Abstract
Blacks are twice as likely to develop and die from multiple myeloma (MM) and are less likely to receive an autologous hematopoietic-cell transplant (AHCT) for MM compared to whites. The influence of race on outcomes of AHCT for MM is not well described. We compared the probability of overall survival, progression-free survival, disease progression and non-relapse mortality among black (N=303) and white (N=1892) recipients of AHCT for MM, who were reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) from 1995 to 2005. The black cohort was more likely to be female, had better Karnofsky performance scores but lower hemoglobin and albumin levels at diagnosis. Black recipients were younger and more likely to be transplanted later in their disease course. Disease stage and treatment characteristics prior to AHCT were similar between the two groups. Black and white recipients had similar probabilities of 5-year overall survival (52% vs. 47%, P=0.19) and progression-free survival (19% vs. 21%, P=0.64) as well as cumulative incidences of disease progression (72% vs. 72%, P=0.97) and non-relapse mortality (9% vs. 8%, P=0.52). In multivariate analyses, race was not associated with any of these endpoints. Black recipients of AHCT for MM have similar outcomes compared to whites, suggesting that the reasons underlying lower rates of AHCT in blacks need to be studied further to ensure equal access to effective therapy.
Keywords: Autologous hematopoietic cell transplantation, multiple myeloma, race, survival, progression-free survival
Background
Multiple myeloma (MM) remains an incurable disease, although prognosis has improved in the past decade.1,2 It is the most common hematologic malignancy among blacks and is the only hematologic malignancy that is more frequent in this racial group compared with whites. In the United States, myeloma and its precursor disease monoclonal gammopathy of undetermined significance (MGUS) are twice as common in blacks (annual incidence of 14.4/100,000 in men and 9.8/100,000 in women compared with 6.6/100,000 in white men and 4.1/100,000 in white women).1,3–7 Proposed factors to explain the increased incidence among blacks include socioeconomic factors, greater exposure to hazardous materials, genetic predisposition, greater degree of background antigenic stimulation, and a greater prevalence of obesity.8–10 Mortality rates from MM in the United States are twice as high for blacks compared to whites (8.3/100,000 for men and 6.0/100,000 for women compared to 4.3/100,000 and 2.8/100,000 for white men and women, respectively).11
Socioeconomic factors that may impact access to cancer therapy and therapeutic choices include place of residence, distance from care centers, unemployment, availability and quality of health insurance, poor nutrition, exposure to infectious agents, lower educational level and annual income.12,13 Prior comparisons have drawn conflicting conclusions on treatment outcomes among blacks compared with white patients with MM. Savage et al found that black patients had shorter survival times following similar therapy for MM. Presentation at later stages of disease, socioeconomic factors or differential access to care were thought to explain this disparity.13,14 Other investigators have suggested that these disparities in outcomes are primarily due to biological characteristics.15,16
Randomized clinical trials support the use of AHCT as a standard therapy for MM.17,18 We have previously shown that blacks are less likely to receive AHCT for MM compared with their age and sex matched white counterparts.19 In the current study, we compared outcomes between black and white patients receiving AHCT for MM to determine if disparate post transplant outcomes validate lower AHCT use in blacks.
Patients and Methods
The Center for International Blood and Marrow Transplant Research (CIBMTR) consists of a voluntary working group of more than 450 transplant centers worldwide. Centers contribute detailed data on consecutive allogeneic and autologous transplants to a statistical center at either the Medical College of Wisconsin in Milwaukee or the National Marrow Donor Program Coordinating Center in Minneapolis. Subjects are followed longitudinally, with yearly follow-up. Computerized checks for errors, physicians’ review of submitted data and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are done with a waiver of informed consent and in compliance with HIPAA regulations as determined by the Institutional Review Board and the Privacy Officer of the Medical College of Wisconsin.
Patients
The study included 2195 (303 black and 1892 white) adult (age ≥ 18 years) recipients of AHCT for MM who were transplanted between January 1995 and June 2005 (Table 1). Only recipients of peripheral blood AHCT were included in this study; patients who had received planned tandem AHCT (N=582) were excluded. Centers obtained information about patient race and then reported it to the CIBMTR.
Table 1.
