Abstract
The BRIDGES (Biological Response Indicator Devices Gauging Environmental Stressors) bio-analytical tool was developed in response to the need for a quantitative technology for assessing the toxicity of environmentally relevant contaminant mixtures. This tool combines passive samplers with the embryonic zebrafish model. When applied in an urban river it effectively linked site specific, bioavailable contaminant mixtures to multiple biological responses. Embryonic zebrafish exposed to extracts from lipid-free passive samplers that were deployed at five locations, within and outside of the Portland Harbor Superfund Megasite, displayed different responses. Six of the eighteen biological responses observed in 941 exposed zebrafish were significantly different between sites. This demonstrates the sensitivity of the bio-analytical tool for detecting spatially distinct toxicity in aquatic systems; bridging environmental exposure to biological response.
Keywords: Passive sampling device, zebrafish, bioavailable, mixture toxicity, Superfund
Introduction
Human and ecosystem exposure to contaminants generally involves complex mixtures of chemicals. Determining the concentrations of a wide range of chemicals in an environmental matrix is limited to the detection of known compounds and may often exclude toxicologically relevant chemicals. Toxicological studies tend to focus on the effects of exposure to a pure chemical or specific class of chemicals. Mixture toxicity is not well understood but recent studies demonstrate non-additive toxic effects elicited by chemical mixtures (Incardona et al., 2004; Wassenberg and Di Giulio, 2004; Boobis et al., 2008; Duan et al., 2008). Present day risk assessment models are inadequate for predicting toxic effects of complex chemical mixtures because they do not take into account interactions between components that cause synergistic, potentiating or inhibiting effects (Dardenne et al., 2008).
There is a need for environmental assessment methods that address the issue of determining the toxicity of environmentally relevant complex mixtures (Eggen et al., 2004; Collins et al., 2008). In response to this need Biological Response Indicator Devices Gauging Environmental Stressors (BRIDGES) was developed to bridge the gap between real-life exposure scenarios and toxicity. We demonstrate the feasibility of conjoining two established technologies, passive sampling devices and the embryonic zebrafish model, to create a rapid throughput bio-analytical tool that assesses multiple biological responses to environmentally relevant contaminant mixtures in a whole organism vertebrate model.
Passive sampling devices (PSDs) are used extensively for the assessment of contamination in air, water and soil (Mayer et al., 2003). They sequester and concentrate the freely dissolved portion of a variety of hydrophobic organic contaminants (Adams et al., 2007). PSDs mimic bioconcentration mechanisms, such as diffusion through biomembranes and partitioning between an organism and it's medium (Huckins et al., 2006). They are thought to be adequate biological surrogates for the uptake of many organic contaminants and do not present the disadvantages inherent in using organisms for environmental monitoring, such as motility, growth and metabolism (Awata et al., 1999; Wells and Lanno, 2001; Zhang et al., 2006). PSDs provide a time integrated concentration of the freely dissolved, bioavailable, fraction of a wide range of analytes (Huckins et al., 2006). Lipid-free tubing (LFT) is a polyethylene membrane with demonstrated capacity for sequestering organic contaminants from waters. Unlike other PSDs, such as the semipermeable membrane devices (SPMDs), LFTs do not contain triolein or other lipids, which facilitates clean-up, analysis and modeling of results (Anderson et al., 2008).
Bioassays are experiments designed to evaluate the ability of contaminants to cause certain biological responses, their potency in doing so, and the nature of the dose-response relationship (Hill et al., 2005). The embryonic zebrafish has been identified as an ideal organism for in vivo, full organism bioassays (Hill et al., 2005; Usenko et al., 2007; Renner, 2008) and is widely used by researchers in a variety of fields. Zebrafish have many advantages over other vertebrate bioassay models with respect to their size, husbandry and early morphology. The small size of the fish reduces housing costs and allows for larger sample sizes. Zebrafish are very fecund, producing up to 200 eggs per adult every 5-7 days. Furthermore, the embryos are nearly transparent, allowing for clear non-invasive visualization of internal organs (Hill et al., 2005). A number of molecular tools are also in place to permit integrative studies of the mechanisms of action underlying observed non-specific biological responses (Vogel, 2000).
There is a recognized need to connect effective and efficient environmental sampling directly to toxicity evaluations and risk assessment (Eggen et al., 2004; Collins et al., 2008). Research to chemically characterize the Portland Harbor Superfund Megasite has been ongoing for many years (Sethajintanin et al., 2004; Sower and Anderson, 2008; Integral et al., 2009). This study does not seek to present additional chemical data but rather to demonstrate the potential advantage of utilizing a complementary bioassay tool in combination with a fit-for-purpose sampling methodology for environmental and risk assessment. A limited number of publications address the possibility of using environmental samples obtained from PSDs in toxicity bioassays (Parrott and Tillitt, 1997; Parrott et al., 1999; Sabaliunas et al., 2000; Heinis et al., 2004; Petty et al., 2004; Ma et al., 2005; Ke et al., 2007; Springman et al., 2008). However, the majority of these studies use in vitro assays or assess only a single biological effect. This present study is the first report of coupling passive sampler technology with the assessment of multiple developmental biological responses in a whole organism vertebrate model. The toxicity of environmentally relevant chemical mixtures was assessed using the embryonic zebrafish model and LFT passive samplers deployed in a model river system. Furthermore, we evaluate differences in the biological responses observed in the zebrafish model related to the spatial deployment of LFT in the river system; Superfund versus upriver or downriver sites, in an extract concentration-dependent manner.
