Abstract
Background
Dengue fever is one of the most significant re-emerging tropical diseases, despite our expanding knowledge of the disease, viral tropism is still not known to target heart tissues or muscle.
Methods
A prospective pediatric clinical cohort of 102 dengue hemorrhagic fever patients from Colombia, South America, was followed for 1 year. Clinical diagnosis of myocarditis was routinely performed. Electrocardiograph and echocardiograph analysis were performed to confirm those cases. Immunohistochemistry for detection of dengue virus and inflammatory markers was performed on autopsied heart tissue. In vitro studies of human striated skeletal fibers (myotubes) infected with dengue virus were used as a model for myocyte infection. Measurements of intracellular Ca2+ concentration as well as immunodetection of dengue virus and inflammation markers in infected myotubes were performed.
Results
Eleven children with dengue hemorrhagic fever presented with symptoms of myocarditis. Widespread viral infection of the heart, myocardial endothelium, and cardiomyocytes, accompanied by inflammation was observed in 1 fatal case. Immunofluorescence confocal microscopy showed that myotubes were infected by dengue virus and had increased expression of the inflammatory genes and protein IP-10. The infected myotubes also had increases in intracellular Ca2+ concentration.
Conclusions
Vigorous infection of heart tissues in vivo and striated skeletal cells in vitro are demonstrated. Derangements of Ca2+ storage in the infected cells may directly contribute to the presentation of myocarditis in pediatric patients.
Keywords: dengue, myocarditis, inflammation, calcium transport
Dengue virus (DENV) infection is caused by 1 of 4 antigenically distinct but related single-stranded, positive-sense RNA viruses in the family Flaviviridae. This virus is transmitted by mosquito vectors, primarily Aedes aegypti. Four serotypes (DENV-1, DENV-2, DENV-3, and DENV-4) circulate worldwide.
DENV infections cause a spectrum of illnesses from self-limited fever, severe hemorrhagic manifestations, and increased vascular permeability. In some cases, neuromuscular and cardiac dysfunctions have been reported. In the acute phase of the disease, there is a systemic inflammatory response, a progressive decrease in T-cell function and an increase in apoptosis, which have been correlated with disease severity.1 The source of systemic proinflammatory cytokines and the identification of organs or tissues that support the majority of viral replication are not well established.
We followed a cohort of 102 pediatric dengue hemorrhagic fever (DHF) patients and scored the prevalence of cardiac involvement. Eleven patients were clinically diagnosed with myocarditis and further evaluations, including electrocardiogram, echocardiography, and analysis of serum values of creatine phosphokinase-MB were conducted. In one of these patients with cardiac involvement who succumbed to the disease, we detected direct viral infection of cardiomyocytes and endothelial cells as well as the presence of proinflammatory mediators in an autopsy sample of the heart. On the basis of the presence of high myocardial infection in this case, it appeared that striated muscles were a viral target.
To study the effects and consequences of dengue infection in muscle fibers, we used an in vitro system of infected human myotubes. We measured proinflammatory markers, including IP-10, and demonstrate increased levels in the infected cells and in the secreted cell culture media. We further measured resting intracellular Ca2+ concentration ([Ca2+]i) and demonstrate that the infected myotubes had significant changes in [Ca2+]i. Given that skeletal muscle is among the largest organ in the human body, we postulate that its infection by DENV may be an important source of the systemic increase of proinflammatory cytokines known to contribute to dengue pathology.
Materials and Methods
Patients
We conducted a prospective study of febrile children admitted to the Department of Pediatrics of the Hospital Universitario Hernando Moncaleano Perdomo (HUHMP) in Neiva, Colombia, between September 2005 and May 2008 This cohort (age, 1–13 years) included outpatients seeking medical care for an acute febrile illness and referred by triage from a nurse or internist physician. The entry criteria for inclusion in the present study were the presence of fever (more than 38.5°C) for at least 3 days and absence of other known infectious diseases. The severity of illness of all referred patients was graded according to the WHO criteria for DHF. The ethical committee at HUHMP approved the clinical study protocol and written informed consent was obtained from the parents or guardian of all patients before entry into the study.