Patient Characteristics
| White | Black | ||
|---|---|---|---|
| Variable | N (%) | N (%) | P-valueb |
| Number of patientsa | 1892 | 303 | |
| Age median (range), years | 57 (27–80) | 55 (27–74) | <0.001 |
| Age group at transplant, years | 0.002 | ||
| <50 | 396 (21) | 88 (29) | |
| 50–64 | 1111 (59) | 172 (57) | |
| ≥ 65 | 385 (20) | 43 (14) | |
| Male sex | 1136 (60) | 164 (54) | 0.05 |
| Karnofsky score pre-transplant | 0.005 | ||
| ≥ 90 | 1153 (61) | 210 (69) | |
| Hypertension | <0.001 | ||
| Yes | 471 (25) | 143 (47) | |
| Diabetes | <0.001 | ||
| Yes | 169 (9) | 50 (17) | |
| Body Mass Index | 0.01 | ||
| Underweight/Normal (<25) | 557 (29) | 67 (22) | |
| Overweight (25–29.9) | 741 (39) | 120 (40) | |
| Obese/Morbidly Obese (≥ 30) | 594 (31) | 116 (38) | |
| Disease related | |||
| Durie-Salmon stage at diagnosis | 0.25 | ||
| I | 203 (11) | 25 (8) | |
| II | 562 (30) | 101 (33) | |
| III | 1127 (60) | 177 (58) | |
| Immunochemical subtype of myeloma | 0.34 | ||
| IgG | 1003 (53) | 173 (57) | |
| IgA | 359 (19) | 45 (15) | |
| Light chain | 329 (17) | 54 (18) | |
| Others/Unknown | 125 (11) | 16 (10) | |
| Albumin level at diagnosis | 0.05 | ||
| > 3.5 g/dL | 732 (39) | 101 (33) | |
| Hemoglobin at diagnosis <10 g/dL | <0.001 | ||
| < 10 g/dL | 552 (29) | 135 (45) | |
| Creatinine at diagnosis | 0.09 | ||
| >1.5 mg/dL | 361 (19) | 74 (24) | |
| B-2 microglobulin level at diagnosis | 0.83 | ||
| ≥ 5.5 mg/L | 195 (10) | 31 (10) | |
| Prior chemotherapy regimens | 0.78 | ||
| MP ± others | 334 (18) | 50 (17) | |
| VAD ± others (not MP) | 1104 (58) | 182 (60) | |
| Cy ± others | 300 (16) | 52 (17) | |
| Corticosteroids ± others | 154 (8) | 19 (6) | |
| Number of lines of chemotherapyd | 0.29 | ||
| 1 | 1125 (59) | 167 (55) | |
| 2 | 536 (28) | 99 (33) | |
| >2 | 231 (12) | 37 (12) | |
| Sensitive to chemotherapy prior to transplant | 0.83 | ||
| Sensitive | 1434 (76) | 228 (75) | |
| Disease status at time of transplant | 0.67 | ||
| Complete Remission/Partial Remission | 1396 (74) | 231 (76) | |
| Treatment related | |||
| Time from diagnosis to transplant median (range), months | 8 (<1–249) | 9 (2–217) | <0.001 |
| Time from diagnosis to transplant | <0.001 | ||
| <12 months | 1364 (72) | 190 (63) | |
| ≥ 12 months | 528 (28) | 113 (37) | |
| Conditioning regimen | 0.7 | ||
| Melphalan only | 1417 (75) | 223 (74) | |
| Melphalan + TBI ± others | 204 (11) | 35 (12) | |
| Bu-Cy ± others (not TBI, not melphalan) | 271 (15) | 45 (15) | |
| Median follow-up of survivors, median (range) | 61 (<1–145) | 51 (<1–132) | |
Abbreviations: MP=Melphalan+Prednisone VAD = vincristine+dexamethasone+adriamycin; Cy = cyclophosphamide; Bu = busulfan; TBI = total body irradiation; Eval = evaluable.
Statistical Methods
Patient-, disease- and treatment-related factors were compared between the black and white cohorts, using Chi-square test for categorical and Kruskal-Wallis test for continuous variables. Outcomes analyzed included non-relapse mortality (NRM), relapse/progression, progression-free survival (PFS) and overall survival (OS). NRM was defined as death occurring in the absence of relapse or progression of MM following AHCT. Responses, relapse/progression were defined according to standard criteria20. Chemotherapy sensitivity was defined as achievement of a partial or complete response to pretransplant therapy. PFS was defined as survival without disease progression or relapse. Patients alive and with no evidence of disease progression or relapse were censored at the time of last follow-up. The survival interval variable was defined as time from the date of transplant to the date of death or last contact and summarized by a survival curve. Probabilities of OS and PFS were calculated using the Kaplan-Meier estimator.21,22 NRM and relapse/progression were calculated using cumulative incidence estimates. The log-rank test was used for univariate comparisons.