Materials and Methods
PSD deployment and processing
Study area
Like many urban rivers, the lower Willamette River, Portland, OR, has been the site of heavy industrial use. The area between river miles (RM) 3.5 and 9.2 was designated a Superfund Megasite in 2000 due to contamination with a number of urban and industrial contaminants including metals, polyaromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), dioxins and organochlorine pesticides (USEPA, 2000). Remediation within the Superfund Megasite is ongoing. A sediment cap was placed over 23 acres of creosote contaminated sediment at the McCormick and Baxter Superfund site at RM 7 east (E) in 2004. Over 11,500 m3 of coal tar was removed from RM 6.3 west (W), the GASCO site within the Portland Harbor Megasite in 2005 (Sower and Anderson, 2008). The Willamette River is populated by resident and migratory fish populations and extensively used by sport and subsistence anglers and recreational boaters (Sethajintanin et al., 2004; Sower and Anderson, 2008). The Portland Harbor Superfund Megasite is a representative river system to investigate the availability and developmental health consequences of urban and industrial compounds to aquatic organisms and, ultimately, to humans.
The study area consists of five locations; upstream (RM 17E), within (RMs 3.5E, 7W, 7E) and downstream (RM 1E) of the Portland Harbor Superfund Megasite (Figure 1). The site locations were selected to coincide with past studies that quantify freely dissolved fractions of PAHs (Anderson et al., 2008; Sower and Anderson, 2008), PCBs and organochlorine pesticides (Anderson et al., 2008) in the surface water using passive sampling devices.
Figure 1.
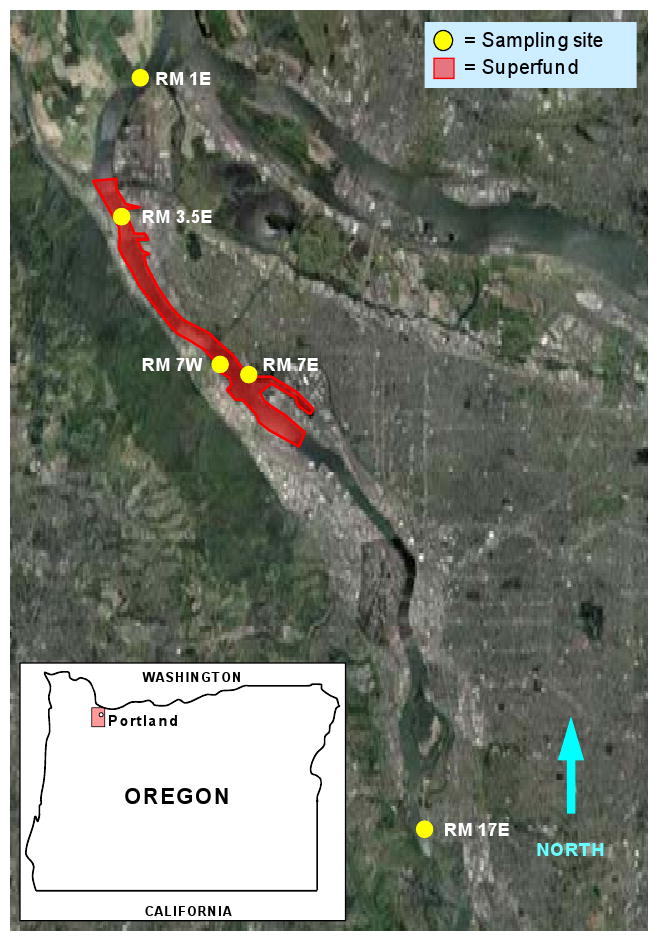
The Lower Willamette River, OR (north flowing). LFT passive samplers were deployed in the water column, 10 ft above the substrate, at the sites indicated by the yellow circles. The Portland Harbor Superfund Megasite is outlined in red. The McCormick and Baxter Superfund site is located on the east bank at river mile 7 (RM 7E).
Sample collection
The PSDs deployed in the lower Willamette River were lipid-free tubing (LFT). Details about LFT preparation, deployment and extraction can be found in Anderson et al (2008). Briefly, additive-free low-density polyethylene membrane (lay-flat tubing) was cleaned with optima grade hexanes then heat sealed at both ends (final dimensions 2.7 × 100 cm). Unspiked LFT (not containing performance reference compounds) were deployed at 5 sites in the lower Willamette River for 21 days in May, 2006. Five LFT were co-deployed in a single stainless steel cage at each sampling site. Following exposure, LFTs were transported to the lab in coolers, extracted into hexanes and split. One part of the split LFT extract was solvent exchanged to dimethyl sulfoxide (DMSO) for the embryonic zebrafish exposures, while the other was kept in hexanes for chemical analysis.
Zebrafish rearing and preparation
Embryos were collected from the Tropical 5D strain of zebrafish (Danio rerio) reared in the Sinnhuber Aquatic Research Laboratory (SARL) at Oregon State University. Adults were kept at standard laboratory conditions of 28 °C on a 14 h light/10 h dark photoperiod. Fish water (FW) consisted of reverse osmosis water supplemented with a commercially available salt solution (0.6% Instant Ocean©). Zebrafish were group spawned and embryos were collected and staged as described by Kimmel et al. (1995).
Zebrafish embryos were exposed to extract solutions via FW exposure. Embryo exposures were initiated between 4-6 hours post fertilization (hpf), prior to the commencement of organogenesis (Kimmel et al., 1995). Preceding exposure, the chorion, an acellular envelope surrounding the embryo, was removed by pronase treatment to minimize blockage of chemical uptake (Mizell and Romig, 1997). Dechorionation was carried out at 2-4 hpf, after which embryos were maintained for one hour then assessed for viability.
