Abstract
Purpose. To elucidate the influence of ionizing radiation (IR) on the oncolytic activity of Parvovirus H-1 (H-1PV) in human high-grade glioma cells. Methods. Short term cultures of human high-grade gliomas were irradiated at different doses and infected with H-1PV. Cell viability was assessed by determining relative numbers of surviving cells. Replication of H-1PV was measured by RT-PCR of viral RNA, fluorescence-activated cell sorter (FACS) analysis and the synthesis of infectious virus particles. To identify a possible mechanism for radiation induced change in the oncolytic activity of H-1PV we performed cell cycle analyses. Results. Previous irradiation rendered glioma cells fully permissive to H-1PV infection. Irradiation 24 hours prior to H-1PV infection led to increased cell killing most notably in radioresistant glioma cells. Intracellular levels of NS-1, the main effector of H-1PV induced cytotoxicity, were elevated after irradiation. S-phase levels were increased one day after irradiation improving S-phase dependent viral replication and cytotoxicity. Conclusion. This study demonstrates intact susceptibility of previously irradiated glioma-cells for H-1PV induced oncolysis. The combination of ionizing radiation followed by H-1PV infection increased viral cytotoxicity, especially in radioresistant gliomas. These findings support the ongoing development of a clinical trial of H-1PV in patients with recurrent glioblastomas.
1. Introduction
Malignant gliomas have remained a malignancy with an extremely unfortunate prognosis [1, 2]. Recent improvements of standard therapies including, when feasible, surgical resection followed by radio-chemotherapy have only extended the 50% survival from 12 months to 16 months [3–5]. Long-term survival is rare, only 5% of patients are alive after 5 years. As a consequence, alternative therapies have to be investigated.
One new strategy is the use of replication competent oncolytic viruses that specifically target and destroy tumor cells while leaving normal cells intact. A number of candidate oncolytic viruses for glioma therapy are currently under investigation including genetically modified Herpesviruses [6], Adenoviruses [7], or Poliovirus [8], and wildtype viruses such as Reovirus [9], Vesicular-stomatitis virus [10], and Measles virus [11]. We previously reported the efficient killing of glioma cells of human and rat origin by Parvovirus H-1 (H-1PV), a single stranded nonenveloped DNA virus of 5.1 kb. H-1PV induced lytic infection of glioma cells even when the cells were resistant to agents inducing apoptosis [12, 13]. In animal experiments, H-1PV infection of rats bearing large intracranial gliomas led to tumor regression and prolonged survival (Geletneky et al., accepted, 2010). The natural host of H-1PV is the rat; however, the virus can efficiently infect cells of other species including humans. In contrast to some other wildtype viruses under investigation for glioma-treatment, H-1PV does not cause any pathology in rodents or humans [14].
Radiation therapy prolongs survival in patients with malignant gliomas and is used as standard treatment of primary high-grade glial tumors [15]. However, as high grade gliomas are resistant to radiation therapy and a clear dose-limitation exists due to cytotoxic effects on the surrounding brain tissue, this treatment modality is not curative and strategies to improve radiation efficiency are under investigation. The combination of radiation therapy with the oral alkylating agent temozolomide has already proved to be superior to either therapy alone and has become the standard of care for the majority of patients with newly diagnosed glioblastoma multiforme [3, 5]. Recent studies of the role of radiation therapy for recurrent gliomas that were already irradiated as part of the primary treatment demonstrated some effect when radiation was applied as a boost to smaller tumor regions [4]. Therefore, depending on the individual situation of the patient, radiation therapy can also be an option for recurrent tumors, but methods to augment the limited therapeutic effect would clearly be beneficial.
Clinical trials with different oncolytic viruses were able to demonstrate the safety of this novel therapeutic approach; however, the positive therapeutic effects were restricted to individual patients [16]. Therefore, the combination of oncolytic viruses with standard therapeutics has become one important focus to improve viral cytotoxicity. Radiation therapy is a part of therapeutic protocols for the majority of malignancies, and an enhanced effect of the oncolytic activity of viruses by radiation therapy could be observed for tumor cells of various histology [17]. This is of particular interest for gliomas, as both treatments, radiation therapy and virotherapy, are primarily designed as regional therapies. For glioma cells, the oncolytic effect of Herpesvirus [18], Adenoviruses [19], Reovirus [20], and Measles virus [21] was shown to be enhanced by IR.
The aim of this study was to assess the influence of IR on the oncolytic activity of H-1PV in glioma cells. The possible interaction of IR with H-1PV oncolytic virotherapy could be twofold: (i) as the use of an oncolytic virus in glioma patients would preferably include the treatment of recurrent tumors originating from previously irradiated remaining tumor cells, it has to be shown whether previous radiation therapy would interfere with viral oncolysis or replication in pretreated gliomas and (ii) administration of radiation therapy together with H-1PV oncolytic virotherapy could lead to improved efficacy of either treatment alone. We therefore investigated the treatment of early-passage glioma cells with IR before or after infection with H-1PV and assessed cytotoxicity, viral replication, and treatment-induced changes of the cell cycle. These findings are important to define patient populations for a clinical trial of oncolytic virotherapy of malignant gliomas and to possibly use H-1PV to increase radiation efficacy in this dismal tumor entity.