Multivariate Cox proportional hazards regression was used to examine the outcomes between black and white patient cohorts and to identify risk factors associated with outcomes.23 A stepwise forward selection multivariate model was built to identify covariates that influenced outcomes. Any covariate with a P-value < 0.05 was considered significant. The proportionality assumption for Cox regression was tested by adding a time-dependent covariate for each risk factor and each outcome. Tests indicated that all variables met the proportional hazards assumption. Results were expressed as relative risks (RR). Any risk factors found to be significant were adjusted in the final Cox model. The main effect tested (i.e. black vs. white) was included in all models. The variables considered in multivariate analyses are summarized in table 2. Analyses were performed using SAS software, version 9.1 (SAS Institute, Cary, NC).
Table 2.
Variables tested in multivariate analysis.
| Main effect variable: |
| Race/ethnicity: White* vs. Black |
| Patient-related variables: |
| Age: < 50* vs. 50–64 vs. ≥ 65 |
| Gender: Male* vs. Female |
| Karnofsky performance status at transplant: <90% vs. ≥ 90%* vs. missing |
| Body Mass Index: underweight/normal* vs. overweight vs. obese/morbidly obese |
| Hypertension anytime prior to transplant: Yes* vs. No |
| Diabetes anytime prior to transplant: Yes* vs. No |
| History of smoking prior to transplant: Yes* vs. No |
| Creatinine >1.5 mg/dL vs ≤ 1.5* mg/dL at diagnosis |
| MM subtype: IgG vs. IgA vs. Light chain vs. Others/Unknown |
| Disease-related variables: |
| Durie-Salmon stage at diagnosis: I* vs. II vs. III |
| Number of lines of chemotherapy: 0 vs. 1* vs. 2 vs. >2 |
| Sensitivity to chemotherapy prior to transplant: sensitive* vs. Others |
| Disease status prior to transplant: complete remission/partial remission* vs. others (includes minimal response, no response, stable disease, relapse/progressive disease and unknown) |
| Prior chemotherapy regimens: MP* vs. VAD vs. Cy ± others vs. Corticosteroids ± others |
| Transplant-related variables: |
| Time from diagnosis to transplant: <12 months* vs. Others |
| Conditioning regimen: Melphalan only* vs. melphalan + TBI ± others vs. Bu-Cy ± others (not TBI, not melphalan) |
| Purging: yes* vs no |
| Year of transplant: 1995–2001 vs. 2002–2005* |
Abbreviations: MP=Melphalan+Prednisone VAD = vincristine+dexamethasone+adriamycin; Cy = cyclophosphamide; Bu = busulfan; TBI = total body irradiation
Reference group
Results
Patient Characteristics
Table 1 shows the characteristics of all patients evaluated. Median ages at AHCT were 55 years for black compared to 57 years for white patients (p<0.001). The black cohort had a higher proportion of females and patients with Karnofsky performance status scores (KPS) > 90 (69 % vs. 61%, p=0.005). Blacks were more likely to have co-morbidities such as hypertension (47% vs. 25%, p<0.001), diabetes mellitus (17% vs. 9%, p<0.001), and obesity (38%vs. 31%, p=0.01). No statistically significant differences in disease stage or MM subtype were identified. Blacks were also more likely to have a lower hemoglobin (Hb <10 g/dL in 45% vs. 29%, p<0.001) at diagnosis. No significant differences in the levels of serum creatinine, beta-2 microglobulin, calcium or marrow plasmacytosis were identified. The cohorts did not differ with respect to the type and number of prior therapies or sensitivity to therapies applied before transplantation. Blacks were transplanted later in the disease course, with 37% receiving AHCT a year or more from diagnosis vs. 28% in whites (p<0.001). There were no significant differences in conditioning regimens used or the receipt of a salvage second AHCT.