Zebrafish exposure scenarios and biological response assessment
At approximately 6 hpf, dechorionated embryos were transferred individually to wells of a 96-well glass-coated plate (Sunsri Systems) containing 100 μl of LFT extract in FW solution. Static extract solution exposures were carried out at three LFT extract concentrations: 1%, 0.5%, and 0.3% of the original LFT extract solution diluted from 100% to 1% DMSO in FW. Control embryos were exposed to 1% DMSO, to account for vehicle effects, and FW to ensure embryo batch quality. Exposures to 1% blank LFT (never deployed in the field) extract concentrations were also carried out to control for possible toxic effects of membrane extracts. Three replicates of 24 fish were exposed per treatment level, totaling a maximum of 72 fish per group. Embryos were assessed immediately after transfer to the wells to confirm viability. Fish that did not survive transfer were not included in the assessment scoring.
Visual observations of developmental endpoints were performed using a stereo microscope at 30 and 126 hpf. Each zebrafish was binary scored (‘present’ or ‘absent’) in vivo for biological responses including mortality or morphologic malformations (Table 1). A ‘present’ scoring indicates malformation or abnormal development compared to normal embryonic zebrafish of the same age as described by Kimmel et al (1995).
Table 1.
Developmental endpoints assessed for abnormal development compared to normal embryonic zebrafish of the same age. The embryonic zebrafish metric (EZM) is based on a 24 point scale and is obtained by summing the EZM scores for every endpoint observed in an individual fish. The sum of all sublethal endpoints is less than the score associated with mortality.
| Toxic Endpoint | EZM Score |
|---|---|
| Mortality at 30 hpf | 24 |
| notochord at 30 hpf | 1.275 |
| mortality at 126 hpf | 21.6 |
| notochord at 126 hpf | 1.275 |
| heart | 1.275 |
| brain | 1.275 |
| yolk sac | 1.275 |
| body | 1.275 |
| circulation | 1.275 |
| eye | 1.275 |
| jaw | 1.275 |
| tail | 1.275 |
| somites | 1.275 |
| caudal fin | 1.275 |
| pectoral fin | 1.275 |
| snout | 1.275 |
| body axis | 1.275 |
| otic vessels | 1.275 |
Eighteen individual developmental endpoints were assessed in each embryo. Furthermore, an integrative embryonic zebrafish metric (EZM), adapted from the EZM for nanomaterial toxicity (EZMNT) (Harper et al., 2008) was used to assess overall toxic effects. Briefly, the EZM is based on a 0 to 24 point metric scale, relating to the 24 fish treatment grouping. The maximum EZM score for any individual fish is 24, which indicates mortality at the first assessment point. Mortality at the later assessment point has a score of 21.6 and the sum of all other sublethal developmental endpoints is 21. The assignation of the relative values for the sublethal biological responses was non-hierarchical; only mortality was valued higher than other endpoints. The use of the EZM allows for comparison of individual organisms with multiple different endpoints through a single integrative score. A list of all the developmental endpoints assessed in this study and their associated EZM values is presented in Table 1.
Chemical Analysis
The LFT extracts were screened for chemical classes of concern using standard methods on gas chromatography mass spectrometry (GC/MS) and gas chromatography electron capture detection (GC/ECD). The chemicals screened for included parent and substituted PAHs, PCBs and pesticides; all of which have been previously reported for the Portland Harbor Superfund Megasite (Sower and Anderson, 2008; Integral et al., 2009). More than forty compounds were identified in the LFT extracts including legacy and current use pesticides, PCBs, 10 of the U.S. Environmental Protection Agency's 16 priority PAHs, 2 other parent PAHs, 3 oxy-PAHs and 7 methyl-PAHs. These results are in accordance with prior reports and demonstrate the presence of complex chemical mixtures in the environment.
Statistical Analysis
Results from the observations of the embryos were grouped according to the 5 LFT deployment sites (RM) and the 3 LFT extract concentration levels (1%, 0.5%, 0.3%) for each toxic endpoint or EZM. Comparison of EZM integrative response scores between groups was carried out by Kruskal-Wallis (n = 59 to 70). Comparison tests of individual binary scored effects were performed using multiple logistic regressions, likelihood ratio (n = 941). A p-value of <0.05 was considered statistically significant. All statistical calculations were performed using Sigmaplot v. 11 (Systat Software Inc., San Jose, CA, USA).
Results
Sublethal biological responses included malformations of the heart, yolk sac, tail and notochord, among others not pictured (Figure 2). No significant differences were observed between the three control groups; FW, 1% DMSO and 1% blank LFT extract. This indicates that the LFT extract does not elicit biological responses above basal levels.
Figure 2.
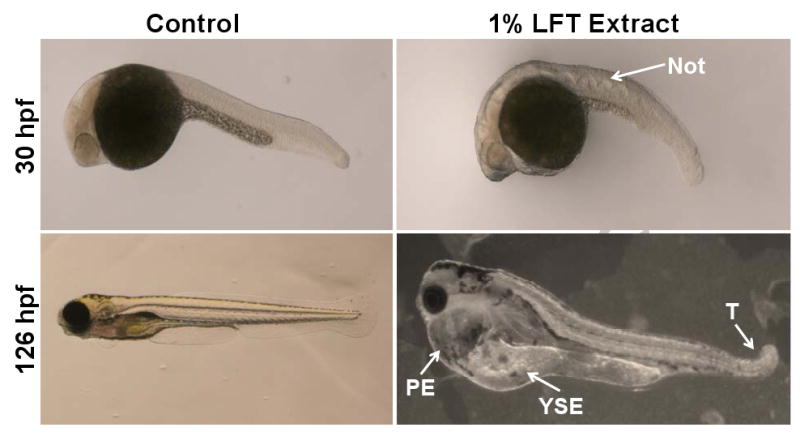
Abnormal developmental morphological endpoints observed in embryonic zebrafish exposed to contaminant mixtures from extracts of LFTs deployed in the lower Willamette River, Spring 2006. Dechorionated embryos were exposed to LFT extract solution or 1% DMSO (vehicle control) in fish water at approximately 6 hours post fertilization. Representative sublethal toxic effects, observed by stereo microscope at 30 and 126 hpf, included notochord waviness (Not), pericardial edema (PE), yolk sac edema (YSE), and bent tail (T).
