artemisinin was extracted from dried shoot material and measured (µg g−1 shoot DW) by HPLC using the method of Towler and Weathers (2007).
To measure the kinetics of the optimum DMSO concentration on artemisinin production, shoots were inoculated, rooted, and grown as described above and fresh medium with 0 (control) or 0.25% (v/v) DMSO was fed to the roots of A. annua plantlets. Flasks containing the rooted shoots were placed on the orbital shaker at 90 rpm and harvested at 0, 1, 2, 3, 5, and 7 days post-DMSO treatment along with corresponding controls. Plantlets were separated into roots and shoots and FW was taken. Shoots were immediately extracted and assayed for artemisinin and three other related metabolites (AA, AB, and DHAA) in this case using liquid chromatography coupled to mass spectrometry (LC/MS). There were four replicates for each condition.
LC/MS analysis of artemisinic metabolites
Artemisia annua (0.5 g FW) ground in liquid nitrogen was extracted by sonication in toluene following the protocol of Towler and Weathers (2007). Extracts were semi-purified by flash chromatography through silica gel (60Å, 220–440 mesh) before being dried under a stream of nitrogen and resuspended in acetonitrile. Samples were separated using a high-pressure binary gradient system (Agilent) using a flow rate of 400 µL min−1, a Zorbax SB-C18 column (Agilent) (30 × 4.6 mm, 1.6 µm) with the following solvents: A, 5 mM ammonium formate; and B, 95% acetonitrile with 5 mM ammonium formate. Gradient elution was linear over 25 min beginning with 95% solvent A and 5% solvent B, and ending with 100% solvent B, followed by an equilibration time of 5 min in the beginning gradient solvent mixture. The mass spectrometer was set to detect each metabolite in SIM scan mode following AP-ESI. Relevant metabolites were identified via their retention times and mass spectra as compared to external standards (AN, Sigma Chemical, St. Louis MO; AA, Apin Chemical, Abingdon, UK; AB, Walter Reed Army Research Institute, Silver Spring MD; DHAA, synthesized by K. Erickson, Clark University). Quantification was done based on a standard curve of peak areas.
ROS assay and vitamin C counter effect
Two grams FW of 14- to 21-day-old, 1- to 2-cm-tall shoot cultures of A. annua were inoculated into 250-mL Erlenmeyer flasks containing 15 mL rooting medium (see above) and placed on an orbital shaker as previously described. At day 14 the medium was replenished with 0 or 2% (v/v) DMSO rooting medium. After 24 h incubation in DMSO, the fourth to seventh leaves from the shoot apical meristem (SAM) of each plantlet were harvested and stained for ROS following the modified procedure of Thordal-Christensen et al. (1997). Leaves were placed in a solution of 1 mg mL−1 (4.67 mM) 3,3′-diaminobenzidine-HCl (DAB, Sigma, cat #D-8001), pH 3.8 (a low pH is necessary in order to solubilize DAB) and incubated in the dark at 25°C for 7 h. To subsequently visualize the ROS stain, leaf chlorophyll was bleached by submersion in 96% (v/v) boiling ethanol for 5 min. DAB is specific for H2O2, which was visualized in the leaves as reddish brown spots under a light microscope. A 7-h incubation time in DAB was determined to be optimum for visualizing H2O2 stain.
To determine how DMSO affects ROS and whether vitamin C (L-ascorbic acid, AsA) alters that response, rooted 14-day-old plantlets were treated with 0, 0.25, 0.5 and 2% (v/v) DMSO for 24 h as previously described. The fourth to seventh leaves were again harvested and placed in DAB for 7 h, chlorophyll bleached out, and the leaves observed using light microscopy. In a second set of experiments, 14-day-old rooted shoots were exposed to 0 or 2% (v/v) DMSO, so chosen because there was a better visual ROS response than at lower concentrations. After 24 h, leaves of both treatments were harvested as described above, placed in DAB having 0, 10 or 20 mM AsA incubated for 7 h, chlorophyll bleached, and then visualized using light microscopy. Both 10 and 20 mM AsA in DAB had almost the same effect in reducing ROS response, so 10 mM AsA was selected for further analysis. To determine the combined effects of DMSO, 10 mM AsA, and pH (AsA decreases the pH of the media) on the ROS response in rooted shoots, plantlets were washed with sterile distilled water and 15 mL of freshly prepared rooting media with ±10 mM AsA and ±2% DMSO was added to the cultures. The experiment was performed at both pH 5.2 and 3.1 (after medium was autoclaved) to determine the effect of pH on ROS response after 24 h. Adjustments of pH of control media were through addition of either HCl or NaOH. It was previously determined that maximum artemisinin production occurred 72 h after incubation in DMSO, so after 72 h, a second set of identically treated cultures but growing only at pH 5.2 was also harvested for extraction and analysis by HPLC as previously described. Although pH had no effect on H2O2 staining, when grown longer than 24 h, plants appeared healthier at pH 5.2.
Analysis of ADS and CYP mRNA transcripts
To measure the effect of DMSO on mRNA expression, culture medium of 14-day-old plantlets was replaced with 15 mL freshly prepared liquid rooting media with either 0 or 0.25% DMSO (3 flasks/concentration/treatment) fed to the roots of A. annua plantlets. Flasks were placed on an orbital shaker at 90 rpm and samples were harvested for analysis of ADS and CYP at 1, 2, 3, 4 and 7 days after treatment with DMSO. ADS was also measured at 4, 8 and 16 h after DMSO addition.
For RNA isolation, 50–100 mg of ground plant tissue was homogenized with 1 mL TRIzol reagent (Invitrogen, Carlsbad, CA). The solution was incubated for 15 min at room temperature, then 200 µL of chloroform was added, shaken vigorously, and incubated for 15 min at room temperature. Cellular debris was removed by centrifuging at 12,000 × g for 10 min. The aqueous phase was transferred to a fresh 1.5-mL Eppendorf tube and mixed with 0.5 mL isopropanol. RNA was allowed to precipitate out of solution for 10 min and then centrifuged at maximum speed for 10 min. The pellet was washed briefly with 1 mL 75% ethanol and then allowed to air-dry. After 5 min, the pellet was resuspended in DEPC-treated water and the concentration of RNA was quantified at 260 nm.