Non-relapse Mortality and Relapse/Progression
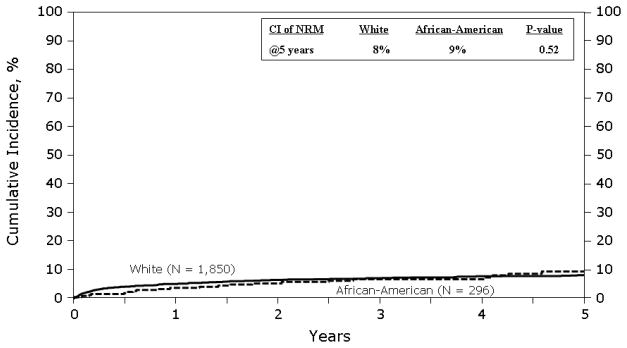
Figure 1 shows the cumulative incidence of NRM. The cumulative incidence of NRM was similar in both groups. At 1 year was 5% (95% CI 4–6%) in whites vs. 3% (95% CI 2–6%) in blacks. At 5 years it was 8% (95% CI 7–9%) vs. 9% (95% CI 6–14%) in whites and blacks respectively. In multivariate analysis (Table 3), race was not associated with NRM. Factors associated with an increased risk of NRM were age ≥ 65 years, KPS <90 and AHCT prior to 2002.
Figure 1.
Cumulative incidence of non-relapse mortality
Table 3.
Multivariate analysis for relapse and non-relapse mortality
| Relapse | Non-Relapse mortality | |||||
|---|---|---|---|---|---|---|
| Variable | N | RR | P-value | N | RR | P-value |
| Race | ||||||
| White | 1850 | 1.00a | 1850 | 1.00a | ||
| Black | 296 | 0.92 (0.78–1.08) | P = 0.28 | 296 | 1.16 (0.75–1.80) | P = 0.51 |
| Patient Age | ||||||
| < 50 | 475 | 1.00a | P < 0.001 | |||
| 50–64 | 1253 | 1.55 (1.01–2.39) | P = 0.05 | |||
| ≥ 65 | 418 | 3.50 (2.17–5.65) | P < 0.001 | |||
| Karnofsky Score prior to conditioning | ||||||
| <90 | 815 | 1.00a | 815 | 1.00a | ||
| ≥ 90 | 1331 | 0.88 (0.79–0.98) | P = 0.02 | 1331 | 0.72 (0.53–0.98) | P = 0.03 |
| Durie-Salmon stage at diagnosis | ||||||
| I | 222 | 1.00a | P < 0.001 | 222 | 1.00a | P = 0.004 |
| II | 652 | 1.23 (1.00–1.51) | P = 0.05 | 652 | 0.61 (0.35–1.06) | P = 0.08 |
| III | 1272 | 1.54 (1.27–1.87) | P < 0.001 | 1272 | 1.16 (0.71–1.88) | P = 0.56 |
| Number of lines of chemotherapyc | ||||||
| 1 | 1256 | 1.00a | P = 0.001 | |||
| 2 | 628 | 1.12 (0.99–1.27) | P = 0.07 | |||
| > 2 | 262 | 1.39 (1.16–1.66) | P < 0.001 | |||
| Sensitivity to chemotherapy prior to transplant | ||||||
| Other | 522 | 1.00a | ||||
| Sensitive | 1624 | 0.76 (0.67–0.85) | P < 0.001 | |||
| Time from diagnosis to transplant | ||||||
| < 12 months | 1519 | 1.00a | ||||
| ≥ 12 months | 627 | 1.19 (1.04–1.35) | P = 0.009 | |||
| Year of transplant | ||||||
| 1995–2001 | 1331 | 1.00a | 1331 | 1.00a | ||
| 2002–2005 | 815 | 1.17 (1.04–1.31) | P = 0.008 | 815 | 0.56 (0.39–0.81) | P = 0.002 |
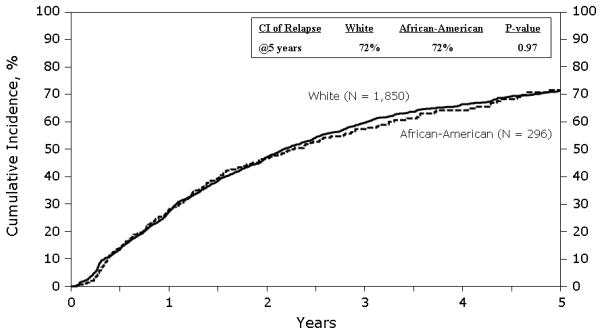
Figure 2 shows cumulative incidence of relapse/progression. The cumulative incidence of relapse/progression was similar in both groups. At 1 year it was 27% (95% CI 25–29%) in whites vs. 28% (95% CI 23–34%) in blacks. At 5 years it was 72% (95% CI 69–74%) vs. 72% (95% CI 65–78%) in whites and blacks respectively. In multivariate analysis (Table 3), race was not associated with disease relapse or progression. Factors associated with an increased risk of relapse included KPS score < 90, Durie-Salmon stage III at diagnosis, receipt of 3 or more lines of chemotherapy before AHCT, lack of chemosensitive disease prior to AHCT, AHCT ≥ 12 months from diagnosis and later year of AHCT.