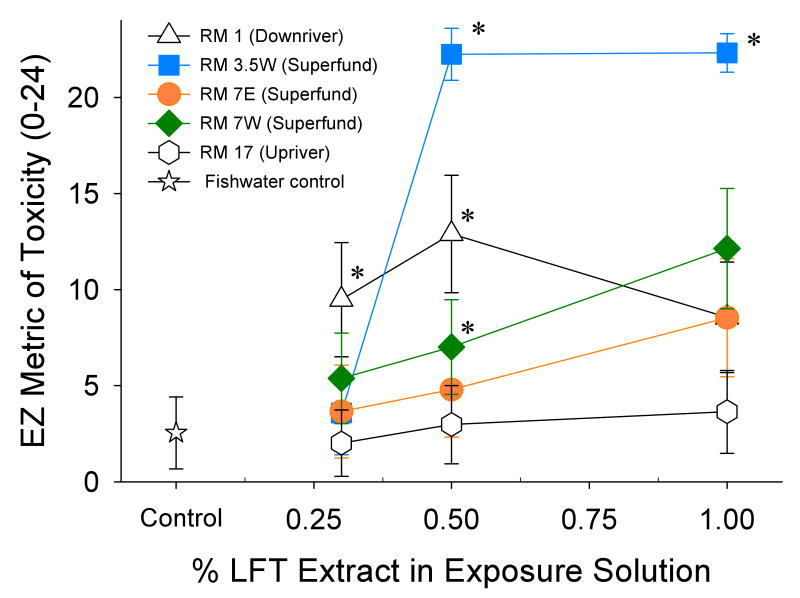
To gain an overview of the general toxicity of the LFT extracts, an initial assessment was performed using the EZM scoring system (Figure 3). The EZM integrates multiple biological responses into a single, non-specific metric that facilitates general comparisons between treatment groups. For the lower Willamette River sites, 6 out of the 15 EZM scores from the 5 sites and 3 exposure concentrations were significantly greater than the control group (p<0.05). The two highest LFT extract concentrations (1% and 0.5%) from RMs 3.5E and 7W, as well as the 0.5% and 0.3% concentrations from RM 1E had greater EZM scores than the DMSO control (Figure 3). None of the extract dilutions from RM 17E, the site located upriver from the Superfund Megasite, generated an EZM greater than the control.
Figure 3.
Comparison of the integrative EZM (mean ± 95% CI) of embryonic zebrafish exposed to different concentrations of extract solution obtained from LFTs deployed in the lower Willamette River, Spring 2006 (n=941). Asterisks (*) indicate significant differences relative to control embryos (1% DMSO). Sampling sites located within the Superfund area, river mile (RM) 3.5E, 7E, 7W, are represented by solid symbols.
A LFT extract concentration-response was observed for the three sites located within the Superfund (3.5E, 7E and 7W), but not for the upstream or downstream sites. The two highest concentrations from RM 3.5E elicited significantly higher EZM scores than the 0.3% concentration (p<0.05) from this site. The high EZM observed for RM 3.5E reflects the elevated occurrence of embryonic mortality elicited by these extracts. The highest concentration from RM 7E obtained a higher EZM than the 0.3% concentration, which was the same trend observed for RM 7W (p<0.05).
Significant differences between sites, at the same LFT extract concentrations, were also observed (p<0.05). At the highest LFT extract concentration used in exposures (1%), the EZM for RM 3.5E was significantly greater than all other sites except RM 7W. At that same extract concentration, RM 7W was greater than RM 17E. The EZM for the 0.5% LFT extract, from RM 3.5E was significantly greater than for all other sites. At this concentration, RM 1E was greater than RM 17E. Only one difference between sites was observed at the lowest concentration; RM 1E had a higher EZM than RM 17E.
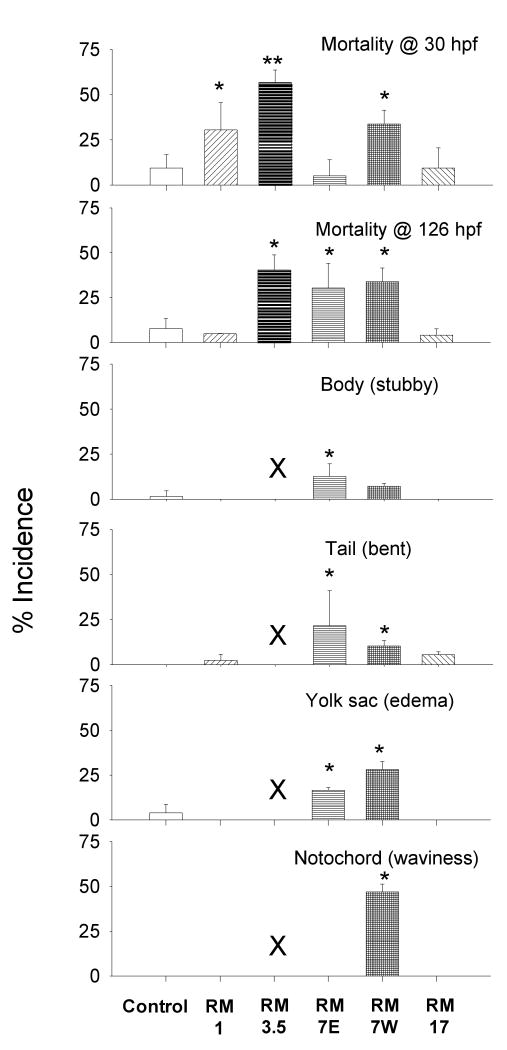
To gain a more detailed understanding of the site-specific biological responses, the occurrence of individual endpoints elicited by the highest LFT extract concentrations (1%) at different sites were compared (Figure 4). Of the 18 individual developmental endpoints observed, 6 had a significantly higher occurrence in zebrafish embryos that were exposed to LFT extracts than the control (p<0.05). The highest mortality at 30 hpf was elicited by extract from RM 3.5E, however RM 1E and 7W were also greater than the control. A higher occurrence of mortality at 126 hpf was observed at the three sites within the Superfund than at the other sites. Because of the high incidence of mortality elicited by the extract from RM 3.5E, this site was not included in the comparisons of sublethal toxic effects. Extracts from RM 7E elicited more underdeveloped bodies (stubby) than any other site. RMs 7E and 7W had a greater occurrence of the bent tail and yolk sac edema developmental endpoints. Finally, notochord waviness was observed only in embryos exposed to extracts from RM 7W. The analysis of individual effects provides different insights into site-specific toxicity than the EZM.