2. Methods and Materials
2.1. Cell Culture
Human short term cultures derived from histologically confirmed glioblastomas (NCH-82, NCH-89, NCH-307) and a gliosarcoma (NCH-37) were established and characterized at the Department of Neurosurgery, Heidelberg, Germany as described previously [12]. NCH-307 is a recurrent glioblastoma cell line that had been irradiated in vivo prior to resection of the recurrent tumor. NCH-37, NCH-89, and NCH-307 were tested at low-passage numbers <30; NCH-82 was tested at a passage number of 100. The ethylnitrosourea-induced rat glioma cell line RG2 was previously shown to be highly susceptibly for H-1PV [12] and was used for virus-titration experiments. All cells were grown in DEME (Sigma-Aldrich, Steinheim, Germany) supplemented with 10% FCS (BiochromKG, Berlin, Germany) and 1% antibiotics (penicillin/streptomycin; Gibca, Invitrogen Corporation, Karlsruhe, Germany) in a 5% CO2 humidified atmosphere at 37°C.
2.2. Treatment with Ionizing Radiation (IR)
Radiation of cell cultures was performed at room temperature in a linear accelerator (Siemens Mevatron KD2, 6-MV photons) at doses of 5 Gy,10 Gy, or 20 Gy as indicated for the respective experiment (dose rate: 3 Gy/min; distance from source to flask: 95 cm). Control cells were transported to the accelerator but not exposed to IR (0 Gy). NCH-307 recurrent glioblastoma cells were not reexposed to IR in vitro.
2.3. H-1 Virus Production and Infection
H-1PV was propagated in human NB324K cells, and purified as described previously [22]. Monolayers of all glioma cell cultures were infected under standard conditions: cells were inoculated with H-1PV diluted in PBS at a multiplicity of infection (MOI) of 5 plaque forming units (PFU) per cell or 100 PFU/cell as indicated for the respective experiment. After 60 minutes, virus suspensions were removed, cells were washed with medium, and cultures were allowed to grow. The corresponding mock-infected cultures were subjected to the same procedure using virus-free PBS instead of virus suspensions.
2.4. Cell Viability
To asses cell viability, glioma cells (NCH-37, NCH-82, NCH-89) were seeded at 3 × 104 cells/well into 12-well dishes and irradiated after 24 hours with 0 Gy, 5 Gy, 10 Gy, or 20 Gy. Cells were infected (MOI = 5 PFU/cell or mock-infection) at 2 different time points: 24 hours post-IR (early infection) or 9 days post-IR (late infection). In vivo irradiated NCH-307 cells were seeded at 3 × 104 cells/well into 12-well dishes and infected (MOI = 5 PFU/cell, 100 PFU/cell or mock-infection) 24 hours postseeding. The MOI of H-1PV was calculated based on counts of living cells immediately prior to infection. Cells were harvested 3 days after H-1PV infection/mock-infection and counted with an electronic counter (Casy, Schaerfe System, Reutlingen, Germany) in triplicate and results were confirmed with the typan blue exclusion test as described previously [12].
2.5. Statistical Analysis
The numbers of living cells were estimated as means and standard deviations of three independent assays. Cell viability (in % +/− standard error) was defined as the number of living treated cells over the number of living untreated cells multiplied by 100; standard error was calculated using the Gaussian law of error propagation. Statistical analysis was performed using a two-way ANOVA with virotherapy and IR as independent factors. Comparative analyses between groups were performed using post-hoc analysis. The SPSS software package (SPSS Inc., Chicago, IL) was used to perform statistical analysis.
2.6. RT-PCR Analysis
For RT-PCR analysis of viral RNA, cells (NCH-37, NCH-82, NCH-89) were plated at a density of 3 × 106 cells in 17 cm2 culture flasks, irradiated (10 Gy) after 24 hours and infected (MOI = 5 PFU/cell or mock-infection) 24 hours post-IR; NCH-307 cells were infected (MOI = 5 PFU/cell or mock-infection) 24 hours postseeding. Briefly, cells were harvested 24 hours p.i., washed with PBS and immediately shock frozen on dry ice. RNA was purified using the High Pure RNA Isolation Kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions. RT-PCR was performed with the C. therm. Polymerase One-Step RT-PCR-System (Roche Diagnostics, Mannheim, Germany) according to the manufacturer's instructions. To detect H-1PV NS-transcripts, the following specific primers were used: sense primer (position nt 1996–2016) 5′-TCA ATG CGC TCA CCA TCT CTG-3′; antisense primer (position nt 2510–2490) 5′-TCG TAG GCT TCG TCG TGT TCT-3′.
2.7. Fluorescence-Activated Cell Sorter (FACS) Analysis of Intracellular NS-1 Protein
Cells (NCH-37, NCH-82, NCH-89) were plated at a density of 3 × 106 cells in 17 cm2 culture flasks, irradiated (0 Gy, 10 Gy) after 24 hours and infected (MOI = 5 PFU/cell or mock-infection) 24 hours post-IR (early infection). For late infection, cells were infected 9 days post-IR. Recurrent glioblastoma NCH-307 cells were infected (MOI = 5 PFU/cell or mock-infection) 24 hours postseeding. All cell cultures were harvested 24 hours p.i. (postinfection). In short, cells were fixed with 4% paraformaldehyde and 100% methanol and permeabilized with 0.1% Triton-X-100 (Sigma-Aldrich, Taufkirchen, Germany). To identify NS-1 protein, probes were blocked with fetal calf serum and incubated on ice for 30 minutes with a polyclonal rabbit-anti-NS-1 antibody (SP8, courtesy of N. Salome, DKFZ, Heidelberg, Germany) in a concentration of 1: 25. The FITC-conjugated secondary goat-anti-rabbit-antibody (Jackson ImmunoResearch, Suffolk, UK) was incubated in a 1:250 dilution for 20 minutes on ice. Probes were analyzed for intracellular NS-1 content by measuring of fluorescence intensity, using a cytometer (FACScan flow cytometer, Becton Dickinson, Heidelberg, Germany) at an excitation wavelength of 525 nm. The data were analyzed with the aid of a software program (FlowJo, Tree Star, Olten, Switzerland) with dead cells gated out using pulse processing. A cell was determined as NS-1-positive when its fluorescence intensity (FL1-H) was greater than a certain threshold value of 5% of false positive mock infected cells.
