Abstract
Background
Determining the effects of varying vaccine coverage, compliance, administration rates, prioritization, and timing among employees during an influenza pandemic.
Methods
As part of the Models of Infectious Disease Agent Study (MIDAS) network’s H1N1 influenza planning efforts, an agent-based computer simulation model (ABM) was developed of the Washington, DC metropolitan region, encompassing five metropolitan statistical areas. Each simulation run involved introducing 100 infectious individuals to initiate a 1.3 reproductive rate (R0) epidemic, consistent with H1N1 parameters to date. Another set of scenarios represented a R0=1.6 epidemic.
Results
An unmitigated epidemic resulted in substantial productivity losses (a mean of $112.6 million for a serologic 15% attack rate and $193.8 million for a serologic 25% attack rate), even with the relatively low estimated mortality impact of H1N1. While vaccinating Advisory Committee on Immunization Practices (ACIP) priority groups resulted in the largest savings, vaccinating all remaining workers captured additional savings and, in fact, reduced healthcare workers’ and critical infrastructure workers’ chances of infection. While employee vaccination compliance affected the epidemic, once 20% compliance was achieved, additional increases in compliance provided less incremental benefit. Even though a vast majority of the workplaces in the DC Metro region had fewer than 100 employees, focusing on vaccinating only those in larger firms (≥100 employees) was just as effective in mitigating the epidemic as trying to vaccinate all workplaces.
Conclusions
Timely vaccination of at least 20% of the large company workforce can play an important role in epidemic mitigation.
INTRODUCTION
Employers and workplaces may be crucial to epidemic and pandemic preparedness planning. The labor force (over 154 million in August 2009) constitutes about half the population and a majority of those aged >25 years in the U.S. This group spends large proportions of its days at work (in 2008, employed people spent an average of 7.9 hours per weekday and 5.6 hours per weekend day working) interacting closely with each other and clients.1 As a result, workplaces are potential sources of disease transmission, and, moreover, illness and absenteeism could lead to substantial productivity losses and hamper many businesses’ ability to continue functioning. Better understanding the possible effects of vaccinating employees may be important and can help policymakers and businesses plan vaccine distribution and administration logistics, especially with the current H1N1 influenza vaccine in short supply.
Vaccination may be an effective means of protecting the labor force in the event of an epidemic. Currently H1N1 vaccine production is underway and should generate vaccines for the 2009–2010 influenza season. However, many questions about employee vaccination remain:
What percentage of the labor force needs to be vaccinated to mitigate an epidemic?
What are the impacts of employee compliance, vaccination timing and administration rates on an epidemic?
Do these impacts differ for different professional and industry sectors, especially healthcare workers and critical society infrastructure personnel?
What is the impact of prioritizing larger firms versus smaller businesses?
How will all of the above factors and scenarios affect employers and society?
The answers to these questions may help policymakers and employers develop and implement better strategies (e.g., workplace vaccination campaigns, educational programs, time off for vaccination clinic visits, financial incentives for vaccination).
As part of the NIH Models of Infectious Disease Agent Study (MIDAS) network’s continuing efforts to assist public health officials and policymakers for pandemic H1N1 planning, the University of Pittsburgh MIDAS Modeling Team in cooperation with Research Triangle Institute and the Pittsburgh Supercomputing Center developed an agent-based computer simulation model (ABM) of five Washington, DC metropolitan statistical areas:
Baltimore–Towson Metropolitan Statistical Area
Washington–Arlington–Alexandria, DC–VA–MD–VA Metropolitan Statistical Area
Winchester, VA–WV Metropolitan Statistical Area
Lexington Park, MD Micropolitan Statistical Area
Culpeper, VA Micropolitan Statistical Area.