Figure 4.
Spatial comparison of mortality and sublethal toxic effects in embryonic zebrafish exposed to 1% extract concentration from LFTs deployed at distinct river miles (RM) along the lower Willamette River, OR, Spring 2006 (mean ± 95% CI, n=3 groups, total of 941). Asterisks (*, **) represent significant differences. Due to a high incidence of mortality, sublethal effects were not considered for RM 3.5E (indicated by X).
Discussion
Conventional analytical approaches have not adequately addressed many important human and environmental health questions related to relevant exposure scenarios and biological responses. The gap between environmental measurements and toxicity is further widened as most biological exposure studies are not conducted at environmentally relevant concentrations and are not performed with realistic mixtures typical of contaminated sites (Wright and Welbourne, 2001). The BRIDGES bio-analytical tool is an integrative approach that effectively links site specific, bioavailable contaminant mixtures to multiple biological responses in a whole organism model.
Embryonic zebrafish exposed to extracts from LFT that were deployed at five distinct locations, within and outside of the Portland Harbor Superfund Megasite, displayed six significant developmental endpoints; mortality at both time points, underdeveloped bodies, bent tail, yolk sac edema and notochord waviness. Both the type and frequency of toxic endpoints observed were significantly different between sites (Figure 4). For example, mortality at both time points was significantly higher for the extracts from the Superfund Megasite. Within the Superfund Megasite, incidence of mortality at RM 3.5E was the highest; great enough that this site was excluded from analysis of sublethal effects due to the small number of live embryos. The general site-specific trend in mortality observed in embryonic zebrafish is consistent with measurements of PAHs in the lower Willamette River obtained in a different study during the same period; higher total concentrations were observed within the Superfund Megasite, the highest at RM 3.5E (Sower and Anderson, 2008).
Of particular interest were the differences in the specific endpoints elicited between sites. Notochord waviness was only observed in embryos exposed to the extract from RM 7W, whereas a significantly higher incidence of underdeveloped bodies was only observed for RM 7E. These sites are located at the same river mile, on opposite banks, but showed markedly different toxicity. Differences in the concentrations of PAHs, PCBs, organochlorine pesticides and other chemicals of concern have been detected for these two sites in previous studies (Sower and Anderson, 2008; Integral et al., 2009). Both RM 7 sites had a greater occurrence of bent tails and yolk sac edema than the other sites, including the site located downstream of the Superfund Megasite. These results demonstrate the sensitivity of this rapid throughput, full organism vertebrate model for detecting distinct toxicity in aquatic systems even within the spatially reduced area of the Portland Harbor Superfund Megasite.
The developmental endpoints observed in this study have been associated with exposures to certain individual contaminants or mixtures in previous zebrafish studies, although by the nature of the model the biological responses are non-specific to a mechanism of action. For example, exposure to PAHs leads to mortality, body axis defects and edemas among other effects (Incardona et al., 2004). Early developmental dithiocarbamate exposures cause notochord distortions in exposed zebrafish (Tilton et al., 2006). Perfluorooctanesulfonate (PFOA) exposure leads to yolk sac edema, tail malformation, underdeveloped bodies and spinal curvature (Shi et al., 2008). The endpoints observed in this study cannot at this time be associated with specific contaminants sequestered by the deployed LFT extract. Future research will focus on the determination of the bio-active contaminants responsible for mode(s) of action via the zebrafish model utilizing multiple parallel approaches, such as those previously described by Eide et al (2002) and McDonald et al (2004).
The integrated EZM scoring system allows for a broad overview of general toxicity and facilitates analysis of an extract concentration-response relationship as well as simplifying comparisons between sites (Harper et al., 2008). However, there are some disadvantages to reducing all toxic endpoints observed to a single score. Subtle differences between sites, such as the frequency of specific developmental endpoints, are not apparent when using EZM for comparison and toxic effects that are unique to a particular site cannot be differentiated by the score alone. Furthermore, it is important to recognize that mortality is the most determinant endpoint in the EZM scoring system and differences in sublethal effects have less influence on the score. None the less, the EZM is a valuable initial site comparison assessment tool that provides an important overview of the general toxicity of LFT extracts.