Figure 2.
Cumulative incidence of disease relapse and progression
Progression-free Survival and Overall Survival
Figure 3 shows the probability of PFS. The 1 and 5 year probabilities of PFS were similar in both groups. At 1 year it was 68% (95% CI 66–70%) in whites vs. 68% (95% CI 63–74%) in blacks. At 5 years it was 21% (95% CI 18–23%) vs. 19% (95% CI 14–25%) in whites and blacks respectively. In multivariate analysis (Table 4), race was not associated with PFS.
Figure 3.
Probability of progression-free survival
Table 4.
Multivariate analysis for overall survival and progression-free survival
| Overall Survival | Progression-free survival | |||||
|---|---|---|---|---|---|---|
| Variable | N | RR | P-value | N | RR | P-value |
| Race | ||||||
| White | 1892 | 1.00a | 1850 | 1.00a | ||
| Black | 303 | 0.94 (0.78–1.13) | P = 0.50 | 296 | 0.94 (0.81–1.09) | P = 0.39 |
| Patient Age | ||||||
| < 50 | 484 | 1.00a | Pb < 0.0001 | 475 | 1.00a | P = 0.03 |
| 50–64 | 1283 | 1.26 (1.09–1.46) | P = 0.002 | 1253 | 1.12 (0.99–1.27) | P = 0.08 |
| ≥ 65 | 428 | 1.52 (1.26–1.83) | P < 0.0001 | 418 | 1.24 (1.06–1.46) | P = 0.007 |
| Karnofsky Score prior to conditioning | ||||||
| <90 | 832 | 1.00a | 815 | 1.00a | ||
| ≥ 90 | 1363 | 0.74 (0.66–0.83) | P < 0.0001 | 1331 | 0.87 (0.79–0.97) | P = 0.009 |
| Durie-Salmon stage at diagnosis | ||||||
| I | 228 | 1.00a | Pb < 0.0001 | 222 | 1.00a | P < 0.0001 |
| II | 663 | 1.13 (0.89–1.44) | P = 0.32 | 652 | 1.12 (0.93–1.36) | P = 0.23 |
| III | 1304 | 1.67 (1.34–2.09) | P < 0.0001 | 1272 | 1.49 (1.25–1.79) | P < 0.0001 |
| Number of lines of chemotherapyc | ||||||
| 1 | 1292 | 1.00a | Pb < 0.0001 | 1256 | 1.00a | P = 0.0002 |
| 2 | 635 | 1.10 (0.96–1.27) | P = 0.17 | 628 | 1.13 (1.00–1.27) | P = 0.04 |
| > 2 | 268 | 1.66 (1.37–2.01) | P < 0.0001 | 262 | 1.41 (1.19–1.67) | P < 0.0001 |
| Sensitivity to chemotherapy prior to transplant | ||||||
| Other | 533 | 1.00a | 522 | 1.00a | ||
| Sensitive | 1662 | 0.82 (0.72–0.94) | P = 0.003 | 1624 | 0.76 (0.68–0.85) | P < 0.0001 |
| Time from diagnosis to transplant | ||||||
| < 12 months | 1554 | 1.00a | 1519 | 1.00a | ||
| ≥ 12 months | 641 | 1.16 (1.01–1.34) | P = 0.04 | 627 | 1.16 (1.03–1.31) | P = 0.01 |
Figure 4 shows the probability of overall survival after AHCT. The 1 and 5 year survival rates were also similar between the two cohorts. At 1 year it was 87% (95% CI 85–88%) in whites vs. 90% (95% CI 87–93%) in blacks. At 5 years it was 47% (95% CI 44–49%) vs. 52% (95% CI 45–59%) in whites and blacks respectively. In multivariate analysis (Table 4), race was not a significant predictor of survival.