To remove contaminating genomic DNA from the RNA samples, the Turbo DNA-free kit (Ambion, Austin, TX) was used following manufacturer instructions. A maximum of 10 µg of nucleic acid was added to a 50 µL DNase reaction, containing 1× DNase buffer, DEPC-treated water, and 4 U DNase. The reaction was incubated at 37°C for 30 min, after which an additional 4 U aliquot of DNase was added. The reaction was then incubated for another 30 min at 37°C.
RNA transcripts were reverse-transcribed into cDNA using the DyNAmo cDNA synthesis kit (New England Biolabs, Ipswich, MA) following manufacturer instructions. Random hexamers were used to randomly prime the RNA for cDNA synthesis instead of oligo-dT primers in order to concurrently reverse-transcribe the 18S rRNA transcripts with the mRNA. The reverse transcription reaction was incubated at 37°C for 1 h, and aliquots were added directly to subsequent PCR reactions.
Primers were designed for the two genes using Primer Select (Lasergene, DNAStar, Inc.) based on cDNA sequences specific for A. annua available at NCBI and are listed in Table S1 (online supplementary material). Primer pairs were designed to have similar melting temperatures and to amplify 200–300 bp fragments. PCR was performed with each primer pair to ensure sufficient and specific amplification.
Real-time PCR was performed using a Bio-Rad iCycler real-time PCR system. Reagents used were supplied as part of the iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) following manufacturer instructions. The protocol used was a three-step amplification followed by a melt-curve analysis. For each amplification cycle, there was a denaturation step at 94°C, an annealing step at 53°C, and an extension step at 72°C. Thirty-five cycles were used. Relative fold changes in gene expression were calculated based on the 2−ΔΔCT comparative method (Livak and Schmittgen 2001; Sehringer et al. 2005; Cikos et al. 2007). The 18S ribosomal small subunit gene was used as the normalizing factor. Normalized levels of target gene amplification in DMSO-treated plantlets were expressed as fold changes of target gene expression relative to normalized levels of target gene amplification for control experiments.
Statistical analyses
All experiments were done at least in triplicate, except for ROS staining for which there were 20 leaves reviewed per condition. Numerical data were statistically analyzed using ANOVA.
Results
Only rooted shoots of A. annua increase artemisinin in response to DMSO
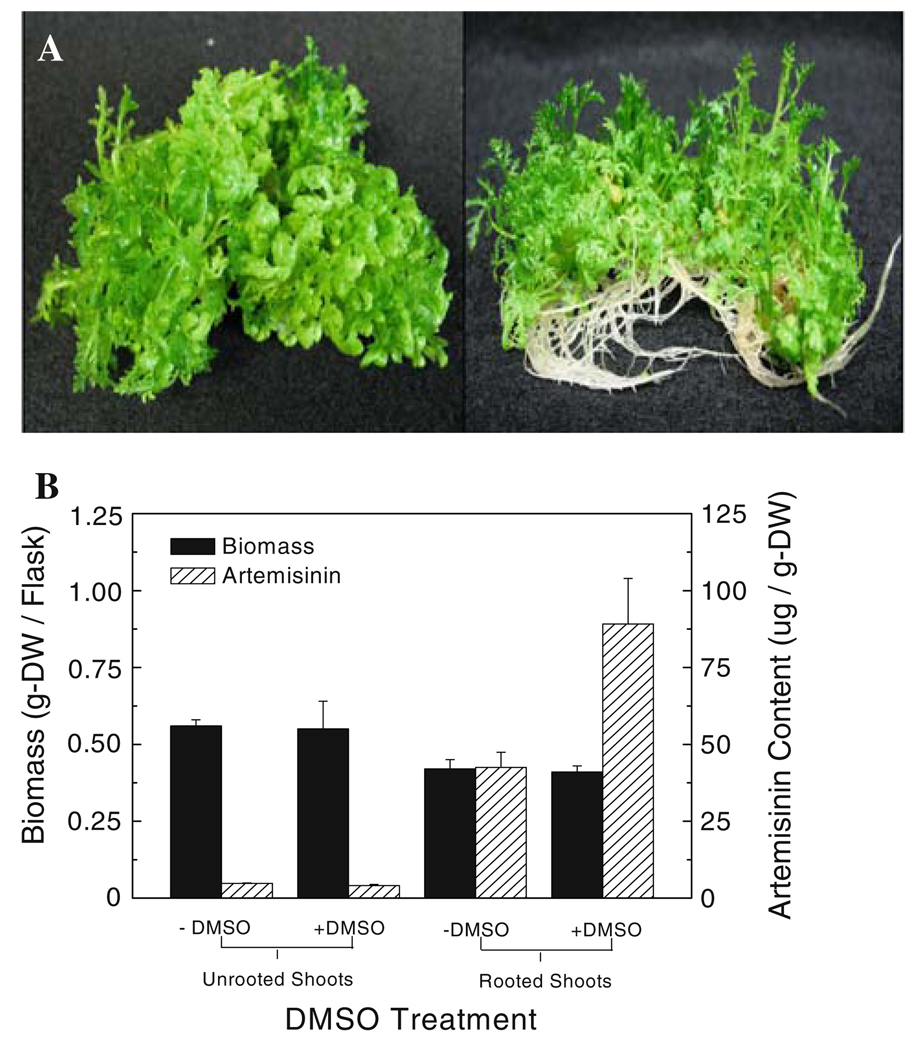
The roots of A. annua have been reported to play a key, but as yet unknown, role in the production of artemisinin in the shoots (Ferreira and Janick 1996). Previously, Towler and Weathers (2007) showed that when DMSO was added to young A. annua seedlings (Yugoslavian, YU, strain) grown in shake flasks, artemisinin production was significantly stimulated compared to controls. Subsequently, the foliage of A. annua plants grown in soil in a growth chamber was sprayed with DMSO to determine if artemisinin production was enhanced, but it was not (data not shown). Further experiments were done using in vitro cultures of a Chinese strain of A. annua in which rooting is readily controlled by changing the culture medium composition (Fig. 2a). The concentration of artemisinin in shoots of rooted cultures is about eight times that of unrooted shoots of this CH strain (Fig. 2b). When DMSO was applied, the rooted shoot cultures doubled their artemisinin yield (Fig. 2b). The total amount of artemisinin produced by rooted cultures treated with DMSO is thus almost 12 times that measured in unrooted shoots and about twice as much as rooted cultures without DMSO (Fig. 2b).