Using the EZM, an exposure concentration-response was observed for LFT extracts from the three sites in the Superfund. The highest LFT extract concentrations from sites within the Superfund Megasite were associated with the greatest EZM scores. In contrast, a relationship between concentration and EZM was not observed for the upstream and downstream extracts. The inclusion of a wider range of concentrations in future studies could help to produce a more defined concentration-response curve. Furthermore, EZM allowed for differentiation between sites. The highest EZM scores were observed for the two highest concentrations from RM 3.5E, which was driven by mortality. Other differences were observed between extracts from sites; in general superfund sites or the downstream location (RM 1E) elicited higher EZM scores than the upstream locations (RM 17E). There is potential for this simple integrated scoring system to be refined and adapted to specific research goals and site assessment in the future.
Along with the results of this study, PSD extracts have proven feasible for linking bioavailable contaminant concentrations to biological responses in several promising proof-of-concept bioassay studies (Ma et al., 2005; Ke et al., 2007). Parrott et al. (1999) and Parrot and Tillitt (1997) investigated EROD (ethoxyresorufin-O-deethylase) induction in fish liver cell lines upon exposure to SPMD extracts. Petty et al. (2004) have reported endocrine effects with the VGT (vitellogenin) and the yeast estrogen screen (YES) assays using PSD extracts. Standard toxicity and genotoxicity bioassays, such as the Daphtox kit, the Ames mutagenicity test, and the Microtox test, as well as whole organism bioassays have been reported to be compatible with exposure to SPMD extracts (1998; Sabaliunas et al., 2000; Huckins et al., 2006; Springman et al., 2008). Prior to this study, multiple developmental responses elicited by PSD extracts in a whole organism model had not been assessed.
Other researchers have observed toxic responses above basal levels as a result of bioassay exposure to field and laboratory blank SPMD extracts (Sabaliunas et al., 1998; Sabaliunas et al., 1999; Sabaliunas et al., 2000; Springman et al., 2008). This has been attributed to co-dialyzed impurities in the SPMD, such as polyethylene oligomers, oleic acid, methyl oleate and elemental sulfur (Petty et al., 2000). Whole organism bioassays and standard toxicity tests are affected by impurities found in SPMD dialysis blanks (Sabaliunas et al., 2000), which may be minimized by purchasing high purity triolein (Springman et al., 2008) and more extensive laboratory clean-up procedures requiring chlorinated solvents. Petty et al.(2000) suggest that residual methyl oleate may be removed through diffusion during field deployment, which was observed by Springman et al. (2008). In this present study, exposure of blank laboratory prepped LFT did not elicit a toxic response different from the fish water and 1% DMSO control groups. One of the distinct advantages in using LFT is that they do not contain oleic acid impurities. Consideration of cost, time and solvent use associated with increased clean-up of other PSDs highlight that the LFT may be better suited to bioassay applications.
The quantitative toxicity data obtained using the BRIDGES bio-analytical tool provides valuable insight into the differential toxicity of environmentally relevant contaminant mixtures. Ideally it would be used as a complementary tool for environmental and risk assessment in conjunction with chemical characterization of sites. Advanced bio-statistical models that provide insight into the association between chemical mixture and bioassay data are under development.
Management decisions are often based solely on single chemical data applied to an additive model using relative hazard quotients, which has been shown to be inadequate in predicting mixture toxicity (Boobis et al., 2008; Dardenne et al., 2008). Application of the BRIDGES bio-analytical tool could inform management actions by providing more accurate information through demonstrated mixture toxicity data. This could be used to validate management actions or, in cases where the results of additive models do not align with demonstrated mixture toxicity, to incite further investigate or reassessment.
Characterization and management of contaminated sites requires effective and biologically validated (Sanchez et al., 2002; Schwartz et al., 2005) quantitative tools to address how contaminants act as components of mixtures both in the environment and upon exposure to organisms and humans (U.S.DOI, 1998; Walker et al., 2001). The BRIDGES bio-analytical tool combines the LFT passive sampler with the embryonic zebrafish model. Passive sampling is a robust and cost-effective technology with the advantages of time-integrated sequestration and concentration of biologically relevant contaminants (Allan et al., 2006). The embryonic zebrafish assay is an ideal whole organism model to screen for biological responses associated with toxicity and relate them to other vertebrate systems. This study demonstrates that BRIDGES is a sensitive bio-analytical tool capable of assessing the toxicity of site specific, environmentally relevant contaminant mixtures.