Clinical Myocarditis
The diagnosis of clinical myocarditis was established after clinical evaluation of the patient by a pediatric cardiologist on the basis of history, physical examination, and investigation results in the absence of an endomyocardial biopsy. To further assess heart function, electrocardiogram and echocardiography were performed in the referred patients with myocarditis. If echocardiograms were abnormal, a test for serum creatine phosphokinase-MB fraction was performed using a commercial kit from Bayer, Inc.
Immunohistochemistry of Autopsy Tissues
Formalin fixed tissues from 1 case of fatal DHF were processed, embedded in paraffin, and were cut in 5-μm sections. Sections were then stained with hematoxylin and eosin for routine histologic evaluation. To examine the distribution of viral antigen, formalin-fixed paraffin-embedded tissues were similarly sectioned. Tissues were immunostained using an avidin-biotin horseradish peroxidase complex technique with diaminobenzidine chromogen as previously described.2,3 Following deparaffinization antigen retrieval was performed using proteinase K pretreatment. Viral antigen was detected using a murine IgG2A monoclonal anti-DV complex (MAB8705; clone D3–2H2–9-21; Millipore, Billerica, MA) diluted 1:200 in phosphate-buffered saline (PBS) and incubated overnight. A biotinylated horse antimouse secondary antibody was followed by incubation with avidin-biotin complex and diaminobenzidine chromogen. A nonspecific isotype-matched control monoclonal antibody was used on infected tissue, and staining of normal noninfected tissue was used as an additional control. All were negative for nonspecific staining. Additional phenotypic markers evaluated on myocardium included CD86 for macrophage detection and CD3 for lymphocyte detection. MCP-1 was evaluated using a polyclonal goat antihuman MCP-1 (R&D Systems AF-279-NA), and MHC II antigen was evaluated using HLA-DQ, P, R (DAKO, Carpinteria, CA). Both are previously described markers of inflammation.4
Infection of Myotubes In Vitro
Human myoblasts were plated on 10-cm plates and, after reaching ∼70% confluence in growth media (Hams F10, 20% FCS, 0.5 ng/mL bFGF), were induced to differentiate into myotubes by growth factor withdrawal accomplished by culture in medium with 2% heat inactivated horse serum (HIHS) for 3 days. Once myotubes formed, they were infected with DV2 strain New Guinea C (NGC) (m.o.i. of 2) for 2 hours at 37°C. Twenty-four hours after infection the cells were trypsinized and centrifuged twice at 700g for 5 minutes and stored at −70°C until RNA extraction.
Measurement of [Ca2+]i in Human Myotubes
[Ca2+]i was measured by means of double-barreled Ca2+-selective microelectrodes in noninfected and DV infected human skeletal myotubes. Microelectrodes were prepared as described previously.5–7 They were backfilled with the neutral carrier ETH 129 (Fluka, Ronkonkoma, NY) and then with pCa 7. Each Ca2+-selective microelectrode was individually tested as described previously5 and only those with a linear relationship between pCa3 and pCa7 (Nernstian response, 28.5 mV per pCa unit at 23°C) and a response of 16 to 20 mV between pCa 7 and pCa 8 were used experimentally. All electrodes were recalibrated after taking measurements of [Ca2+]i. Three criteria were used as key elements to accept or reject individual measurements of [Ca2+]i: (1) the calibration of the electrode before and after the [Ca2+]i determination had to agree with one another within 2.5 mV; (2) an instantaneous change in membrane resting potential (Vm and VCae) had to occur during the cell impalement, followed by a stable recording for at least 40 seconds; and (3) resting membrane potential Vm were less than −60 mV. The calcium sensitivity of the Ca2+-selective microelectrodes was not affected by any of the solutions used in the present study.
ELISA
Detection of MB creatine phosphokinase in serum was performed using a specific detection kit (Bayer). For ELISAs using cell-culture supernatants, the media covering the cells was removed 24 hours after infection, the culture supernatant was centrifuged for 10 minutes at 1000g to separate any dead cells, collected and stored at −70°C for IP-10 measurements (R&D Systems).