2.8. Release of Infectious Viral Particles
Cells (NCH-37, NCH-82, NCH-89) were seeded at a density of 1.5 × 105 cells/well in 6-well dishes, irradiated 24 hours postseeding (10 Gy), and infected (MOI = 5 PFU/cell or mock-infection) 24 hours post-IR. NCH-307 was infected (MOI = 5 PFU/cell or mock-infection) 24 hours postseeding. The quantity of infectious viral particles in the supernatant of glioma cells was determined 1 hour and 3 days p.i. by titration on highly susceptible RG2 cells.
2.9. Cell Cycle Analysis
Cells (NCH-37, NCH-82, NCH-89) were plated at a density of 3 × 106 cells in 17 cm2 culture flasks and irradiated (0 Gy, 10 Gy) after 24 hours. Cells were harvested 24 hours and 48 hours post-IR and fixed in 80% ice-cold ethanol at 4°C overnight. After fixation, cells were incubated in 2 mg/mL RNase (Sigma-Aldrich, Taufkirchen, Germany), and 0,1 mg/mL PI (Sigma-Aldrich, Taufkirchen, Germany) for 30 minutes in the dark. Samples of 10,000 cells were analyzed for DNA content by flow cytometry (FACScan flow cytometer, Becton Dickinson, Heidelberg, Germany), and cell cycle phase distributions were analyzed with FlowJo software using the Dean-Jett-Fox model [23].
3. Results
3.1. Susceptibility of Irradiated Glioma Cells to Infection with H-1PV
As previous radiation therapy of tumor cells induces genetic alterations that could interfere with the susceptibility and efficiency of H-1PV infection, we infected glioma cells with a delay of 9 days after IR (late infection) when the cells had reentered the cell cycle. The goal of this experiment was to mimic and evaluate the possibility of H-1PV virotherapy of recurrent tumors after completion of radiation therapy as part of the initial standard treatment. In addition to testing tumor cells from primary gliomas (NCH-37, NCH-82, NCH-89), we also included NCH-307 cells that were established from a recurrent glioblastoma that had been irradiated several months prior to secondary surgical resection.
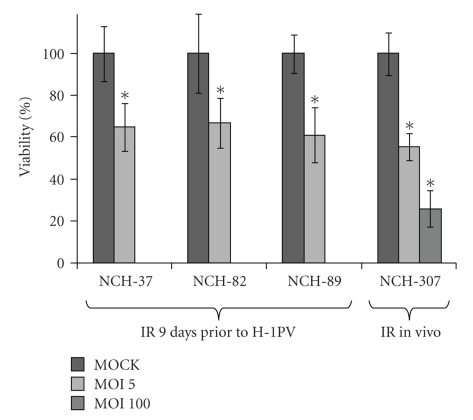
Cells (NCH-37, NCH-82, and NCH-89) were treated with IR of 10 Gy and had resumed to proliferate 9 days after radiation therapy. However, the growth rate was clearly reduced compared with untreated controls indicating persisting long-term effects of the treatment. In comparison, cell cultures of NCH-37 cells were less affected by radiation-induced growth retardation than NCH-82, and NCH-89 cells. Upon late infection with H-1PV with an MOI of 5 PFU/cell, all cells (NCH-37, NCH-82, and NCH-89) showed a significant (P < .001) reduction of surviving cells 3 days p.i.. Cell viability of infected cells was 65.04 (+/−11.5) % in NCH-37 cultures, 67.00 (+/−11.9) % in NCH-82 cells, and 61.04 (+/−13.8) % in NCH-89 cell cultures indicating intact susceptibility to H-1PV induced cell killing (Figure 1).
Figure 1.
Effects of late parvovirus H-1 (H-1PV) infection on human high-grade glioma cells surviving ionizing radiation (IR). Short-term cultures of human gliosarcoma NCH-37, human glioblastoma NCH-82, and human glioblastoma NCH-89 were seeded at 30,000 cells/well, irradiated with 10 Gy and infected with H-1PV at an MOI of 5 PFU/cell 9 days post-IR (MOI 5) and compared to mock-infected cells surviving IR (MOCK). The short-term culture of in vivo irradiated recurrent glioblastoma NCH-307 was seeded at 30,000 cells/well and infected with H-1PV at low (MOI = 5 PFU/cell: MOI 5) or high (MOI = 100 PFU/cell: MOI 100) virus doses and compared to mock-infected cells. All experiments were performed in three independent assays. Viability (%) was assessed as the number of living treated cells over the number of living untreated cells three days post (mock-) infection. Error bars represent the respective standard error. (*) indicates significant differences (P < .001) between the number of living infected and the corresponding number of living MOCK-infected cells.
NCH-307 recurrent glioma cells were infected with a low (5 PFU/cell) and a high (100 PFU/cell) MOI (Figure 1). Cell viability of NCH-307 cells was significantly (P < .001) reduced to 55.39 (+/− 6.6) % (low MOI) and 25.97 (+/− 8.8) % (high MOI) indicating dose-dependent cytotoxicity of H-1PV also in recurrent glioma cells.