Acknowledgments
The project described was supported in part by Award Numbers P42 ES016465, and ES00210 from the National Institute of Environmental Health Sciences. Sarah E. Allan was supported in part by NIEHS Training Grant Fellowship T32 ES007060 from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health. We appreciate valuable input from Stacey Harper and Jennifer Field assistance with chemical analysis from Glenn Wilson.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams RG, Lohmann R, Fernandez LA, MacFarlane JK. Polyethylene Devices: Passive Samplers for Measuring Dissolved Hydrophobic Organic Compounds in Aquatic Environments. Environ Sci Technol. 2007;41:1317–1323. doi: 10.1021/es0621593. [DOI] [PubMed] [Google Scholar]
- Allan IJ, Vrana B, Greenwood R, Mills GA, Roig B, Gonzalez C. A “toolbox” for biological and chemical monitoring requirements for the European Union's Water Framework Directive. Talanta. 2006;69:302–322. doi: 10.1016/j.talanta.2005.09.043. [DOI] [PubMed] [Google Scholar]
- Anderson KA, Sethajintanin D, Sower G, Quarles L. Field trial and modeling of uptake rates on in situ lipid-free polyethylene membrane passive sampler. Environ Sci Technol. 2008;42:4486–4493. doi: 10.1021/es702657n. [DOI] [PubMed] [Google Scholar]
- Awata H, Johnson KA, Anderson TA. Passive sampling devices as surrogates for evaluating bio availability of aged chemicals in soil. Toxicological & Environmental Chemistry. 1999;73:25–42. [Google Scholar]
- Boobis AR, Ossendorp BC, Banasiak U, Hamey PY, Sebestyen I, Moretto A. Cumulative risk assessment of pesticide residues in food. Toxicol Lett. 2008 doi: 10.1016/j.toxlet.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Collins FS, Gray GM, Bucher JR. Transforming Environmental Health Protection. Science. 2008;319:906–907. doi: 10.1126/science.1154619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardenne F, Nobels I, De Coen W, Blust R. Mixture toxicity and gene inductions: can we predict the outcome? Environ Toxicol Chem. 2008;27:509–518. doi: 10.1897/07-303.1. [DOI] [PubMed] [Google Scholar]
- Duan Z, Zhu L, Zhu L, Yao K, Zhu X. Individual and joint toxic effects of pentacholorophenol and bisphenol A on the development of zebrafish (Danio rerio) embryo. Ecotoxicol Environ Safe. 2008;71:774–780. doi: 10.1016/j.ecoenv.2008.01.021. [DOI] [PubMed] [Google Scholar]
- Eggen RIL, Behra R, Burkhardt-Holm P, Escher BI, Schweigert N. Challenges in Ecotoxicology. Environ Sci Technol. 2004;38:58A–64A. doi: 10.1021/es040349c. [DOI] [PubMed] [Google Scholar]
- Eide I, Neverdal G, Thorvaldsen B, Grung B, Kvalheim OM. Toxicological evaluation of complex mixtures by pattern recognition: Correlating chemical fingerprints of mutagenicity. Environ Health Perspect. 2002;110:985–988. doi: 10.1289/ehp.02110s6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper SL, Lee S, Tanguay RL. An EZ metric for evaluation nanomaterial biological interactions. Society of Toxicology 47th Annual Meeting.2008. [Google Scholar]
- Heinis LJ, Highland TL, Mount DR. Method for Testing the Aquatic Toxicity of Sediment Extracts for Use in Identifying Organic Toxicants in Sediments. Environ Sci Technol. 2004;38:6256–6262. doi: 10.1021/es049661c. [DOI] [PubMed] [Google Scholar]
- Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- Huckins JN, Petty JD, Booij K. Monitors of Organic Chemicals in the Environment: Semipermeable Membrane Devices. Springer; New York: 2006. [Google Scholar]
- Incardona JP, Collier TK, Scholz NL. Defects in cardiac function precede morphological abnormalities in fish embryos exposed to polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol. 2004;196:191–205. doi: 10.1016/j.taap.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Integral, Windward, Kennedy/Jenks, Anchor-QEA. Portland Harbor RI/FS Remedial Investigation Report. IC09-0003. Prepared for the Lower Willamette Group, Portland OR. Integral Consulting Inc.; Portland, OR: Windward Environmental LLC, Inc.; Seattle, WA: Kennedy/Jenks Consultants; Portland, OR: Anchor QEA LLC; Seattle, WA, Portland Oregon: 2009. [Google Scholar]
- Ke R, Li J, Qiao M, Xu Y, Wang Z. Using semipermeable membrane devices, bioassays, and chemical analysis for evaluation of bioavailable polycyclic aromatic hydrocarbons in water. Archives of Environmental Contamination and Toxicology. 2007;53:313–320. doi: 10.1007/s00244-006-0158-4. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of Embryonic Development of the Zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Ma M, Wang C, Wang Z. Assessing toxicities of hydrophobic organic pollutants in Huaihe River by using two types of sampling. Journal of Environmental Science and Health, Part A. 2005;40:331–342. doi: 10.1081/ese-200045544. [DOI] [PubMed] [Google Scholar]
- Mayer P, Tolls J, Hermens JLM, Mackay D. Equilibrium Sampling Devices. Environ Sci Technol. 2003;37:184A–191A. doi: 10.1021/es032433i. [DOI] [PubMed] [Google Scholar]
- McDonald JD, Eide I, Seagrave J, Zielinska B, Whitney K, Lawson DR, Mauderly JL. Relationship between composition and toxicity of motor vehicle emission samples. Environ Health Perspect. 2004;112:1527–1538. doi: 10.1289/ehp.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizell M, Romig ES. The aquatic vertebrate embryo as a sentinel for toxins: zebrafish embryo dechorionation and perivitelline space microinjection. Int J Dev Biol. 1997;41:411–423. [PubMed] [Google Scholar]
- Parrott JL, Backus SM, Borgmann AI, Swyripa M. The use of semipermeable membrane devices to concentrate chemicals in oil refinery effluent on the Mackenzie river. Arctic. 1999;52:125–138. [Google Scholar]