Simpler models (e.g., compartment models) represent populations as relatively homogenous collections of individuals that interact equally among each other without important spatial detail. Such models are limited in their ability to examine the effects of vaccinating specific populations at specific locations. By contrast, the current ABM consisted of a virtual population of computer agents, each having a set of sociodemographic characteristics and behaviors, which, like virtual people, moved among virtual households, workplaces, schools, and other locations every day and interacted with each other through simulated social networks. An integrated Susceptible–Exposed–Infected–Recovered (SEIR) disease framework allowed us to simulate various influenza epidemic scenarios and employee vaccination strategies. The current model incorporated many methods from other previously published MIDAS simulation models.2,3
MATERIALS AND METHODS
Model Structure and Synthetic Census-Based Population
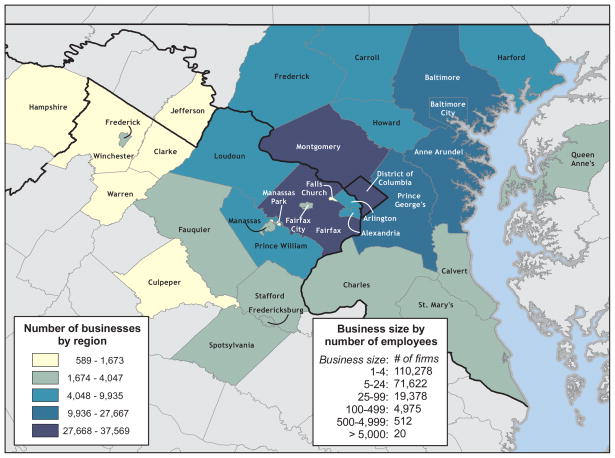
Figure 1 illustrates the simulated Washington, DC, metropolitan region. The region encompassed five census metropolitan statistical areas listed above. The model contained a total of 7,414,562 virtual people, called computer commuter “agents”, each agent having a set of sociodemographic characteristics and daily behaviors (e.g., age, gender, employment status, occupation, household location, household membership, school assignment for students and teachers, work location assignment for employed adults, work status as employed or unemployed, and disease status).4 A total of 2,044,283 agents were aged <18 years (2,525,546 aged <24 years) and 726,930 were aged >65 years. In this population, 272,806 individuals fell into the Advisory Committee on Immunization Practices (ACIP)–defined vaccination priority groups for the H1N1 vaccine, which currently includes pregnant women, healthcare workers, children aged 6–24 months, people who live with or care for children aged <6 months, and people who are at risk for influenza complications due to underlying medical conditions.5,6 To determine the agents in the population who are at increased risk for influenza complications, the 2006–2008 National Health Interview Surveys (NHIS) was utilized to determine what percentage of each age group would have relevant comorbidities: 1.5% for those aged ≤5 months; 4.2% for those aged 6–24 months; 8.8% for those aged 2–4.9 years; 11.8% for those aged 5–18 years; 12.4% for those aged 19–24 years; 15.7% for those aged 25–49 years; 30.6% for those aged 50–64 years; and 47.0% for those aged ≥65 years.7 A method modified from that developed by Beckman, et. al. helped extract the agent population from U.S. Census Bureau’s Public Use Microdata files (PUMs) and Census aggregated data.8,9 The synthesized household population was generated using Public Use Microdata Samples (PUMS) and census aggregated population counts by block group using the method detailed in Wheaton, et. al.4 Synthetic households closely matched census-aggregated counts for selected variables including income, age of head of household, and household size. For example, households occupied by agents were established such that the age of the head of the household matched census-aggregated counts at the block-group level, which aimed to generate appropriate distributions of family demographics (e.g., young families and older empty-nesters). Both family and nonfamily (i.e., unrelated roommates who live together) types of households were included. Assignments of school-age children to schools and working adults to workplaces were based on business data (locations and sizes of businesses) and commuting pattern data. Assignments of working adults to workplaces are based on business data (locations and sizes of businesses) and commuting pattern data. In addition, when assigning workers to workplaces, only a percentage of people aged ≥65 years were assigned to workplaces under the assumption that most people aged ≥65 years are no longer in the workforce. In all cases, demographic assignments were based on underlying available data.
Figure 1.