Fig. 2.
Only rooted shoots of A. annua respond to elicitation by 0.25% DMSO. a Left to right unrooted versus rooted A. annua shoots. b Biomass and artemisinin content of unrooted shoots and rooted shoots grown in shake flasks ± DMSO. Error bars are SE and n = 3
Effect of optimum DMSO concentration and exposure time on artemisinin production
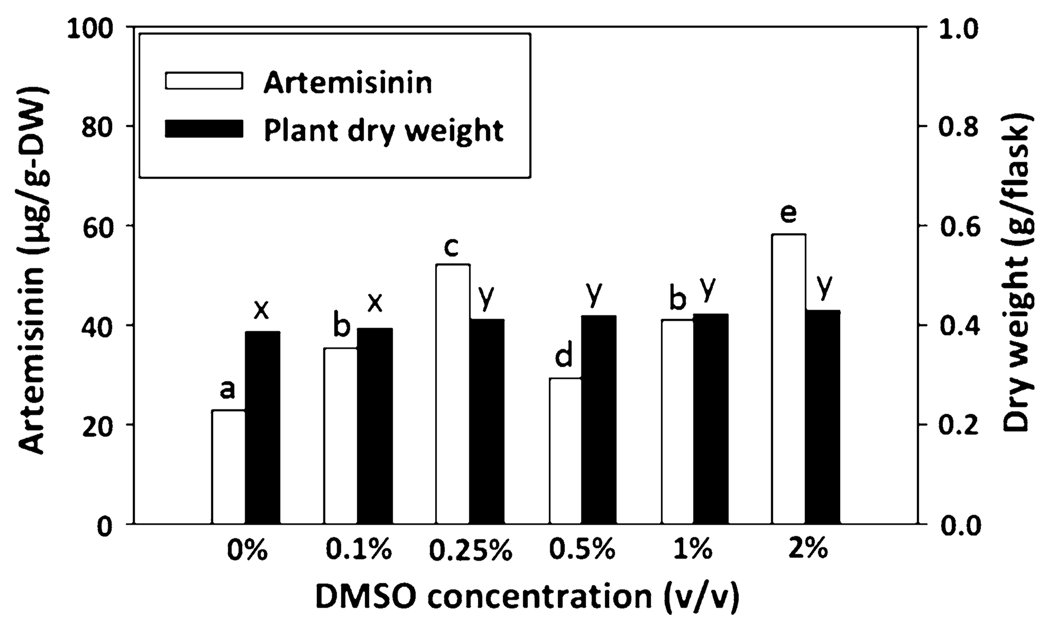
In our original study (Towler and Weathers 2007), seedlings of A. annua (strain YU) were exposed to 0.5% DMSO. In this study, rooted shoot cultures of a Chinese (CH) variety of A. annua were used to determine if there is an optimum DMSO concentration where growth was unaffected but artemisinin levels increased. Using concentrations of DMSO ranging between 0 and 2%, rooted shoot cultures of A. annua showed a biphasic response in artemisinin production compared to the untreated controls (Fig. 3). This biphasic response was reproducible in the hands of two different individuals. The first peak of artemisinin was at 0.25% DMSO and was 2.26 times that of the control (Fig. 3), while the second peak in production was at about the same yield, but was obtained with 2% DMSO (Fig. 3). In contrast, at 0.5% there was a significant decrease in artemisinin production relative to that at 0.25 and 2% (Fig. 3).
Fig. 3.
DMSO stimulates a biphasic response in artemisinin production in rooted shoots of A. annua. Rooted shoot cultures of A. annua were exposed to increasing concentration of DMSO [0, 0.1, 0.25, 0.5 1 and 2% (v/v/) DMSO] for 7 days. Letters show statistical significance at P = 0.051; n = 4
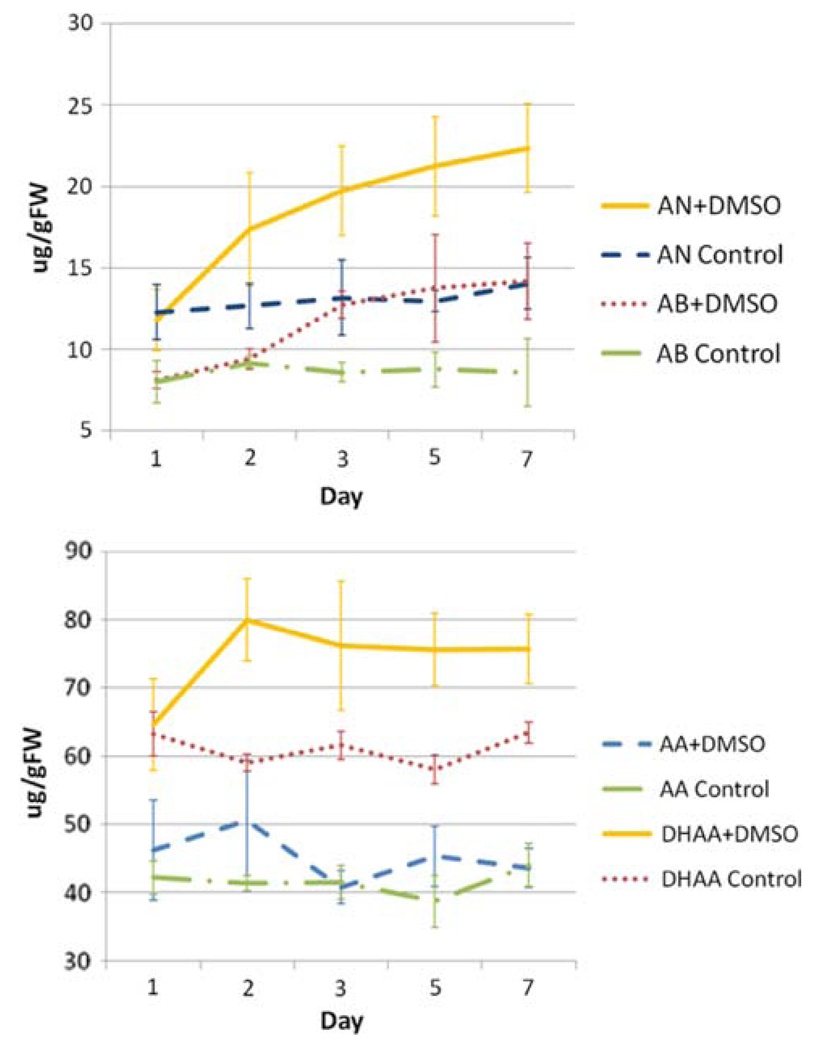
To determine the optimum exposure time needed to obtain a DMSO-induced increase in artemisinin, 0.25% DMSO was used as it was the lowest DMSO concentration that elicited an artemisinin response. When A. annua plantlets were exposed to 0.25% DMSO added to the roots, artemisinin yield continued to increase 7 days after DMSO treatment (Fig. 4); total plant dry weight was also not affected by the presence of DMSO (Fig. 2b). Of particular interest is the increase of AN and AB in the presence of DMSO (Fig. 4). Although AA did not increase substantially, DHAA did, supporting the notion that DHAA may be acting as a ROS scavenger as first hypothesized by Wallaart et al. (1999). These data suggest that ROS induced by DMSO are possibly causing the increase in production of DHAA and providing the extra oxygens required for the final biosynthetic step leading to AN.