Figure 4.
Probability of overall survival
PFS and overall survival were worse in patients with older age at AHCT (>50 years), KPS score <90, higher Durie-Salmon stage, those who received two or more lines of therapy prior to AHCT, AHCT ≥ 12 months from diagnosis and chemotherapy resistant disease (Table 4). Overall survival was also lower in patients who underwent AHCT prior to 2002.
The major cause of mortality in both cohorts was relapse or progression of MM that accounted for 72% of all deaths.
Discussion
Our analysis establishes that black and whites have very similar outcomes after AHCT for MM. These results concur with observations in other studies of non-transplant therapy that the disparity in outcomes for MM disappears when blacks receive identical therapy.28
Several investigators have shown that blacks have outcomes similar to whites when given the same non-transplant treatment for MM. Rohatgi et al showed that blacks were less likely to receive chemotherapy but they responded with similar outcomes when given similar non-transplant therapy for MM.24 In the pre-transplant era, Modiano et al. retrospectively evaluated the impact of race in the results of the SWOG 8829 study of conventional chemotherapy for MM.25 From 99 study sites in the US, 116 black and 467 white patients were shown to have similar median survival (32 and 30 months, respectively). There were no differences by stage or MM subtype. A smaller study from the Department of Defense equal access health care system, reported on the outcomes of 36 black and 55 white newly diagnosed patients receiving AHCT for MM and observed comparable outcomes between the two groups.26 In their study, there were no differences in the stage, hemoglobin, calcium or creatinine levels, although blacks did have higher CRP levels and a trend for less skeletal involvement. The authors recommended a larger retrospective study such as the current one. Other single center analyses comparing black and white recipients of AHCT for MM have drawn conflicting conclusions. Khaled et al. analyzed 101 black patients and concluded that they were likely to relapse earlier after AHCT.27 Survival was not compared in this study. Saraf et al. in their comparative study that included 38 black and 32 white AHCT recipients, found that black patients had more prolonged responses and greater event free survival.28
Unfortunately, there is ample evidence that blacks are less likely to receive chemotherapy for MM as well as AHCT. Rohatgi et al. reviewed patterns of chemotherapy use for patients with MM outside the clinical trial setting.24 From a population based retrospective cohort of 49,021 patients of age 65 years or older with stage II or III MM, they found that only 52% received chemotherapy. Blacks were less likely to receive chemotherapy compared to whites (47.6% vs. 52.8%) despite evidence that use of chemotherapy decreased all cause mortality, myeloma specific mortality and increased survival. 24 The reasons for the disparate access are unclear, since controlling for socioeconomic status did not eliminate the disparity in the receipt of chemotherapy.
These disparities in the receipt of therapy occur in the transplant setting as well. Joshua et al, in a previous study from the CIBMTR demonstrate that whites are more likely to receive AHCT for newly diagnosed MM compared to an age and sex adjusted black population.19 Using data from the SEER and CIBMTR registries, the study showed that age and sex adjusted odds of receiving AHCT for MM is 1.72 times greater in whites compared to blacks. Although our study cannot address the reasons for this under utilization of AHCT in blacks, interesting conclusions can be drawn regarding AHCT for MM in black patients.
It has been proposed that reduced access to treatment for myeloma may be related to actual or perceived worse outcomes in black patients. Our study clearly shows that outcomes are not different between blacks and whites receiving AHCT for MM suggesting this treatment modality should be offered to all patients when medically appropriate. These results are in accordance with a meta-analysis of patients treated for 14 different cancers, where survival in the majority of cancers was similar between races when comparable treatment was given.29
The pre-transplant characteristics of black recipients of AHCT are interesting. The black cohort was younger and had better performance status than the white cohort, despite higher rates of anemia and other co- morbidities at diagnosis. These differences likely indicate a selection bias operating against older black patients with lower KPS scores with regard to referral for consideration of AHCT. Black patients were also likely to have had a longer time between diagnosis and transplantation compared to whites, while receiving a similar number of chemotherapy regimens and having similar responses. This suggests delayed referral for consideration of AHCT. A referral bias favoring only the healthiest black patients for transplant may be in effect, while patients with less favorable clinical features may only be offered non-transplant or even non-treatment options.