Infections
Monolayers of myotubes maintained in medium +2% HIHS were washed with fresh cell-culture medium at the time of infection. DENV2 strain New Guinea C (NGC), previously grown in Aedes albopictus C6/36 cell monolayers (ATTC CRL-1660) and titrated in Vero cells, was added to confluent monolayers of muscle cells at an m.o.i. of 1 to 2 and placed in the 37°C incubator. After addition of insect cell media (mock infection) or that containing virus, the supernatants were removed at 2 hours post infection from the monolayers and the cells were carefully washed 3 times at room temperature with PBS. Fresh growth medium containing 2% HIHS was added to each well for 24 hours of culture.
Quantitative Reverse Transcriptase–PCR
Taqman quantifications of dengue viral RNA were performed as previously described.8 Results were calculated using the standard curve method for in vitro infections and delta-delta CT method for clinical samples. Ten to 100 ng of total cellular RNA was reverse transcribed using TaqMan reverse transcription reagents (Applied Biosystems) in the presence of random hexamers. Beta-actin was used as an endogenous control for normalization levels of RNA. All primers and probes were obtained commercially through Applied Biosystems.
Immunostaining of Myotubes
Immunostaining for DENV was performed as described by.9 Briefly, myoblasts were plated on 22 mm Thermonox cover-slips in 10 cm plates, and transformed into myotubes then infected with DENV2 NGC (m.o.i. of 2) for 2 hours at 37°C as described above. At 24 hours postinfection, cells were washed and fixed in cold methanol for 15 minutes at −20°C, rinsed with PBS, blocked with PBS +1% BSA for 1 hours at room temperature, and then incubated overnight at 4°C with either an anti-DENV capsid or DENV NS1 monoclonal antibody at a 1:200 dilution. Cells were washed and then incubated for 1 hour with Alexa Fluor 514 goat antimouse antibody (Invitrogen). DAPI was used for nuclear visualization. Images of stained cells were obtained using a Nikon TE 2000 microscope equipped with a Yokogawa spinning disc confocal system. Images were deconvoluted using iterative deconvolution software (Image J, National Institutes of Health) and the point spread function was calculated for each objective lens using calibrated fluorescent beads. Brightness and contrast were adjusted off-line to improve clarity for each image; no data were added or deleted.
Statistics
In Vivo
Clinical signs were analyzed by nonparametric chi square method.
In Vitro
All values are expressed as mean ± SE indicating the number of the samples in each group. Correlation coefficients, 1-way ANOVA and Tukey multiple comparison tests were used to compare values of [Ca2+]i among infected and uninfected myotubes.
Results
Clinical Evidence of Myocarditis in Dengue
A total of 102 children with the diagnosis of viral febrile illness were admitted to the pediatric unit of HUHMP for further diagnosis and study. The median age of the 102 patients was 72 months (6 years), ranging from 13 to 120 months of age. The patients were admitted for hospitalization between day 4 and 5 days of fever, and for 3 to 8 consecutive hospitalization days the patients were monitored and received supportive treatment. All the patients admitted to the hospital had fever, in addition to some of the following symptoms: vomiting, abdominal pain, myalgias, arthralgia, bleeding, and wheezing. The patients were clinically diagnosed with dengue fever (23 patients) or dengue hemorrhagic fever (DHF) with positive chest roentgenograms diagnostic of pleural effusion (79 patients). Eleven of the DHF patients presented with clinical myocarditis, one of whom had a fatal outcome at day 7 of the disease. Nine of the 11 myocarditis patients' electrocardiogram indicated sinus bradycardia while 2 had tachycardia (Table 1). Seven myocarditis patients showed T-wave inversions. Of the 7 echocardiograms studied, pericardial effusion and diastolic function impairment was present in 5 and 2 patients, respectively. In addition, pathologically elevated concentrations of creatine phosphokinase myocardial band (muscle specific) (CPK-MB) levels were found in 6 patients with symptoms of myocarditis (data not shown).
TABLE 1.