Fig. 4.
At low concentrations, maximum artemisinin production occurs 3 days after incubation in 0.25% DMSO. Rooted shoots were incubated in 15 mL rooting media containing 0.25% (v/v) DMSO. AN artemisinin, AB arteannuin B, AA artemisinic acid, DHAA dihydroartemisinic acid. Data are the average of at least three replicates
DMSO affects some of the genes in the artemisinin biosynthetic pathway
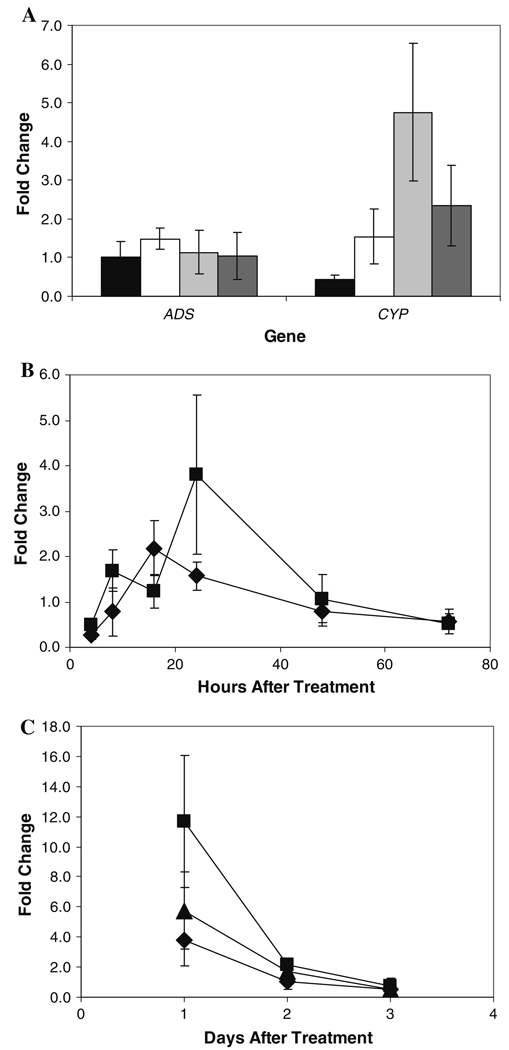
Artemisinin levels rise in response to DMSO (Fig. 2, Fig. 4), so a direct effect on the genes in the pathway was anticipated. Using real-time PCR, mRNA transcript levels of both ADS and CYP (Fig. 1) were measured in shoots of A. annua rooted shoot cultures. Within 24 h, only CYP showed a significant response to DMSO (Fig. 5); ADS, the gene encoding the first enzyme in the pathway, showed no response. Interestingly, the greatest number of CYP transcripts was observed at the DMSO concentration (0.5%) at which artemisinin levels were lowest. Indeed, the overall CYP response was inversely related to the amount of artemisinin produced in the shoots.
Fig. 5.
Only CYP71AV1 responds at the transcript level to DMSO; ADS does not. a Levels of ADS and CYP71AV1 mRNA after 24 h exposure to 0% DMSO (black column), 0.25% DMSO (white column), 0.5% DMSO (light grey column), and 2.0% DMSO (dark grey column). b Levels of ADS (diamonds) and CYP71AV1 (squares) mRNA in response to 0.25% DMSO over time. c Levels of CYP71AV1 mRNA after exposure to 0.25% DMSO (diamonds), 0.5% DMSO (squares), and 2.0% DMSO (triangles) over time. Error bars indicate ± SD. ADS amorphadiene synthase, CYP cytochrome P450 CYP71AV1
DMSO increases ROS in A. annua shoots
Artemisinin is a small molecule with five oxygen atoms. Prior work in our lab showed that in a highly oxygenated environment more artemisinin is produced than in a hypoxic one (Weathers et al. 1999) consistent with the hypothesis by Wallaart et al. (1999, 2001) that radical oxygen is required for the last steps in the biosynthesis of the drug. To determine if ROS is involved in the production of artemisinin, the fourth to seventh leaves from the apical meristem of rooted shoots were harvested from 20 rooted in vitro-grown plantlets and stained with DAB for a common form of ROS in plants, H2O2. Increasing DMSO concentrations did not affect growth (Fig. 6a), but increased the level of DAB staining in the leaves of rooted plantlets (Fig. 6b) indicating an increased in situ production of H2O2 in the foliage. Unrooted shoots showed no ROS formation in the presence of DMSO indicating that roots were required for the ROS response (Fig. 6c).