The major strength of our study is the broad representation of transplant centers making it very likely that these results are applicable to the transplant community as a whole. In this analysis we are unable to draw any conclusions about factors associated with non-receipt of transplant in blacks since a non-transplant population is not represented. The characteristics of the population of black MM patients not receiving AHCT need to be analyzed to identify the causes of a under utilization of AHCT. It is possible that many blacks who are not receiving stem-cell transplantation for myeloma are forgoing the transplant by choice. However it is also possible that referral bias, unequal access to tertiary care, compliance gap, reluctance to enter clinical trials and socioeconomic disparities account for some of the differences in utilization of AHCT for patients with MM. With the demonstration of equal outcomes for black with MM, further study and definitive action to ensure better awareness and delivery of transplant options for the black population is warranted.
Acknowledgments
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Aetna; American Society for Blood and Marrow Transplantation; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Association of Medical Microbiology and Infectious Disease Canada; Astellas Pharma US, Inc.; Baxter International, Inc.; Bayer HealthCare Pharmaceuticals; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Canadian Blood and Marrow Transplant Group; Celgene Corporation; CellGenix, GmbH; Centers for Disease Control and Prevention; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Cubist Pharmaceuticals; Cylex Inc.; CytoTherm; DOR BioPharma, Inc.; Dynal Biotech, an Invitrogen Company; Enzon Pharmaceuticals, Inc.; European Group for Blood and Marrow Transplantation; Gambro BCT, Inc.; Gamida Cell, Ltd.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Infectious Diseases Society of America; Kiadis Pharma; Kirin Brewery Co., Ltd.; Merck & Company; The Medical College of Wisconsin; MGI Pharma, Inc.; Michigan Community Blood Centers; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; New York Blood Center; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka Pharmaceutical Development & Commercialization, Inc.; Pall Life Sciences; PDL BioPharma, Inc; Pfizer Inc; Pharmion Corporation; Saladax Biomedical, Inc.; Schering Plough Corporation; Society for Healthcare Epidemiology of America; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex; Teva Pharmaceutical Industries; The Marrow Foundation; THERAKOS, Inc.; Vidacare Corporation; Vion Pharmaceuticals, Inc.; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting typesetting and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kyle RA, Rajkumar SV. Epidemiology of the plasma-cell disorders. Best Pract Res Clin Haematol. 2007 Dec;20(4):637–664. doi: 10.1016/j.beha.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008 Mar 1;111(5):2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown LM, Gridley G, Check D, Landgren O. Risk of multiple myeloma and monoclonal gammopathy of undetermined significance among white and black male United States veterans with prior autoimmune, infectious, inflammatory, and allergic disorders. Blood. 2008 Apr 1;111(7):3388–3394. doi: 10.1182/blood-2007-10-121285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landgren O, Gridley G, Turesson I, et al. Risk of monoclonal gammopathy of undetermined significance (MGUS) and subsequent multiple myeloma among black and white veterans in the United States. Blood. 2006 Feb 1;107(3):904–906. doi: 10.1182/blood-2005-08-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ries L, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: 1975–2005. [accessed January 2009]. http://seer.cancer.gov/csr/1975_2005/, based on November 2007 SEER data submission, posted to the SEER web site, 2008. last . [Google Scholar]
- 6.Singh J, Dudley AW, Jr, Kulig KA. Increased incidence of monoclonal gammopathy of undetermined significance in blacks and its age-related differences with whites on the basis of a study of 397 men and one woman in a hospital setting. J Lab Clin Med. 1990 Dec;116(6):785–789. [PubMed] [Google Scholar]
- 7.Landgren O, Katzmann JA, Hsing AW, et al. Prevalence of monoclonal gammopathy of undetermined significance among men in Ghana. Mayo Clin Proc. 2007 Dec;82(12):1468–1473. doi: 10.1016/S0025-6196(11)61089-6. [DOI] [PubMed] [Google Scholar]
- 8.Samanic C, Gridley G, Chow WH, Lubin J, Hoover RN, Fraumeni JF., Jr Obesity and cancer risk among white and black United States veterans. Cancer Causes Control. 2004 Feb;15(1):35–43. doi: 10.1023/B:CACO.0000016573.79453.ba. [DOI] [PubMed] [Google Scholar]
- 9.Benjamin M, Reddy S, Brawley OW. Myeloma and race: a review of the literature. Cancer Metastasis Rev. 2003 Mar;22(1):87–93. doi: 10.1023/a:1022268103136. [DOI] [PubMed] [Google Scholar]
- 10.Friedman GD, Herrinton LJ. Obesity and multiple myeloma. Cancer Causes Control. 1994 Sep;5(5):479–483. doi: 10.1007/BF01694762. [DOI] [PubMed] [Google Scholar]
- 11. [accessed January 2009]; http://seer.cancer.gov/statfacts/html/mulmy.html. last .