Workplaces in Washington DC Metropolitan Region
Each simulation weekday, agents moved among their respective households, their assigned workplaces (or schools depending on their age), and various locations in the community, where they interacted with other proximal agents based on the rates in Table 1. (Since at the time of the modeling effort, InfoUSA had no information about how many employees may be telecommuters, telecommuting was not factored into the analysis.) The model included all workers that were labeled as members of the Federal, State and local government officials and supporting staff as labeled by the 2000 census occupational data.10 Agents interacted more frequently with agents with whom they had closer relationships (e.g., family members, household members, classmates, and office mates). Large firm employees interacted more closely with their office mates but also encountered people who worked in different offices of the same firm. Workers in firms that have only one office repeatedly contact the same people each day. On weekends, schools and many workplaces closed, prompting agents to increase their community interactions by 50%.11 A minority (20%) of employees continued to work on weekends.12
TABLE 1.
Model transmission and person-to-person contact parameter values
| Transmission parameters | |||
|---|---|---|---|
| Contact Group | Infected | Susceptible | Transmission probabilitya |
| Household | Adult | Adult | 0.4 |
| Household | Child | Adult | 0.3 |
| Household | Adult | Child | 0.3 |
| Household | Child | Child | 0.6 |
| Elementary school | Student | Student | 0.0435 |
| Middle school | Student | Student | 0.0375 |
| High school | Student | Student | 0.0315 |
| Workplace | Adult | Adult | 0.0575 |
| Hospital | HCW | HCW | 0.0575 |
| Hospital | HCW | Patient | 0.01 |
| Hospital | Patient | HCW | 0.01 |
| Neighborhood | All | Child | 0.0000145 |
| Neighborhood | All | Adult | 0.000725 |
| Community | All | Child | 0.00003175 |
| Community | All | Adult | 0.00018125 |
| Social network parameters | |||
|---|---|---|---|
| Name | Participant | Mean contacts per day | Social Network |
| Classroom | Student and teachers | 10 | School |
| School outside of classrooms | Student | 10 | School |
| School outside of school | Teacher student | 6.5 | Community |
| Weekend activity | Student | 9.5 | Community |
| Per office | Worker | 4 | Workplace |
| Per firm | Worker | 3.45 | Workplace |
| Community | All (including students) | 9.2 | Community |
| Per hospital/clinic ward | HCW | 4 | Workplace |
| Per hospital/clinic building | HCW | 3.45 | Workplace |
| Doctor seeing patient | HCW that sees patients | 30 | Workplace |
For all infections (symptomatic and asymptomatic)
HCW, healthcare worker
Disease Parameters and Model Calibration
Disease parameters and assumptions came from previous MIDAS models.12–18 An underlying Susceptible–Exposed–Infectious–Recovered (SEIR) disease model governed disease progression and transmission. Based on information from the special July 2009 H1N1 ACIP Meeting, the model assumed that 34% of individuals aged >65 years had residual immunity from 1957 pandemic.19 At the start of each simulation run, all unvaccinated people who did not have any residual immunity were initially susceptible (S) to influenza. On Simulation Day 1, 100 agents, based on an estimate of the number of daily incident cases in the DC Metropolitan region on September 1, 2009, were randomly chosen for initial infection. (Note: In order to test the robustness of this assumption, sensitivity analyses varied this initial seed from 1 case to 500 cases and found no significant difference). Every susceptible individual who contacted an infectious individual had a probability of contracting influenza (Table 1), derived from prior studies of the 1957–8 Asian influenza pandemic.2 Each newly infected person then moved to the exposed (E) state for the duration of the disease’s incubation period and then to the infectious state (I) where the person could infect others. (All people in the E state moved to the I state.) The mean incubation period length was 2 days, and the mean length of infectiousness was 7 days. Two thirds of infectious patients exhibited symptoms, 50% of symptomatic students and workers stayed home with no community contacts unless they saw a doctor, and 40% of symptomatic patients visited a clinic or emergency room.2,12,20 Each day a symptomatic worker has a 50% chance of staying at home and not going to work.2,12 Moreover. asymptomatic yet infectious individuals remained unaware of their infection and continued to go to work. Following the infectious period, the agent proceeded to the recovered state (R), in which he or she was immune to subsequent infections. Vaccination had a probability (i.e., vaccine efficacy) of transforming a susceptible (S) individual into a recovered (R) individual. Initial model calibration utilized the Ferguson et al. approach with data from historic (1957–58, 1968–69) influenza pandemics and targeted an epidemic with a 35% serologic attack rate (AR) seen in the 1957–1958 pandemic.2