Fig. 6.
DMSO increases a ROS response that is countered by vitamin C in shoots of A. annua plantlets. a Plants at different DMSO concentrations show similar growth. b ROS increases with increasing DMSO concentrations and vitamin C counteracts the DMSO ROS response. c ROS does not increase in unrooted shoots treated with DMSO. d pH did not affect the ROS or vitamin C response. Plantlets were treated ± 0–2% DMSO ± 10 or 20 mM ascorbate (AsA) for 24 h, then 20 leaves were harvested and stained with DAB for 7 h, bleached with ethanol for 5 min, and viewed with light microscopy. The DAB stain is specific for H2O2 and forms a dark precipitate. Data are representative of 20 leaves from each condition
Vitamin C counters the ROS response and reduces DMSO-induced artemisinin production
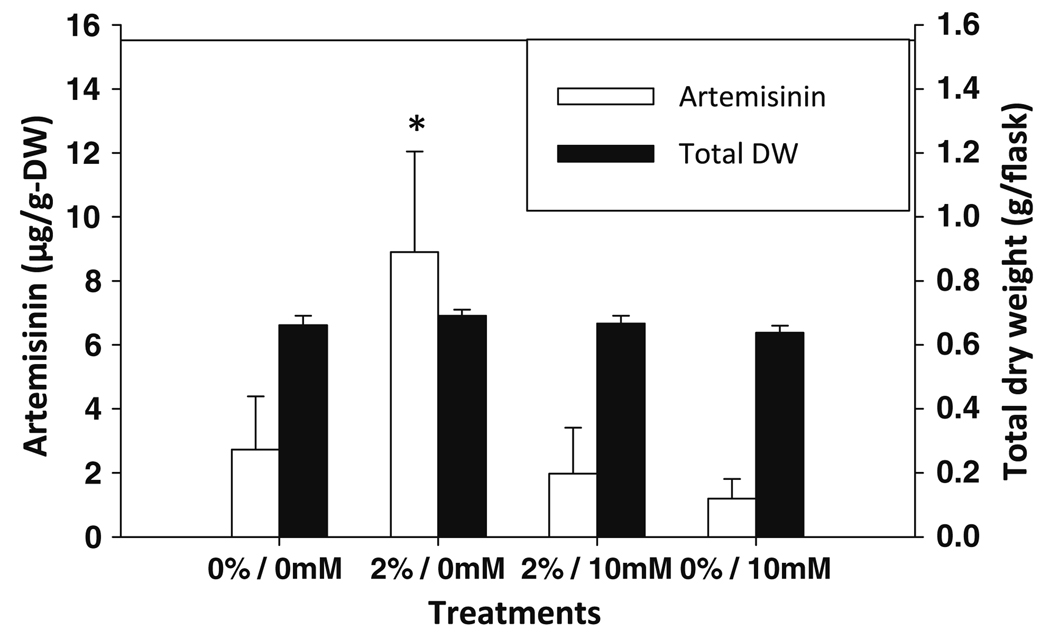
If DMSO was inducing a ROS response, then it was posited that a naturally abundant ROS scavenger (intracellular concentrations in plants are between 2 and 25 mM; Noctor and Foyer 1998), such as l-ascorbic acid (AsA, vitamin C), in the plants may counter the effect. When either 10 or 20 mM AsA was added to the DMSO-treated plants, the DMSO-induced ROS response was effectively eliminated (Fig. 6b). Since the most evident ROS staining was observed at 2% DMSO (Fig. 6b), this concentration was used to further test the effect of AsA on ROS induction by DMSO. AsA addition to the cultures resulted in a significant decrease in pH from the normal 5.2 of the culture medium to 3.1. To ensure that the AsA effect on ROS was not a pH effect, cultures were treated with AsA with pH adjusted to 5.2 with NaOH. DMSO was also added to cultures with pH adjusted with HCl to 3.1, and compared to DMSO + AsA at both pH 3.1 and 5.2. In the presence of DMSO at either pH, DAB showed a strong ROS response that in both cases was also countered by AsA (Fig. 6d). These results showed that the observed AsA recovery of the DMSO-stimulated ROS response in A. annua is not an artifact of pH. Furthermore, AsA was also shown to inhibit the DMSO-induced artemisinin production in plantlets treated ± DMSO and ± AsA (Fig. 7). This result shows that a known ROS scavenger, AsA, also inhibits artemisinin production.
Fig. 7.
Vitamin C counters the ROS response and reduces DMSO-induced artemisinin production. Plantlets were treated with 2% DMSO ± 10 mM AsA for 24 h then harvested and shoots dried and extracted for assay of artemisinin by HPLC. Asterisk indicates statistical significance at P = 0.051. Error bars are ± 1 SD; n = 4
Discussion
Most reports regarding the biological mechanism of DMSO effects on cells have focused on animal cells where DMSO is identified as a ROS scavenger (see reviews by Yu and Quinn 1994; Kharasch and Thyagarajan 1983). Although plants produce considerable ROS through many processes similar to those in animals, e.g., in defense against pathogens, and response to abiotic stress, some are unique to plants and include their role in photosynthesis and photorespiration (Apel and Hirt 2004).
DMSO is proposed to be a radical oxygen scavenger (Yu and Quinn 1994; Kharasch and Thyagarajan 1983); thus, we would expect it to reduce ROS. In A. annua, however, DMSO increased ROS in shoots, but only if the shoots are rooted. When AsA, a known ROS scavenger especially of peroxide (Noctor and Foyer 1998; Apel and Hirt 2004), was added, the DMSO-induced ROS disappeared. When ROS disappeared, artemisinin also declined substantially. Thus, in contrast to reports in other systems, in A. annua DMSO is not reducing ROS, but rather, increasing it and this may contribute to an increase in oxygen-rich artemisinin. The ROS measured in this study is H2O2 as demonstrated by the hydrogen peroxide-specific DAB staining. Interestingly, an earlier report by Sangwan et al. (1993) showed that in a cell-free system, addition of H2O2 with horseradish peroxidase increased artemisinin production; a result later confirmed by Zhang et al. (2003). There is, however, some evidence that a mixture of peroxide and various peroxidases can yield singlet oxygen (Khan et al. 1983), and is consistent with participation in the last non-enzymatic photo-oxygenic step leading to artemisinin (Sy and Brown 2002).