3.2. Combination of IR and H-1PV Infection
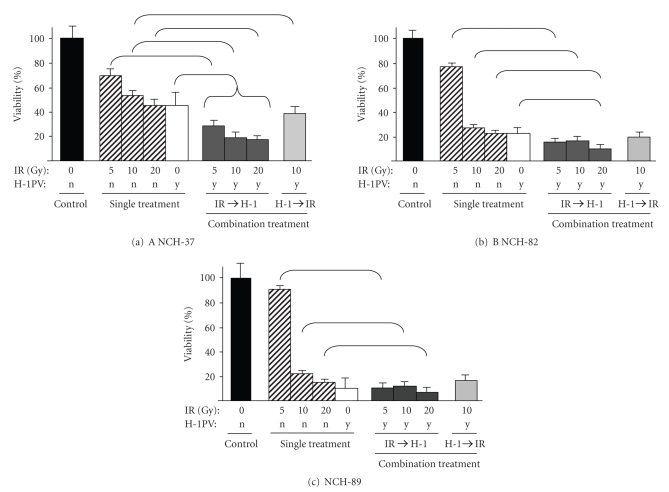
In initial experiments, the effect of radiation therapy or H-1PV infection alone was examined prior to testing combination treatment. At radiation-doses of 5 Gy, growth rates in all cell lines (NCH-37, NCH-82, NCH-89) were only slightly affected: cell viability was 70 (+/−9.9) % in NCH-37, 76 (+/−4.5) % in NCH-82, and 91 (+/−7.0) % in NCH-89. IR with 10 Gy had a strong effect on NCH-82 and NCH-89 cells with a cell viability of 25.64 (+/−1.8) % (NCH-82) and 22.81 (+/−4.7) % (NCH-89). NCH-37 cells were much less sensitive, the cell viability was reduced to 54.25 (+/−7.2) %. A dose of 20 Gy had a slightly stronger effect in all cell cultures: NCH-82 21.53 (+/−3.8) % and NCH-89 15.93 (+/−5.6) % cell viability, however in NCH-37 cultures 45.19 (+/−5.6) % of cells were still alive (Figure 2).
Figure 2.
Effects of ionizing radiation (IR), parvovirus H-1 (H-1PV) infection, and combination of IR and H-1PV infection on human high-grade glioma cells. Short-term cultures of human gliosarcoma NCH-37 (a), human glioblastoma NCH-82 (b), and human glioblastoma NCH-89 (c) were seeded at 30,000 cells/well, irradiated with 5 Gy, 10 Gy, or 20 Gy, and infected with H-1PV at an MOI of 5 PFU/cell 24 hours post-IR (IR→H-1) or cells were infected with H-1PV at an MOI of 5 PFU/cell and irradiated with 10 Gy 24 hours post-infection (H-1→IR). Effects on cell survival were compared to single treatment with IR or single treatment with H-1PV. Control cells were mock-infected and transported to the accelerator but not exposed to IR (0 Gy). All experiments were performed in three independent assays. Viability (%) was assessed as the number of living treated cells over the number of living untreated cells three days post (mock-) infection. Error bars represent the respective standard error. Significant differences (P < .05) between single treatment and combination treatment groups are indicated by brackets.
The infection of glioma cells with H-1PV at an MOI of 5 PFU/cell had a strong cytopathic effect in NCH-82 and NCH-89 cell cultures, with only 22.19 (+/− 3.0) % (NCH-82) and 9.73 (+/− 2.1) % (NCH-89) cell viability. NCH-37 cells were less sensitive to H-1PV; cell viability was 45.94 (+/− 6.0) % (Figure 2).
To assess whether the combination of radiation therapy and H-1PV virotherapy increases the therapeutic efficacy of either treatment alone and to evaluate the influence of the order of treatments on cytotoxicity, we performed two separate experiments: (i) glioma cells were irradiated with 3 different doses and infected 1 day after IR (Figure 2, combination treatment: IR →H-1) and (ii) glioma cells were infected first and subsequently irradiated with a dose of 10 Gy 24 hours p.i. (Figure 2, combination treatment: H-1 → IR, far right column). Two-way-ANOVA showed that in all cell lines tested (NCH-37, NCH-82, NCH-89), both independent factors (IR and H-1PV infection) had a significant influence on the number of surviving cells. There were significant interactions between IR and H-1PV infection for all of the data presented in Figure 2. Comparative analyses between groups revealed the following: in all glioma cell cultures (NCH-37, NCH-82, NCH-89), combination of H-1PV infection 1 day after IR was significantly (P < .05) more effective than IR alone (Figure 2). Compared with H-1PV infection alone, combination treatment was significantly (P < .05) more effective after previous IR with 5 Gy, 10 Gy, or 20 Gy in NCH-37 cells and after previous IR with 20 Gy in NCH-82 cells. When the order of treatments was reversed and H-1PV infection was performed 24 hours prior to IR, combination treatment only led to significantly (P < .05) improved cell killing in NCH-37 when compared to IR alone, but not when compared to H-1PV infection alone or in the other cell lines tested.