Clinical data (n = 11)
| Signs | Range of Values | |
|---|---|---|
| Systolic pressure (mm Hg) | 96 | (87, 100) |
| Diastolic pressure (mm Hg) | 30 | (29, 36) |
| Cardiac frequency (per minute) | 67 | (61, 84) |
| % Pleural effusion | 35 | (0, 50) |
| Platelet counts (per mL) | 45,000 | (29,000, 74,000) |
| % Hematocrit | 38 | (32, 40) |
| PT (s) | 15 | (14, 23) |
| PTT (s) | 45 | (32, 62) |
| AST (UI/mL) | 115 | (71, 200) |
| ALT (UI/mL) | 50 | (27, 64) |
| No. patients with myocarditis presenting symptoms | ||
| Bradycardia | 9 | |
| Tachycardia | 2 | |
| Supraventricular tachycardia | 1 | |
| Inverted T segment | 7 | |
| Pleural effusion* | 5 | |
| Ventricular dysfunction* | 2 |
PT indicates prothrombin time; PTT, partial thromboplastin time; AST, aspartate transaminase; ALT, alanine transaminase.
Echocardiogram performed in 6 patients presenting myocarditis.
Dengue Virus Detected in Human Cardiac Tissue
In the fatal case, the presence of DENV in postmortem samples was studied. Virus detection was determined by immunohistochemistry using a commercial antibody that recognizes the capsid protein of DENV. The myocardium sections appear morphologically normal (Fig. 1A). Immunohistochemistry of cardiac tissue showed distinct perinuclear staining in endothelial cells within the heart as well as in myoblasts (Fig. 1B). Several preparations of the heart tissue revealed dengue antigen within several cell types including cardiomyocytes, myocardial interstitial cells, and endothelial cells. In the infected cardiomyocytes, DENV antigen appeared as small granular deposits within the cytoplasm, often in a perinuclear location (Fig. 1C). Endothelial cells of small myocardial vessels were also frequently positive (Fig. 1C). Scattered cardiac interstitial cells likely representing resident macrophages or dendritic cells were infrequently positive. Infected tissues incubated with a control isotype matched antibody (Fig. 1D) or uninfected tissues stained for the DENV antigen using the antidengue antibody (Fig. 1E) had low background and were uniformly negative. Despite evidence of widespread viral infection in the heart there were minimal cellular infiltrates as evidenced by staining with hematoxylin-eosin or by immunohistochemistry for CD3 and CD8 (data not shown).
FIGURE 1.
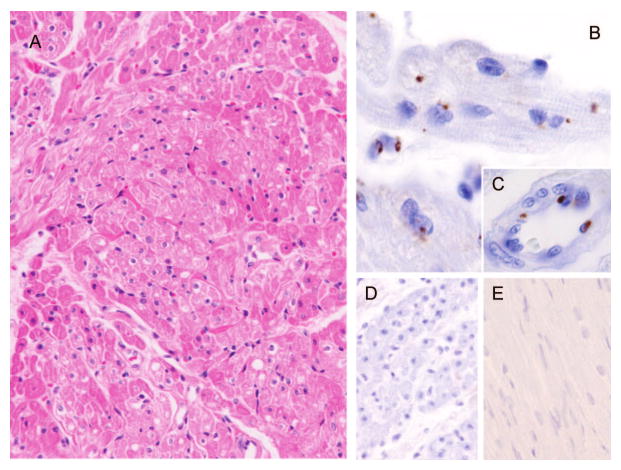
Immunohistochemistry of heart tissue from a fatal DHF case show dengue infection of myocardium. (A) H&E stained sections of myocardium appeared morphologically normal. (B, C) Immunohistochemistry performed with monoclonal anti-DENV antibody MAB8705 revealed intracytoplasmic granular deposits within some cardiomyocytes often in a perinuclear location (arrow, B) and multifocally within interstitial and endothelial cells (C). (D) Immunohistochemistry performed with a monoclonal anti-DENV antibody MAB8705 on non-infected control myocardium tissue was negative for viral antigen. (E) Infected myocardium tissue did not stain with an isotype matched irrelevant antibody.