- Parrott JL, Tillitt DE. The Use of Semipermeadble Membrane Devices (SPMDs) to Concentrate Inducers of Fish Hepatic Mixed Function Oxygenase (MFO) In: Zelikoff JT, editor. Ecotoxicology: Responses, Biomarkers and Risk Assessment, an OECD workshop. SOS Publications; Fair Haven, NJ: 1997. pp. 185–196. [Google Scholar]
- Petty JD, Huckins JN, Alvarez DA, Brumbaugh WG, Cranor WL, Gale RW, Rastall AC, Jones-Lepp TL, Leiker TJ, Rostad CE, Furlong ET. A holistic passive integrative sampling approach for assessing the presence and potential impacts of waterborne environmental contaminants. Chemosphere. 2004;54:695–705. doi: 10.1016/j.chemosphere.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Petty JD, Orazio CE, Huckins JN, Gale RW, Lebo JA, Meadows JC, Echols KR, Cranor WL. Considerations involved with the use of semipermeable membrane devices for monitoring environmental contaminants. J Chromatogr. 2000;879:83–95. doi: 10.1016/s0021-9673(00)00315-0. [DOI] [PubMed] [Google Scholar]
- Renner R. A Tale of Two Fish. Environ Sci Technol. 2008;42:6784–6785. doi: 10.1021/es801813m. [DOI] [PubMed] [Google Scholar]
- Sabaliunas D, Ellington J, Sabaliuniene I. Screening Bioavailable Hydrophobic Toxicants in Surface Waters with Semipermeable Membrane Devices: Role of Inherent Oleic Acid in Toxicity Evaluations. Ecotoxicol Environ Safe. 1999;44:160–167. doi: 10.1006/eesa.1999.1802. [DOI] [PubMed] [Google Scholar]
- Sabaliunas D, Lazutka J, Sabaliuniene I. Acute toxicity and genotoxicity of aquatic hydrophobic pollutants sampled with semipermeable membrane devices. Environ Pollut. 2000;109:251–265. doi: 10.1016/s0269-7491(99)00259-6. [DOI] [PubMed] [Google Scholar]
- Sabaliunas D, Lazutka J, Sabaliuniene I, Sodergren A. Use of semipermeable membrane devices for studying effects of organic pollutants: Comparison of pesticide uptake by semipermeable membrane devices and mussels. Environ Toxicol Chem. 1998;17:1815–1824. [Google Scholar]
- Sanchez F, Thibodeaux L, Valsaraj K, Reible D. Multimedia chemical fate model for environmental dredging. Pract Per Haz Tox and Radioact Waste Managem. 2002:119–128. [Google Scholar]
- Schwartz DA, Weis B, Wilson SH. The Need for Exposure Health Sciences. Environmental Health Perspectives. 2005;113:A650. doi: 10.1289/ehp.113-1281298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethajintanin D, Johnson ER, Loper BR, Anderson KA. Bioaccumulation Profiles of Chemical Contaminants in Fish from the Lower Willamette River, Portland Harbor, Oregon. Arch Environ Contam Toxicol. 2004;46:114–123. doi: 10.1007/s00244-003-2266-8. [DOI] [PubMed] [Google Scholar]
- Shi X, Du Y, Lam PKS, Wu RSS, Zhou B. Developmental toxicity and alteration of gene expression in zebrafish embryos exposed to PFOS. Toxicol Appl Pharmacol. 2008;230:23–32. doi: 10.1016/j.taap.2008.01.043. [DOI] [PubMed] [Google Scholar]
- Sower GJ, Anderson KA. Spatial and Temporal Variation of Freely Dissolved Polycyclic Aromatic Hydrocarbons in an Urban River Undergoing Superfund Remediation. Environ Sci Technol. 2008;42:9065–9071. doi: 10.1021/es801286z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springman KR, Short JW, Lindeberg MR, Maselko JM, Khan C, Hodson PV, Rice SD. Semipermeable membrane devices link site-specific contaminants to effects: Part 1 -Induction of CYP1A in rainbow trout from contaminants in Prince William Sound, Alaska. Mar Environ Res. 2008;66:477–486. doi: 10.1016/j.marenvres.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Tilton F, LaDu JK, Vue M, Alzarban N, Tanguay RL. Dithiocarbamates have a common toxic effect on zebrafish body axis formation. Toxicol Appl Pharmacol. 2006;216:55–68. doi: 10.1016/j.taap.2006.04.014. [DOI] [PubMed] [Google Scholar]
- U.S.DOI. Guidelines for interpretation of the biological effects of selected constituents in biota, water, and sediment. United States Department of the Interior; 1998. [Google Scholar]
- Usenko CY, Harper SL, Tanguay RL. In vivo evaluation of carbon fullerene toxicity using embryonic zebrafish. Carbon. 2007;45:1891–1898. doi: 10.1016/j.carbon.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA. National Priorities List Site Narrative for Portland Harbor. US Environmental Protection Agency; Portland, Oregon: 2000. [Google Scholar]
- Vogel G. Genomics. Sanger will sequence zebrafish genome. Science. 2000;290:1671. [PubMed] [Google Scholar]
- Walker CH, Hopkin SP, Sibly RM, Peakall DB. The fate of organic pollutants in individuals and in ecosystems Principles of ecotoxicology. Taylor & Francis Inc; New York, NY: 2001. pp. 59–89. [Google Scholar]
- Wassenberg DM, Di Giulio RT. Synergistic Embryotoxicity of Polycyclic Aromatic Hydrocarbon Aryl Hydrocarbon Receptor Antagonist with Cytochrome P4501A Inhibitors in Fundulus heteroclitus. Environ Health Perspect. 2004;112:1658–1664. doi: 10.1289/ehp.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JB, Lanno RP. Passive sampling devices (PSDs) as biological surrogates for estimating the bioavailability of organic chemicals in soil. In: Greenberg BM, Hull RN, Roberts MHJ, Gensemer RW, editors. Environmental toxicology and risk assessment: science, policy and standardization - implications for environmental descisions. American Society for Testing and Materials; West Conshohocken, PA: 2001. pp. 253–270. [Google Scholar]
- Wright DA, Welbourne P. Factors affecting toxicity Environmental toxicology. Cambridge University Press; Cambridge: 2001. pp. 218–248. [Google Scholar]
- Zhang B, Smith PN, Anderson TA. Evaluating the bioavailability of explosive metabolites, hexahydro-1-nitroso-3,5-dinitro-1,3,5-triazine (MNX) and hexahydro-1,3,5-trinitroso-1,3,5-triazine (TNX), in soils using passive sampling devices. J Chromatogr. 2006;1101:38–45. doi: 10.1016/j.chroma.2005.10.007. [DOI] [PubMed] [Google Scholar]