3.3. Long-Term Effects of IR Followed by H-1PV Infection
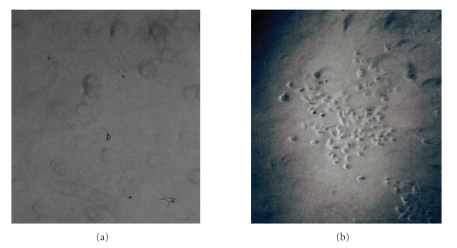
Even though high-dose radiation of NCH-37, NCH-82, and NCH-89 cells with 20 Gy or infection with H-1PV was highly cytotoxic, approximately 2 weeks after single treatment with IR or H-1PV alone, all cell lines resumed to proliferate from surviving clones, albeit at a much reduced rate (Table 1). Thus, neither IR nor H-1PV infection alone was able to eradicate all tumor cells. In contrast, when glioma cell cultures were treated with the combination of IR (20 Gy) and H-1PV infection (MOI = 5 PFU/cell) 24 hours after IR, no surviving tumor cells could be observed on day 21 p.i. or at later time points after treatment in any of the tested cell cultures (NCH-37, NCH-82, NCH-89) indicating long-term efficiency of combination treatment (Table 1 and Figure 3). The experiment was confirmed in triplicate in all cell cultures.
Table 1.
Long-term effect of ionizing radiation (IR) and/or parvovirus H-1 (H-1PV) infection on human high-grade glioma cells cultures.
| cell line | IR | H-1PV | 1 week p.i. | 2 weeks p.i. | 3 weeks p.i. |
|---|---|---|---|---|---|
| NCH-37 | 0 | MOCK | ++++ | ++++ | ++++ |
| 0 | MOI 5 | + | ++ | +++ | |
| 20 | MOCK | + | ++ | ++ | |
| 20 | MOI 5 | + | + | 0 | |
| NCH-82 | 0 | MOCK | ++++ | ++++ | ++++ |
| 0 | MOI 5 | + | ++ | ++ | |
| 20 | MOCK | + | ++ | ++ | |
| 20 | MOI 5 | + | + | 0 | |
| NCH-89 | 0 | MOCK | ++++ | ++++ | ++++ |
| 0 | MOI 5 | + | ++ | ++ | |
| 20 | MOCK | + | + | ++ | |
| 20 | MOI5 | + | + | 0 | |
(++++) confluent cell layer.
(+++) conflating cell clones.
(++) single cell clones.
(+) single cells.
(0) no cells.
Figure 3.
Long-term effect of combined ionizing radiation (IR) and parvovirus H-1 (H-1PV) infection on human high-grade glioma cells. Short-term cultures of human glioblastoma NCH-89 were irradiated with 20 Gy, and infected with H-1PV at an MOI of 5 PFU/cell 24 hours post-IR. 3-week postseeding photographs of culture dishes were taken at a magnification of 400× and no surviving cells could be found (a). In comparison, after mono treatment with H-1PV at an MOI of 5 PFU/cell or after mono treatment with IR (data not shown), proliferating cell clones could be observed (b).
3.4. Replication of H-1PV in Human Glioma Cells after IR
Replication of H-1PV in infected glioma cells was tested (i) by the presence of viral NS-1-specific RNA by RT-PCR, (ii) by the detection of the viral protein NS-1 as the main effector of parvoviral cytotoxicity [14] by FACS analysis, and (iii) by the release of infectious viral particles into the supernatant of cell cultures.
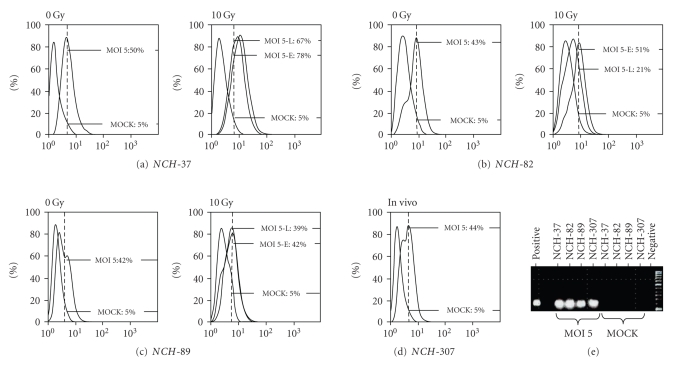
(i) Detection of viral RNA: cells (NCH-37, NCH-82, and NCH-89) were irradiated with 10 Gy and infected with H-1PV at an MOI of 5 pfu/cell 24 hours later. NCH-307 recurrent glioma cells were infected without additional radiation. RNA was extracted 24 hours p.i. and viral transcripts were detected by RT-PCR. In all cells-lines RT-PCR was positive for the presence of viral RNA indicating transcription from viral DNA (Figure 4(e)).
Figure 4.
Replication of parvovirus H-1 (H-1PV) in irradiated human high-grade glioma cells. FACS analysis of intracellular cytotoxic parvoviral protein NS-1 in short-term cultures of human gliosarcoma NCH-37 (a), human glioblastoma NCH-82 (b), and human glioblastoma NCH-89 (c) after infection with H-1PV. To analyse the influence of radiation therapy, glioma cells were irradiated with 10 Gy or transported to the accelerator but not exposed to IR (0 Gy). For early infection experiments, cells were infected at an MOI of 5 PFU/cell 24 hours post-IR (MOI 5-E) or mock-infected (MOCK); for late infection experiments cells were infected at an MOI of 5 PFU/cell 9 days post-IR (MOI 5-L). The short-term culture of in vivo irradiated recurrent glioblastoma NCH-307 (d) was infected with an MOI of 5 PFU/cell (MOI5) or mock-infected (MOCK). All cell cultures were harvested 24 hours p.i. and the percentage of cells positive for intracellular NS-1 was determined by FACS-analysis. (e) Detection of H-1PV RNA by RT-PCR. All cell lines except for NCH-307 were irradiated with 10 Gy. 24 hours post-IR and 24 postseeding for NCH-307, respectively, cells were infected with H-1PV at an MOI of 5 PFU/cell (MOI5). RNA was isolated 24 hours p.i. amplified by RT-PCR and compared with RNA of mock-infected cells (MOCK). For positive control, RNA of H-1PV infected unirradiated highly susceptible RG2 rat glioma cells was used.