Although another reported role of DMSO is as a radical transfer trap (Kharasch and Thyagarajan 1983), that does not seem to be its role in A. annua mainly because the plant’s roots are required to receive the DMSO signal to stimulate ROS and artemisinin production in the shoots. This suggests that there is transport of some unknown intermediate or signal molecule from roots to shoots. It is also unlikely that DMSO is directly contributing its oxygen to the artemisinin reactions because direct application of DMSO to the shoots has no effect on terpene production. Although one could argue that DMSO may not have penetrated the shoots, this seems unlikely because the chemical is an excellent penetrant (Yu and Quinn 1994; Kharasch and Thyagarajan 1983).
Others (Kurina-Sanz et al. 2000; Hernandez et al. 2005) have shown some DMSO elicitation of secondary metabolism in plants; however, the response in A. annua seems to be different. First, the DMSO stimulation of artemisinin in A. annua requires perception of the chemical by the plant’s roots. Second, there is clearly a ROS connection to AN production, a relationship implied in some studies (Smith et al. 1997; Zhang et al. 2005; Lulu et al. 2008; Baldi and Dixit 2008), but not confirmed in planta.
If DMSO were acting at the point of transcript regulation of the artemisinin pathway, then one might expect the number of ADS mRNA transcripts to have increased, but this was not observed. Instead, transcripts of the genes of the second enzyme in the pathway, the P450, CYP, increased, but only at the DMSO concentration where artemisinin level is comparatively reduced. While there is a possibility that DMSO is perhaps easing a possible bottleneck in the pathway, an analysis of pathway intermediates in response to DMSO was performed to attempt to resolve this query.
DMSO clearly stimulated AN, DHAA, and AB production, but not AA. Both AB and AN have a higher number of oxygen atoms in their structures, three and five, respectively, compared to AA and DHAA, each of which has two oxygens. Furthermore, it is the final photo-oxidative step from DHAA to AN that has been hypothesized to involve ROS (Wallaart et al. 1999; Sy and Brown 2002); our data are consistent with that hypothesis. Considering that DMSO increases the amount of all of the metabolites except AA, and that both AB and AN have at least three oxygen atoms, our results are consistent with the hypothesis that DHAA is acting as a ROS sink in A. annua.
This study has shown that the stimulatory effect of DMSO on artemisinin production appears to be linked to stimulation of ROS which when inhibited by a known ROS scavenger, vitamin C, also inhibits artemisinin production. DHAA thus appears to be acting somewhat as a ROS sink in A. annua. The dynamics of the DMSO effect are complicated suggesting there could be several possible mechanisms of action. This can be clearly seen in the breakdown of a dose–response relationship between DMSO and AN. It remains unclear why at certain concentrations of DMSO (0.25 and 2.0%) AN increased significantly, while at other intermediate concentrations (0.5%) the response was mitigated. Our data suggest that ROS is a key factor in the increases observed but may not be the only factor influencing AN production in this system. Indeed, numerous other studies on AN elicitation using diverse agents and conditions may be unified under the umbrella of ROS, but to date no conclusive evidence for this has been presented. Induction of AN production by GA3 (Smith et al. 1997; Zhang et al. 2005), cold stress (Lulu et al. 2008), and methyl jasmonate (Baldi and Dixit 2008) all have possible connections to in situ ROS production and serve to bolster the hypothesis that DHAA acts as ROS sink. To our knowledge, this is the first report of an elicitory treatment (DMSO) being specifically countered by the addition of a ROS scavenger (AsA).
Lastly, the DMSO stimulus is apparently only perceived by the roots of the plant and not the shoots, yet only the shoots produce artemisinin, suggesting a long-distance signal or chemical moving from roots to shoots. Although not fully understood, use of DMSO may prove useful not only in improving the yield of the drug in A. annua, but also in understanding the regulation of the pathway and especially of the last non-enzymatic photo-oxidative steps in biosynthesis of this important sesquiterpene.
Supplementary Material
Acknowledgments
Support was provided in part by NIH 2R15GM069562-02 and the Arkansas Biosciences Institute, the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000. Fellowship support was provided to P. Arsenault from the George Alden Fellowship program at WPI and the George Alden Trust. Fellowship support was also provided to A. Mannan from the International Research Support Initiative Program from the Higher Education Commission, Government of Pakistan. We thank Patrick Covello, Plant Biotechnology Lab, Saskatoon, Canada for the CYP71AV1 gene, Joseph Chappell, University of Kentucky, for the ADS gene, and J Yactayo-Chang for technical assistance with the DAB staining assay.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00299-009-0807-y) contains supplementary material, which is available to authorized users.
Contributor Information
Abdul Mannan, Arkansas Biosciences Institute, Arkansas State University, Jonesboro, AR 72467-0639, USA; Department of Biochemistry, Faculty of Biological Sciences, Quaid-i-Azam University, Islamabad 45320, Pakistan.
Chunzhao Liu, Arkansas Biosciences Institute, Arkansas State University, Jonesboro, AR 72467-0639, USA; National Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, 100080 Beijing, China.
Patrick R. Arsenault, Worcester Polytechnic Institute, Worcester, MA 01609, USA
Melissa J. Towler, Worcester Polytechnic Institute, Worcester, MA 01609, USA
Dan R. Vail, Arkansas Biosciences Institute, Arkansas State University, Jonesboro, AR 72467-0639, USA Worcester Polytechnic Institute, Worcester, MA 01609, USA.
Argelia Lorence, Arkansas Biosciences Institute, Arkansas State University, Jonesboro, AR 72467-0639, USA.
Pamela J. Weathers, Arkansas Biosciences Institute, Arkansas State University, Jonesboro, AR 72467-0639, USA, weathers@wpi.edu Worcester Polytechnic Institute, Worcester, MA 01609, USA.