We also used immunohistochemistry to explore the presence of inflammatory markers MCP-1 and MHC-II in dengue infected tissues. MCP-1 is produced during bacterial and viral infections and has been reported in multiple acute disease sera,4,10–12 where it serves to recruit monocytes, memory T cells, and dendritic cells to sites of tissue injury and infection. MHC-II (HLA-DR, -P, -Q), is a cellular marker of inflammation. Increased expression of MCP-1 antigen was observed in the endothelium of small myocardial vessels and cardiac interstitial cells (Fig. A, B, Supplemental Digital Content 2, http://links.lww.com/INF/A339), as well as myocardial myoblasts (Fig. 2, Supplemental Digital Content 2, http://links.lww.com/INF/A339). In contrast, MCP-1 positive staining was not observed in normal noninfected myocardium (Fig. E, Supplemental Digital Content 2, http://links.lww.com/INF/A339) or tissues stained with irrelevant antibody (data not shown). Expression patterns of MHC-II antigen were comparable to those of MCP-10 (Fig. D, Supplemental Digital Content 2, http://links.lww.com/INF/A339).
Expression of Dengue Infection Markers Increase in Myotubes
Previous studies have identified markers of dengue infection in vivo.13 In addition to MCP-10 and MCH-II, which we have characterized above using immunohistochemistry, these markers include the proinflammatory cytokine IP-10. We have also found a set of genes that are coexpressed, including IP-10 and cell surface protein CD38 (data not shown) after dengue infection in vitro in primary human cells including myotubes. To further characterize dengue infection inflammatory response in muscle cells, we measured levels of these 2 mRNAs normalized by β-actin mRNA. The mRNA levels of CD38 were 100-fold higher in infected cultures. The mRNA and protein product of the IP-10 gene were greatly induced (1000-fold increase in mRNA levels at 24 hours post infection) by dengue virus as measured by qRT-PCR and ELISA. IP-10 protein levels progressively increased in the media of infected myotubes in vitro, compared with mock infected or control media (25-fold increase at 12 hours and 550-fold increase at 24 hours). In the Affymetrix Genechip gene expression analyses (data not shown), IP-10 was one of the cytokines most highly induced by dengue infection for other cells also, including monocytes, B cells, dendritic cells, and Human umbilical cord endothelial cells. Myoblasts and myotubes are novel IP-10 expressing cells as a result of a viral infection, as no prior publications were found describing the presence of IP-10 in muscle cells. In contrast to IP-10 mRNA, the Ryanodine receptor-1 mRNA expression did not changed after infection of dengue at 48 hours postinfection nor were gene expression changes detected for IP3 receptor isoform 1 (data not shown) indicating that at least at the level of transcription, there are not significant changes in the 2 major regulators of intracellular Ca2+ tested by RT-PCR.
DENV Infects Skeletal Myotubes In Vitro and Alters [Ca2+]i
To test the hypothesis that striated muscle is a target of dengue infection and that the myocardial dysfunction associated with dengue infection is the result of alterations of calcium homeostasis, human skeletal myotubes were exposed to DENV and Ca2+ changes were assayed. Myotubes resemble adult skeletal muscle fibers in the expression of intracellular calcium buffers, calcium channels, pumps, and transporters that are involved in Ca2+ homeostasis. Myotube virus infection was robust as shown by the presence DENV Capsid (Fig. A, Supplemental Digital Content 3, http://links.lww.com/INF/A340), and NS1 (Fig. D, Supplemental Digital Content 3, http://links.lww.com/INF/A340) proteins using confocal immunofluorescence microscopy. These data demonstrate that the virus both infects and replicates within the muscle cells. Interestingly, the E protein was detected in the perinuclear region of myotubes (Fig. C, Supplemental Digital Content 3, http://links.lww.com/INF/A340) similar to the subcellular localization of this marker in postmortem myocardial images (Fig. 1B).
[Ca2+]i was measured in skeletal myotubes exposed to DENV after 6, 12, and 24 hours and in nonexposed controls. After 6 hours, [Ca2+]i was similar in both groups of skeletal myotubes. In DENV exposed myotubes, [Ca2+]i was 118 ± 7 nM (range, 109–124; n = 15) and in nonexposed myotubes it was 120 ± 8 nM (range, 112–125; n = 11). In the 12-hour group, [Ca2+]i in the control myotubes was 118 ± 8 nM (range, 108–125; n = 15), while in exposed myotubes, we clearly detected 2 groups of cells with 2 different values. One group showed a normal [Ca2+]i (121 ± 8 nM; range, 105–120; n = 12) and a second showed an abnormal [Ca2+]i (140 ± 8 nM; range, 133–151; n = 8). The second group presumably represented the cells infected by DENV.