(ii) Expression of NS-1 protein: irradiated (10 Gy) or untreated control cells were either H-1PV infected (MOI = 5 pfu/cell) or mock-infected 24 hours post-IR (early infection) or 9 days post-IR (late infection). Recurrent glioblastoma cells (NCH-307) were infected with an MOI of 5 pfu/cell without additional IR. 24 hours after (mock-) infection, intracellular NS-1 was marked with a FITC-conjugated antibody as described above. NS-1 protein expression of unirradiated glioma cells ranged from 50% of NS-1-positive NCH-37 cells to 43% in NCH-82 cells and 42% in NCH-89 cells 24 hours p.i. (Figure 4(a)–4(c)). The recurrent glioblastoma cell line NCH-307 showed NS-1 expression in 44% of cells 24 hours p.i. (Figure 4(d)). When cells were irradiated with 10 Gy and infected with H-1PV 24 hours (early infection) or 9 days (late infection) after IR, ratios of NS-1 expression 24 hours p.i. were as follows: NCH-37 cells showed an increase of NS-1-positive cells to 78% after early infection and to 67% after late infection. In NCH-82 cells, NS-1 expression increased to 51% after early infection and dropped to 21% after late infection. The NS-1 expression level in NCH-89 remained nearly unchanged with 42% of NS-1-positive cells after early infection and 39% after late infection.
(iii) Production of infectious H-1 virus particles: in order to assess whether cytopathic H-1PV infection of irradiated glioma cells resulted in the production of infectious progeny particles, virus yields were determined by titration on highly susceptibly RG2 cells. As demonstrated in Table 2, a 103 log-fold higher virus titer could be detected compared with input virus within 3 days after infection irrespective if cells were irradiated (10 Gy) or not (0 Gy). Results were similar in all cell lines tested (NCH-37, NCH-82, NCH-89), demonstrating persisting assembly of progeny virus after IR.
Table 2.
Titer of infectious virus particles in the supernatant of irradiated (10 Gy) or nonirradiated (0 Gy) human high-grade glioma cell lines 1 hour and 3 days post H-1PV infection.
| 1 hour p.i. | 3 days p.i. | ||
|---|---|---|---|
| NCH-37 | 10 Gy | 1: 100 | 1: 10,000 |
| 0 Gy | 1: 10 | 1: 1,000 | |
| NCH-82 | 10 Gy | 1: 100 | 1: 10,000 |
| 0 Gy | 1: 100 | 1: 10,000 | |
| NCH-89 | 10 Gy | 1: 100 | 1: 10,000 |
| 0 Gy | 1: 100 | 1: 10,000 | |
| NCH-307 | in vivo* | 1: 10 | 1: 1,000 |
*in vivo irradiated recurrent glioblastoma cell line NCH-307 was not irradiated in vitro.
3.5. Cell Cycle Alterations Induced by IR, H-1PV Infection, and Combination Treatment
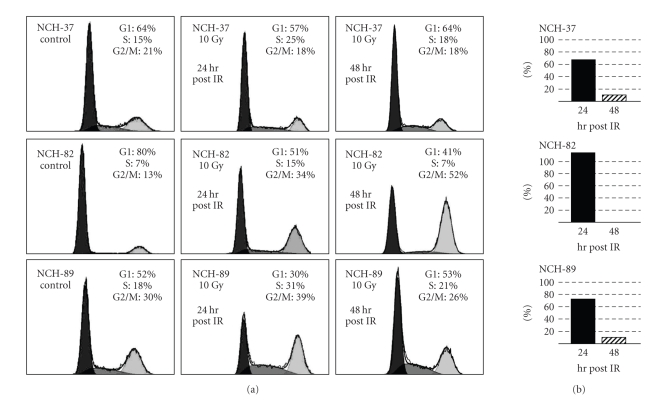
One possible mechanism for an improved cytotoxicity of H-1PV infection after IR could be associated to changes of the cell cycle as H-1PV replication is restricted to cells in S-phase. We therefore analyzed the effect of IR on the cell cycle of glioma cell lines (NCH-37, NCH-82, NCH-89). IR of glioma cell cultures with 10 Gy resulted in an increase of cells in S-phase 24 hours post-IR (Figure 5). In NCH-37 the increase was +67%, in NCH-82 +114%, and in NCH-89 +72% (Figure 5(b)). The increase was transient as 48 hours post-IR; the amount of cells in the S-phase decreased to +12% in NCH-37, to control level in NCH-82, and to +10% in NCH-89. In parallel, at this time, the overall cell cycle distribution of NCH-37 and NCH-89 cells had almost returned to control levels, whereas NCH-82 cells were blocked in G2/M (Figure 5(a)) and reached control levels 4 days post-IR (data not shown). In comparison, upon H-1PV infection, cell cultures showed less homogeneous results. While NCH-82 and NCH-89 cells that were most sensitive to H-1 infection showed increased ratios of cells in S-phase, the amount of less sensitive NCH-37 cells in S-phase was unchanged. The cell cycle changes after combination of radiation therapy and H-1PV infection were identical to H-1PV infection alone (data not shown).
Figure 5.
Effects of ionizing radiation (IR) on the cell cycle of human high-grade glioma cells. Short-term cultures of human gliosarcoma NCH-37, human glioblastoma NCH-82, and human glioblastoma NCH-89 were irradiated with 10 Gy, and cell cycle analyses were performed 24 hours post-IR (a, middle column) or 48 hours post-IR (a, right column). Control cells (a, left column) were transported to the accelerator but not exposed to IR (ctrl.). (b) IR induced increase of S-Phase fraction in percent of unirradiated control cells.