References
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Arsenault PR, Wobbe KK, Weathers PJ. Recent advances in artemisinin production through heterologous expression. Curr Med Chem. 2008;15:2886–2896. doi: 10.2174/092986708786242813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj YPS, Rathore VS, Wittwer SH, Adams MW. Effect of dimethyl sulfoxide on zinc65 uptake, respiration, and RNA and protein metabolism in bean (Phaseolus vulgaris) tissues. Am J Bot. 1970;57:794–799. [Google Scholar]
- Baldi A, Dixit VK. Yield enhancement strategies for artemisinin production by suspension cultures of Artemisia annua. Bioresour Tech. 2008;99:4609–4614. doi: 10.1016/j.biortech.2007.06.061. [DOI] [PubMed] [Google Scholar]
- Bozom MP, Gargadennec A, Andary C, Roussel JL, Gueiffier A. Alkaloid repartition and DMSO effects on alkaloid accumulation in Catharanthus seedlings. J Plant Physiol. 1998;153:534–538. [Google Scholar]
- Brown GD, Sy LK. In vivo transformations of dihydroartemisinic acid in Artemisia annua plants. Tetrahedron. 2004;60:1139–1159. [Google Scholar]
- Brown GD, Sy LK. In vivo transformations of artemisinic acid in Artemisia annua plants. Tetrahedron. 2007;63:9548–9566. [Google Scholar]
- Carswell GK, Johnson CM, Shillito RD, Harms CT. O-acetyl-salicylic acid promotes colony formation from protoplasts of an elite maize inbred. Plant Cell Rep. 1989;8:282–284. doi: 10.1007/BF00274130. [DOI] [PubMed] [Google Scholar]
- Chen Z, Zhang Y. Dimethyl sulfoxide targets phage RNA polymerases to promote transcription. Biochem Biophys Res Comm. 2005;333:664–670. doi: 10.1016/j.bbrc.2005.05.166. [DOI] [PubMed] [Google Scholar]
- Chen G, Wand GY, Li X, Waters B, Davies J. Enhanced production of microbial metabolites in the presence of dimethyl sulfoxide. J Antibiotics. 2000;53:1145–1153. doi: 10.7164/antibiotics.53.1145. [DOI] [PubMed] [Google Scholar]
- Cikos S, Bukovska A, Koppel J. Relative quantification of mRNA: comparison of methods currently used for real-time PCR data analysis. BMC Mol Biol. 2007;8:113–123. doi: 10.1186/1471-2199-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries PJ, Dien TK. Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs. 1996;52:818–836. doi: 10.2165/00003495-199652060-00004. [DOI] [PubMed] [Google Scholar]
- Duke S, Paul RN. Development and fine structure of the glandular trichomes of Artemisia annua L. Int J Plant Sci. 1993;154:107–118. [Google Scholar]
- Efferth T. Willmar Schwabe Award 2006: antiplasmodial and antitumor activity of artemisinin—from bench to bedside. Planta Med. 2007;73:299–309. doi: 10.1055/s-2007-967138. [DOI] [PubMed] [Google Scholar]
- Ferreira JFS, Janick J. Floral morphology of Artemisia annua with special reference to trichomes. Int J Plant Sci. 1995;156:807–815. [Google Scholar]
- Ferreira JFS, Janick J. Roots as an enhancing factor for the production of artemisinin in shoot cultures of Artemisia annua. Plant Cell Tiss Organ Cult. 1996;44:211–217. [Google Scholar]
- Hahne G, Hoffmann F. Dimethyl sulfoxide can initiate cell divisions of arrested callus protoplasts by promoting cortical microtubule assembly. Proc Natl Acad Sci USA. 1984;81:5449–5453. doi: 10.1073/pnas.81.17.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez XE, Orden AA, Giordano OS, Kurina M. Effects of elicitor and copper sulfate on grindelic acid production in submerged cultures of Grindelia pulchella. Eur J Biotechnol. 2005;8:276–283. [Google Scholar]
- Jones-Brando L, D’Angelo J, Posner GH, Yolken R. In vitro inhibition of Toxoplasma gondii by four new derivatives of artemisinin. Antimicrob Agents Chemother. 2006;50:4206–4208. doi: 10.1128/AAC.00793-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AU, Gebauer P, Hager LP. Chloroperoxidase generation of singlet Δ molecular oxygen observed directly by spectroscopy in the 1- to 1.6-µm region. PNAS. 1983;17:5195–5197. doi: 10.1073/pnas.80.17.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharasch N, Thyagarajan BS. Structural basis for biological activities of dimethyl sulfoxide. Annal NY Acad Sci. 1983;411:391–402. doi: 10.1111/j.1749-6632.1983.tb47334.x. [DOI] [PubMed] [Google Scholar]
- Kurina-Sanz M, Hernandez XE, Tonn CE, Guerreiro E. Enhancement of tessaric acid production in Tessaria absinthioides cell suspension cultures. Plant Cell Rep. 2000;19:821–824. doi: 10.1007/s002990000192. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lulu Y, Chang Z, Ying H, Ruiyi Y, Qingping Z. Abiotic stress induced expression of artemisinin biosynthesis genes in Artemisia annua L. Chin J Appl Environ Biol. 2008;14:1–5. [Google Scholar]
- Marks PA, Breslow R. Dimethyl sulfoxide to vorinostat: development of this histone inhibitor an anticancer drug. Nat Biotechnol. 2007;25:84–90. doi: 10.1038/nbt1272. [DOI] [PubMed] [Google Scholar]