Twenty-four hours after DENV exposure, 28 of 47 myotubes had a [Ca2+]i in the normal range (121 ± 10 nM; range, 108–128; n = 28), while 19 had a significantly increased [Ca2+]i (199 ± 15 nM; range, 172–226; n = 19) (Fig. Supplemental Digital Content 4, http://links.lww.com/INF/A341). No change in [Ca2+]i was observed in skeletal muscle myotubes 6, 12, or 24 hours after exposure to insect cell supernatant (mock infection control). These data support the hypothesis that muscle is a target of DENV and that with infection there is a significant dysregulation of intracellular Ca2+ homeostasis.
Discussion
Depression of myocardial function has been reported associated with the hemorrhagic form of DENV infection.14 But, until this study, it was not known whether direct infection of myocardial tissue by DENV could contribute to DHF myocarditis, or whether the condition was caused by the indirect effects of cellular immune responses and/or cytokine mediators released from other DENV target tissues like immune cells or endothelium.8 The present work describes the direct infection of muscle cells by DENV. We also show that DENV directly infects and alters the Ca+2 storage of skeletal muscle cells in vitro. These observations support the hypothesis that cardiac and skeletal muscle dysfunction associated with DHF is the direct result of DENV infection of myocytes and skeletal muscle fibers.
In the present study, we describe 11 patients from a cohort of 102 pediatric cases of dengue referred to HUHMP, who showed evidence of mild-to-severe myocardial involvement on clinical evaluation. Bradycardia, tachycardia, pericardial effusion, and diastolic dysfunction were the most prominent symptoms. In a high percentage of patients with cardiac symptoms, a significant increase in the CPK-MB concentration was found, suggesting destruction of myocardial cells by DENV infection, as CPK-MB is an independent marker of myocardium damage.15
DHF associated atrioventricular conduction disorders (junctional rhythm and atrioventricular block), supraventricular arrhythmias, and myocarditis have been reported.16 Ventricular dysfunction is also associated with the acute phase of DHF and has been described by several authors.17–20 These disorders are probably under diagnosed in clinical practice. During an epidemic in India, 54 children with dengue of varying degrees of severity underwent evaluation of ventricular function. Approximately, 16% had ejection fractions of less than 50%.17 In the same year, other group reported that 12 (70%) of 17 subjects with DHF or dengue shock syndrome had diffuse ventricular hypokinesis, with a mean ejection fraction of 40%.18 In a study involving serial echocardiograms in 24 patients with DHF, a transient reduction in ventricular ejection fraction and cardiac index during acute infection was observed.19
Cultured human skeletal muscle myotubes represent a well established model system for the study muscle cells.5,6 These cells share some characteristics with cardiac myocytes. We found that cultured myotubes were highly susceptible to infections by DENV. Our conclusions are based on confocal immunofluorescence microscopy of the viral proteins C and NSI and suggest that, like the heart, skeletal muscle is a target organ for DENV infection.
An increase in resting [Ca2+]i was one of the consequences of DENV infection that we observed in muscle cells. This increase varied with time after virus exposure (from 1.14-fold at 12 hours to 1.64-fold at 24 hours post DENV infection in a subgroup of infected cells). [Ca2+]i increased from a control level of 118 ± 8 nM to a 24 hour infected value of 199 ± 15 nM for a subgroup of infected cells. We did not detect any change in the initial 6 hours of infection, indicating a certain time is required for dengue infection to induce intracellular Ca2+ raise. The lack of [Ca2+]i change in mock infected cells at any time was an important experimental control.
Based on these findings in skeletal muscle cells, it is probable that increased resting (diastolic) Ca2+ is present in infected myocardium and may be responsible for the arrhythmias and altered contractile function of the myocardium documented in vivo.