4. Discussion
Oncolytic virotherapy is a promising new approach for the treatment of a variety of malignancies including malignant brain tumors. In early phase clinical trials, intracerebral infection of patients with oncolytic viruses of different genera was well tolerated. However, only in a few patients tumor regression and a prolongation of progression-free survival could be demonstrated. This led to numerous attempts not only to further improve the oncolytic activity of viruses but also to investigate the combination treatment of viral infection of tumor cells with established conventional therapies.
Radiation therapy is a standard treatment of patients with high-grade malignant gliomas. It is administered locally to the tumor region and the surrounding brain tissue. Recurrences occur in 70 to 80% of cases within 2 cm of the primary tumor site and will therefore develop from cells that were already hit by a radiation dose of up to 60 Gy. As radiation therapy is known to induce long-term changes in cellular genomes, this could potentially lead to an altered effect of virus infection compared with unirradiated primary glioma cells. In addition to other studies, we therefore specifically investigated the oncolytic potential of H-1PV in glioma cells that grew from irradiated clones.
A cell culture established from a recurrent glioma that was irradiated with 56 Gy during the initial treatment of the patient, and that had its origin in the radiation field, was fully permissive to H-1PV infection and cell killing was dose-dependent. This finding was confirmed in primary glioma cultures that were irradiated in vitro with a sublethal dose, allowed to regrow, and infected with H-1PV in a time interval of several days. The intact susceptibility of glioma cells to H-1PV infection after IR is of clinical significance as patients with recurrent gliomas who face an even worse prognosis with oftentimes less therapeutic options are prime candidates for experimental therapies. As a consequence, the group of patients with recurrent gliomas is usually the main patient population of early clinical trials of oncolytic virotherapy. However, to our knowledge the response of previously irradiated glioma cells to the oncolytic infection has never been specifically addressed for other oncolytic viruses.
When H-1PV infection was performed early after IR, our data show improved killing of glioma cells, most pronounced in the most radioresistant cell line tested. These findings are in line with studies conducted with other oncolytic viruses that is, Herpesvirus [18], Adenovirus [24], Reovirus [20], and Measles virus [21] that also showed an enhanced efficacy of viral oncolysis in combination with radiation therapy. Whether this effect can also be demonstrated in vivo was beyond this proof of concept study and should be addressed in future experiments.
When radiation treatment was performed one day before H-1PV infection, combination treatment was significantly better in all cell lines than single treatment. Virus infection followed by IR was less efficient in all cell cultures and had a reduced cytotoxic effect. One possible reason for the improved cytotoxicity of H-1PV after IR is the increased level of glioma cells positive for NS-1 expression 24 hours after early infection. Previous studies revealed that NS-1 is the key-mediator of parvoviral cytotoxicity and its expression is strongly S-Phase dependent [14, 25]. Cell cycle analyses revealed that in all primary glioma cell cultures tested, the rate of cells in S-phase was increased 24 hours post-IR. Even though improved viral cytotoxicity may depend on several factors, this altered cell cycle distribution supports the finding of increased viral transcription and increased cell killing. As a consequence, when cells were infected when the cell cycle had returned to control levels (late infection), NS-1 expression decreased.
The glioma cell cultures in our study, like other glioma cell lines, were relatively radio-resistant and even after treatment with 20 Gy remaining cell clones continued to grow. Recent data suggests that stem-like cells exist within high-grade gliomas which are radioresistant and capable of initiating tumour regrowth. This is considered to be due to upregulated DNA damage checkpoint pathways [26]. In our system, neither radiation therapy nor H-1PV infection was able to kill all tumor cells. However, combined treatment with a high-radiation dose resulted in complete cytotoxicity in all cell cultures, indicating improved efficacy also in relatively resistant clones. These results may warrant to test whether the combination of radiation and H-1PV infection could also overcome the resistance of glioma cells expressing stem cell markers thereby offering a new treatment opportunity in these therapy refractory cells.
In conclusion, irradiated glioma-cells show intact susceptibility for H-1PV infection with even improved cell killing by combining IR with H-1PV, most pronounced in radioresistant glioma cells. These results further support the ongoing development of a phase-I clinical trial for the use of H-1PV in malignant gliomas, allowing for the inclusion of pretreated patients into the study population.
Conflicts of Interest Notification
The authors DO NOT have a financial interest/arrangement or affiliation with one or more organisations that could be perceived as a real or apparent conflict of interest in the context of the subject of this article.
Acknowledgment
Karsten Geletneky and Andreas D. Hartkopf contributed equally to this work.