- 12.Abou-Jawde RM, Baz R, Walker E, et al. The role of race, socioeconomic status, and distance traveled on the outcome of black patients with multiple myeloma. Haematologica. 2006 Oct;91(10):1410–1413. [PubMed] [Google Scholar]
- 13.Savage D, Lindenbaum J, Van Ryzin J, Struening E, Garrett TJ. Race, poverty, and survival in multiple myeloma. Cancer. 1984 Dec 15;54(12):3085–3094. doi: 10.1002/1097-0142(19841215)54:12<3085::aid-cncr2820541246>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 14.Cella DF, Orav EJ, Kornblith AB, et al. Socioeconomic status and cancer survival. J Clin Oncol. 1991 Aug;9(8):1500–1509. doi: 10.1200/JCO.1991.9.8.1500. [DOI] [PubMed] [Google Scholar]
- 15.Lyn D, Cherney BW, Lalande M, et al. A duplicated region is responsible for the poly(ADP-ribose) polymerase polymorphism, on chromosome 13, associated with a predisposition to cancer. Am J Hum Genet. 1993 Jan;52(1):124–134. [PMC free article] [PubMed] [Google Scholar]
- 16.Cao J, Hong CH, Rosen L, et al. Deletion of genetic material from a poly(ADP-ribose) polymerase-like gene on chromosome 13 occurs frequently in patients with monoclonal gammopathies. Cancer Epidemiol Biomarkers Prev. 1995 Oct-Nov;4(7):759–763. [PubMed] [Google Scholar]
- 17.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996 Jul 11;335(2):91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 18.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003 May 8;348(19):1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 19.Joshua TV, Rizzo JD, Zhang MJ, Horowitz MM. Access to hematopoietic stem cell transplantation: Effect of race and gender. Biol Blood Marrow Transplant. 2007;13(Suppl 2):22. [Google Scholar]
- 20.Blade J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998 Sep;102(5):1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 22.Klein JP, Moeschberger ML. Survival analysis: techniques for censored and truncated data. 2. New York: Springer Verlag; 2003. [Google Scholar]
- 23.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 24.Rohatgi N, Du XL, Coker AL, Moye LA, Wang M, Fang S. Chemotherapy and survival for patients with multiple myeloma: findings from a large nationwide and population-based cohort. Am J Clin Oncol. 2007 Oct;30(5):540–548. doi: 10.1097/COC.0b013e3180592a30. [DOI] [PubMed] [Google Scholar]
- 25.Modiano MR, Villar-Werstler P, Crowley J, Salmon SE. Evaluation of race as a prognostic factor in multiple myeloma. An ancillary of Southwest Oncology Group Study 8229. J Clin Oncol. 1996 Mar;14(3):974–977. doi: 10.1200/JCO.1996.14.3.974. [DOI] [PubMed] [Google Scholar]
- 26.Verma PS, Howard RS, Weiss BM. The impact of race on outcomes of autologous transplantation in patients with multiple myeloma. Am J Hematol. 2008 May;83(5):355–358. doi: 10.1002/ajh.21139. [DOI] [PubMed] [Google Scholar]
- 27.Khaled Y, Abidi MH, Janakiraman N, et al. Outcomes after auto-SCT in blacks with multiple myeloma. Bone Marrow Transplant. 2009 Jun;43(11):845–851. doi: 10.1038/bmt.2008.401. [DOI] [PubMed] [Google Scholar]
- 28.Saraf S, Chen YH, Dobogai LC, et al. Prolonged responses after autologous stem cell transplantation in black patients with multiple myeloma. Bone Marrow Transplant. 2006 Jun;37(12):1099–1102. doi: 10.1038/sj.bmt.1705392. [DOI] [PubMed] [Google Scholar]
- 29.Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, Begg CB. Survival of blacks and whites after a cancer diagnosis. Jama. 2002 Apr 24;287(16):2106–2113. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]