- Merali S, Meshnick SR. Susceptibility of Pneumocystis carinii to artemisinin in vitro. Antimicrob Agents Chemother. 1991;35:1225–1227. doi: 10.1128/aac.35.6.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Nam W, Tak J, Ryu JK, Jung M, Yook JI, Kim HJ, Cha IH. Effects of artemisinin and its derivatives on growth inhibition and apoptosis of oral cancer cells. Head Neck. 2007;29:335–340. doi: 10.1002/hed.20524. [DOI] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Ann Rev Plant Physiol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Romero MR, Efferth T, Serrano MA, Castano B, Macias RI, Briz O, Marin JJ. Effect of artemisinin/artesunate as inhibitors of hepatitis B virus production in an “in vitro” replicative system. Antiviral Res. 2005;68:75–83. doi: 10.1016/j.antiviral.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Sangwan RS, Agarwal K, Luthra R, Thakur RS, Singh-Sangwan N. Biotransformation of arteannuic acid into arteannuin-B and artemisinin in Artemisia annua. Phytochemistry. 1993;34:1301–1302. [Google Scholar]
- Santos NC, Figueira-Coelho J, Martins-Silva J, Saldanha C. Multidisciplinary utilization of dimethyl sulfoxide: pharmacological, cellular, and molecule aspects. Biochem Pharmacol. 2003;65:1035–1041. doi: 10.1016/s0006-2952(03)00002-9. [DOI] [PubMed] [Google Scholar]
- Schmid WE. On the effects of DMSO in cation transport by excised barley roots. Am J Bot. 1968;55:757–761. [Google Scholar]
- Schramek N, Wang H, Römisch-Margl W, Wang H, Keil B, Radykewicz T, Winzenhörlein B, Li G, Ye H, Rohdich F, Bacher A, Liu B, Eisenreich W. In vivo study of artemisinin using 13CO2 as a precursor. TERPNET 2007 8th international meeting: biosynthesis and function of isoprenoids in plants, microorganisms and parasites; 2007. Abstracts P53. [Google Scholar]
- Sehringer B, Zahradnik HP, Deppert WR, Simon M, Noethling C, Schaefer WR. Evaluation of different strategies for real-time RT-PCR expression analysis of corticotropin-releasing hormone and related proteins in human gestational tissues. Anal Bioanal Chem. 2005;383:768–775. doi: 10.1007/s00216-005-0067-9. [DOI] [PubMed] [Google Scholar]
- Simó R, Pedrós-Alió C, Malin G, Gimalt JO. Biological turnover of DMS, DMSP, and DMSO in contrasting open-sea waters. Mar Ecol Prog Ser. 2000;203:1–11. [Google Scholar]
- Singh NP, Lai HC. Artemisinin induces apoptosis in human cancer cells. Anticancer Res. 2004;24:2277–2280. [PubMed] [Google Scholar]
- Smith TC, Weathers PJ, Cheetham RD. Effects of gibberellic acid on hairy root cultures of Artemisia annua: growth and artemisinin production. In Vitro Cell Dev Biol Plant. 1997;33:75–79. [Google Scholar]
- Sy L-K, Brown GD. The mechanism of the spontaneous autoxidation of dihydroartemisinic acid. Tetrahedron. 2002;58:897–908. [Google Scholar]
- Teoh KH, Polichuk DR, Reed DW, Nowak G, Covello PS. Artemisia annua L. (Asteraceae) trichome-specific cDNAs reveal CYP71AV1, a cytochrome P450 with a key role in the biosynthesis of the antimalarial sesquiterpene lactone artemisinin. FEBS Lett. 2006;580:1411–1416. doi: 10.1016/j.febslet.2006.01.065. [DOI] [PubMed] [Google Scholar]
- Teoh KH, Polichuk DR, Reed DW, Covello PS. Molecular cloning of an aldehyde dehydrogenase implicated in artemisinin biosynthesis in Artemisia annua. Botany. 2009;87:635–642. [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997;11:1187–1194. [Google Scholar]
- Towler MJ, Weathers PJ. Evidence of artemisinin production from IPP stemming from both the mevalonate and the nonmevalonate pathways. Plant Cell Rep. 2007;26:2129–2136. doi: 10.1007/s00299-007-0420-x. [DOI] [PubMed] [Google Scholar]
- Utzinger J, Xiao S, N’Goran EK, Bergquist R, Tanner M. The potential of artemether for the control of schistosomiasis. Int J Parasitol. 2001;31:1549–1562. doi: 10.1016/s0020-7519(01)00297-1. [DOI] [PubMed] [Google Scholar]
- Wallaart TE, Van Uden W, Lubberink HGM, Woerdenbag HJ, Pras N, Quax WJ. Isolation and identification of dihydroartemisinic acid from Artemisia annua and its possible role in the biosynthesis of artemisinin. J Nat Prod. 1999;62:430–433. doi: 10.1021/np980370p. [DOI] [PubMed] [Google Scholar]
- Wallaart TE, Pras N, Beekman AC, Quax WJ. Seasonal variation of artemisinin and its biosynthetic precursors in plants of Artemisia annua of different geographical origin: proof for the existence of chemotypes. Planta Med. 2000;66:57–62. doi: 10.1055/s-2000-11115. [DOI] [PubMed] [Google Scholar]
- Wallaart TE, Bouwmeester HJ, Hille J, Popping L, Maijers NC. Amorpha-4, 11-diene synthase: cloning and functional expression of a key enzyme in the biosynthetic pathway of the novel antimalarial drug artemisinin. Planta. 2001;212:460–465. doi: 10.1007/s004250000428. [DOI] [PubMed] [Google Scholar]
- Weathers PJ, Wyslouzil BE, Wobbe KK, Kim YJ, Yigit E. The biological response of hairy roots to O2 levels in bioreactors. In Vitro Cell Dev Biol Plant. 1999;35:286–289. [Google Scholar]
- Yu ZW, Quinn PJ. Dimethyl sulfoxide: a review of its applications in cell biology. Biosci Rep. 1994;14:259–281. doi: 10.1007/BF01199051. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ye H, Li G. Effect of horseradish peroxidase on the biosynthesis of artemisinin in Artemisia annua in vitro. Chin J Appl Environ Biol. 2003;9:616–618. [Google Scholar]
- Zhang YS, Ye HC, Liu BY, Wang H, Li GF. Exogenous GA3 and flowering induce the conversion of artemisinic acid to artemisinin in Artemisia annua plants. Russ J Plant Physiol. 2005;52:68–73. [Google Scholar]
- Zhang Y, Teoh KH, Reed DW, Maes L, Goossens A, Olson DJH, Ross ARS, Covello PS. The molecular cloning of artemisinic aldehyde Δ11(13) reductase and its role in glandular trichome-dependent biosynthesis of artemisinin in Artemisia annua. J Biol Chem. 2008;283:21501–21508. doi: 10.1074/jbc.M803090200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.