References
- 1.Maher EA, Furnari FB, Bachoo RM, et al. Malignant glioma: genetics and biology of a grave matter. Genes and Development. 2001;15(11):1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 2.Zhu Y, Parada LF. The molecular and genetic basis of neurological tumours. Nature Reviews Cancer. 2002;2(8):616–626. doi: 10.1038/nrc866. [DOI] [PubMed] [Google Scholar]
- 3.DeAngelis LM. Chemotherapy for brain tumors—a new beginning. The New England Journal of Medicine. 2005;352(10):1036–1038. doi: 10.1056/NEJMe058010. [DOI] [PubMed] [Google Scholar]
- 4.Combs SE, Debus J, Schulz-Ertner D. Radiotherapeutic alternatives for previously irradiated recurrent gliomas. BMC Cancer. 2007;7, article 167 doi: 10.1186/1471-2407-7-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stupp R, Dietrich P-Y, Kraljevic SO, et al. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. Journal of Clinical Oncology. 2002;20(5):1375–1382. doi: 10.1200/JCO.2002.20.5.1375. [DOI] [PubMed] [Google Scholar]
- 6.Markert JM, Yancey Gillespie G, Weichselbaum RR, Roizman B, Whitley RJ. Genetically engineered HSV in the treatment of glioma: a review. Reviews in Medical Virology. 2000;10(1):17–30. doi: 10.1002/(sici)1099-1654(200001/02)10:1<17::aid-rmv258>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 7.Bischoff JR, Kirn DH, Williams A, et al. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274(5286):373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 8.Gromeier M, Lachmann S, Rosenfeld MR, Gutin PH, Wimmer E. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(12):6803–6808. doi: 10.1073/pnas.97.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilcox ME, Yang W, Senger D, et al. Reovirus as an oncolytic agent against experimental human malignant gliomas. Journal of the National Cancer Institute. 2001;93(12):903–912. doi: 10.1093/jnci/93.12.903. [DOI] [PubMed] [Google Scholar]
- 10.Stojdl DF, Lichty B, Knowles S, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nature Medicine. 2000;6(7):821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 11.Allen C, Paraskevakou G, Liu C, et al. Oncolytic measles virus strains in the treatment of gliomas. Expert Opinion on Biological Therapy. 2008;8(2):213–220. doi: 10.1517/14712598.8.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrero YCM, Cornelis JJ, Herold-Mende C, Rommelaere J, Schlehofer JR, Geletneky K. Parvovirus H-1 infection of human glioma cells leads to complete viral replication and efficient cell killing. International Journal of Cancer. 2004;109(1):76–84. doi: 10.1002/ijc.11626. [DOI] [PubMed] [Google Scholar]
- 13.Di Piazza M, Mader C, Geletneky K, et al. Cytosolic activation of cathepsins mediates parvovirus H-1-induced killing of cisplatin and TRAIL-resistant glioma cells. Journal of Virology. 2007;81(8):4186–4198. doi: 10.1128/JVI.02601-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rommelaere J, Cornelis JJ. Autonomous parvoviruses. In: Hernaiz DP, Rabkin SD, editors. Replication-Competent Viruses for Cancer Therapy. Vol. 22. Basel, Switzerland: Karger; 2001. pp. 100–129. (Monographs in Virology). [Google Scholar]
- 15.Laperriere N, Zuraw L, Cairncross G. Radiotherapy for newly diagnosed malignant glioma in adults: a systematic review. Radiotherapy and Oncology. 2002;64(3):259–273. doi: 10.1016/s0167-8140(02)00078-6. [DOI] [PubMed] [Google Scholar]
- 16.Aghi M, Martuza RL. Oncolytic viral therapies—the clinical experience. Oncogene. 2005;24(52):7802–7816. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- 17.Advani SJ, Mezhir JJ, Roizman B, Weichselbaum RR. ReVOLT: radiation-enhanced viral oncolytic therapy. International Journal of Radiation Oncology Biology Physics. 2006;66(3):637–646. doi: 10.1016/j.ijrobp.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 18.Advani SJ, Sibley GS, Song PY, et al. Enhancement of replication of genetically engineered herpes simplex viruses by ionizing radiation: a new paradigm for destruction of therapeutically intractable tumors. Gene Therapy. 1998;5(2):160–165. doi: 10.1038/sj.gt.3300546. [DOI] [PubMed] [Google Scholar]
- 19.Chu RL, Post DE, Khuri FR, Van Meir EG. Use of replicating oncolytic adenoviruses in combination therapy for cancer. Clinical Cancer Research. 2004;10(16):5299–5312. doi: 10.1158/1078-0432.CCR-0349-03. [DOI] [PubMed] [Google Scholar]
- 20.Twigger K, Vidal L, White CL, et al. Enhanced in vitro and in vivo cytotoxicity of combined reovirus and radiotherapy. Clinical Cancer Research. 2008;14(3):912–923. doi: 10.1158/1078-0432.CCR-07-1400. [DOI] [PubMed] [Google Scholar]
- 21.Liu C, Sarkaria JN, Petell CA, et al. Combination of measles virus virotherapy and radiation therapy has synergistic activity in the treatment of glioblastoma multiforme. Clinical Cancer Research. 2007;13(23):7155–7165. doi: 10.1158/1078-0432.CCR-07-1306. [DOI] [PubMed] [Google Scholar]
- 22.Faisst S, Faisst SR, Dupressoir T, et al. Isolation of a fully infectious variant of parvovirus H-1 supplanting the standard strain in human cells. Journal of Virology. 1995;69(7):4538–4543. doi: 10.1128/jvi.69.7.4538-4543.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox MH. A model for the computer analysis of synchronous DNA distributions obtained by flow cytometry. Cytometry. 1980;1(1):71–77. doi: 10.1002/cyto.990010114. [DOI] [PubMed] [Google Scholar]
- 24.Zeng M, Cerniglia GJ, Eck SL, Stevens CW. High-efficiency stable gene transfer of adenovirus into mammalian cells using ionizing radiation. Human Gene Therapy. 1997;8(9):1025–1032. doi: 10.1089/hum.1997.8.9-1025. [DOI] [PubMed] [Google Scholar]
- 25.Wolter S, Richards R, Armentrout RW. Cell cycle-dependent replication of the DNA of minute virus of mice, a parvovirus. Biochimica et Biophysica Acta. 1980;607(3):420–431. doi: 10.1016/0005-2787(80)90152-5. [DOI] [PubMed] [Google Scholar]
- 26.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]