Other viruses have previously been shown to induce increases in [Ca2+]i and to interfere with Ca2+ homeostasis. These include HSV and CMV.21,22 In addition, human immunodeficiency virus exerts an acute cytopathic effect on T cells through a mechanism accompanied by intracellular Ca2+ accumulation.23 Ca2+ release from intracellular stores has been identified as one of the major signals that can induce the mitochondrion-dependent pathway of apoptotic cell death. Increased Ca2+ has been shown to produce mitochondrial pore opening, cytochrome c release, caspase activation, and nuclear apoptosis in cells exposed to apoptotic signals.24
Given that skeletal muscle has a large tissue volume in the human body, we postulate that its infection by DENV may be an important source of the systemic increase of proinflammatory cytokines observed in dengue infections. Production of proinflammatory cytokines by muscle cells has previously been documented.9,25 Endothelium has also been postulated to contribute to circulating proinflammatory mediators in dengue.8,26
Supplementary Material
Acknowledgments
The authors thank the personnel at Hospital Universitario de Neiva, Colombia, for the clinical evaluation of the patients and laboratory tests performed and Fernando Bosch, MD, for critical analysis of the electrocardiograms. We are grateful to the Gehrke lab for their gene expression data of human myoblast and myotubes.
Supported by NIAID, U19 AI057319.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.pidj.com).
References
- 1.Myint KS, Endy TP, Mongkolsirichaikul D, et al. Cellular immune activation in children with acute dengue virus infections is modulated by apoptosis. J Infect Dis. 2006;194:600–607. doi: 10.1086/506451. [DOI] [PubMed] [Google Scholar]
- 2.Yearley JH, Pearson C, Carville A, et al. SIV-associated myocarditis: viral and cellular correlates of inflammation severity. AIDS Res Hum Retroviruses. 2006;22:529–540. doi: 10.1089/aid.2006.22.529. [DOI] [PubMed] [Google Scholar]
- 3.Yearley JH, Pearson C, Shannon RP, et al. Phenotypic variation in myocardial macrophage populations suggests a role for macrophage activation in SIV-associated cardiac disease. AIDS Res Hum Retroviruses. 2007;23:515–524. doi: 10.1089/aid.2006.0211. [DOI] [PubMed] [Google Scholar]
- 4.Hendricks EE, Lin KC, Boisvert K, et al. Alterations in expression of monocyte chemotactic protein-1 in the simian immunodeficiency virus model of disseminated Mycobacterium avium complex. J Infect Dis. 2004;189:1714–1720. doi: 10.1086/383324. [DOI] [PubMed] [Google Scholar]
- 5.Lopez JR, Alamo L, Caputo C, et al. Determination of ionic calcium in frog skeletal muscle fibers. Biophys J. 1983;43:1–4. doi: 10.1016/S0006-3495(83)84316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marban E, Rink TJ, Tsien RW, et al. Free calcium in heart muscle at rest and during contraction measured with Ca2+ -sensitive microelectrodes. Nature. 1980;286:845–850. doi: 10.1038/286845a0. [DOI] [PubMed] [Google Scholar]
- 7.Yang T, Allen PD, Pessah IN, et al. Enhanced excitation-coupled calcium entry in myotubes is associated with expression of RyR1 malignant hyperthermia mutations. J Biol Chem. 2007;282:37471–37478. doi: 10.1074/jbc.M701379200. [DOI] [PubMed] [Google Scholar]
- 8.Warke RV, Xhaja K, Martin KJ, et al. Dengue virus induces novel changes in gene expression of human umbilical vein endothelial cells. J Virol. 2003;77:11822–11832. doi: 10.1128/JVI.77.21.11822-11832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warke RV, Becerra A, Zawadzka A, et al. Efficient dengue virus (DENV) infection of human muscle satellite cells upregulates type I interferon response genes and differentially modulates MHC I expression on bystander and DENV-infected cells. J Gen Virol. 2008;89:1605–1615. doi: 10.1099/vir.0.2008/000968-0. [DOI] [PubMed] [Google Scholar]
- 10.Liprandi A, Bartoli C, Figarella-Branger D, et al. Local expression of monocyte chemoattractant protein-1 (MCP-1) in idiopathic inflammatory myopathies. Acta Neuropathol. 1999;97:642–648. doi: 10.1007/s004010051041. [DOI] [PubMed] [Google Scholar]
- 11.Porter JD, Guo W, Merriam AP, et al. Persistent over-expression of specific CC class chemokines correlates with macrophage and T-cell recruitment in mdx skeletal muscle. Neuromuscul Disord. 2003;13:223–235. doi: 10.1016/s0960-8966(02)00242-0. [DOI] [PubMed] [Google Scholar]
- 12.Wachtman LM, Gualtieri L, Wanke C, et al. Viral and host correlates of serum resistin in simian AIDS. AIDS Res Hum Retroviruses. 2008;24:34–42. doi: 10.1089/aid.2007.0154. [DOI] [PubMed] [Google Scholar]
- 13.Fink J, Gu F, Ling L, et al. Host gene expression profiling of dengue virus infection in cell lines and patients. PLoS Negl Trop Dis. 2007;1:e86. doi: 10.1371/journal.pntd.0000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalayanarooj S, Vaughn DW, Nimmannitya S, et al. Early clinical and laboratory indicators of acute dengue illness. J Infect Dis. 1997;176:313–321. doi: 10.1086/514047. [DOI] [PubMed] [Google Scholar]
- 15.Blum A, Safori G, Hous N, et al. The prognostic value of high-sensitive C-reactive protein and cardiac troponin T in young and middle-aged patients with chest pain without ECG changes. Eur J Intern Med. 2003;14:310–314. doi: 10.1016/s0953-6205(03)00099-2. [DOI] [PubMed] [Google Scholar]
- 16.Khongphatthallayothin A, Chotivitayatarakorn P, Somchit S, et al. Morbitz type I second degree AV block during recovery from dengue hemorrhagic fever. Southeast Asian J Trop Med Public Health. 2000;31:642–645. [PubMed] [Google Scholar]
- 17.Kabra SK, Juneja R, Madhulika JY, et al. Myocardial dysfunction in children with dengue haemorrhagic fever. Natl Med J India. 1998;11:59–61. [PubMed] [Google Scholar]
- 18.Wali JP, Biswas A, Chandra S, et al. Cardiac involvement in dengue haemorrhagic fever. Int J Cardiol. 1998;64:31–36. doi: 10.1016/s0167-5273(98)00008-4. [DOI] [PubMed] [Google Scholar]
- 19.Khongphatthanayothin A, Suesaowalak M, Muangmingsook S, et al. Hemodynamic profiles of patients with dengue hemorrhagic fever during toxic stage: an echocardiographic study. Intensive Care Med. 2003;29:570–574. doi: 10.1007/s00134-003-1671-9. [DOI] [PubMed] [Google Scholar]
- 20.Malheiros SM, Oliveira AS, Schmidt B, et al. Dengue. Muscle biopsy findings in 15 patients. Arq Neuropsiquiatr. 1993;51:159–164. doi: 10.1590/s0004-282x1993000200001. [DOI] [PubMed] [Google Scholar]
- 21.Cheshenko N, Del Rosario B, Woda C, et al. Herpes simplex virus triggers activation of calcium-signaling pathways. J Cell Biol. 2003;163:283–293. doi: 10.1083/jcb.200301084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Himpens B, Proot P, Neyts J, et al. Human cytomegalovirus modulates the Ca2+ response to vasopressin and ATP in fibroblast cultures. Cell Calcium. 1995;18:111–119. doi: 10.1016/0143-4160(95)90002-0. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki M, Uchiyama J, Ishikawa H, et al. Induction of apoptosis by calmodulin-dependent intracellular Ca2+ elevation in CD4+ cells expressing gp 160 of HIV. Virology. 1996;224:18–24. doi: 10.1006/viro.1996.0502. [DOI] [PubMed] [Google Scholar]
- 24.Szalai G, Krishnamurthy R, Hajnoczky G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. EMBO J. 1999;18:6349–6361. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Rossi M, Bernasconi P, Baggi F, et al. Cytokines and chemokines are both expressed by human myoblasts: possible relevance for the immune pathogenesis of muscle inflammation. Int Immunol. 2000;12:1329–1335. doi: 10.1093/intimm/12.9.1329. [DOI] [PubMed] [Google Scholar]
- 26.Avirutnan P, Malasit P, Seliger B, et al. Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. J Immunol. 1998;161:6338–6346. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