Our simulation runs employed the latest estimates of the current H1N1 pandemic, including a basic reproductive rate (R0) of 1.3, which is the expected number of secondary cases that a typical infected individual will produce in completely susceptible population.21,22 Case fatality rates were estimates of the H1N1 pandemic as of September 2009: 0.0615 deaths per 100 H1N1 cases.22–25
The model also depicted the region’s individual households, schools, workplaces, and healthcare facilities, with agents assigned to each using previously described methods and the following data sources:
Schools and school assignments: U.S. Department of Education National Center for Education Statistics (public schools data) and Private data vendor (private schools)
Workplaces and workplace assignments: U.S. Census Standard Tabulation Product (STP64) commuting pattern data and ESRI Business Analyst (InfoUSA business data)
The DC Metro region included 204, 691 workplaces with a total of 3,714,125 employees. The distribution of firm sizes was as follows: 5,924 had more than 100 employees (47% of the workforce), 9,829 from 50 to 99 employees (16% of the workforce), 11,038 from 20 to 49 employees (11% of the workforce), 26,397 from 10 to 19 employees (7% of the workforce), and 151,503 less than 10 employees (20% of the workforce). The healthcare work force encompassed 81,836 individuals. Critical infrastructure employees as defined by the Department of Homeland Security amounted to 272,806 individuals, which include employees that belong to the following sectors: health care, emergency services, critical government, utilities, and information technologies.26
Employee Vaccination Scenarios and Strategies
Experiments assumed that the vaccine would be a one-dose vaccine for those aged ≥10 years, conferring 80% immunity and a two-dose vaccine for those aged <10 years with one dose conferring 50% immunity and two doses conferring 80% immunity.27 Since the clinical effectiveness and efficacy of H1N1 vaccine is currently unknown, the current values mirrored those of the seasonal influenza vaccine.28–31 Each dose took 2 weeks after administration to achieve its effects. The current baseline scenario assumed that healthcare workers and all higher-priority groups (i.e., pregnant women, children, and people with high-risk conditions) would be vaccinated before healthy adult workers.24 Different sets of simulation runs explored various combinations of the following employee vaccination scenarios:
Employee Vaccination Timing: Employer policies and employee attitudes may affect when employees are vaccinated. For example, on-site employer vaccination campaigns, educational programs, or time-off to visit health clinics may expedite employee vaccination. Therefore, a set of experiments explored the impact of vaccination timing.
Employee Vaccination Coverage and Compliance: These experiments explored the effects of changing vaccine coverage and compliance among different groups.
Vaccine Administration Speed: These experiments assessed the impact of varying the speed at which vaccines were administered to the population.
Employer Sector: These experiments focused on vaccination coverage, compliance, and timing within different key employment sectors. One set of experiments looked at hospital-based healthcare workers. Another set studied critical infrastructure workers as defined by the USDHHS
Model Outcomes
Each simulation run generated daily disease incidence, prevalence, work absenteeism, clinic visits, hospitalizations, and deaths. The following assumptions and parameters translated these outputs into costs: median hourly wage $15.57, median length of tenure at a workplace 4.1 years, median age of workers 36.7 years, and median life expectancy 44 years.32,33 Costs and earnings were updated to 2009 U.S. dollars. A net rate of 3% (growth minus inflation) discounted future costs (i.e., lost wages due to death) to 2009 costs.34,35
Our model assumed the employers’ perspective, determining the potential productivity losses of each scenario and the effects of vaccination in mitigating these losses. Hourly wage distributions by sector came from the Bureau of Labor Statistics.1 Absenteeism resulted in 8 hours of lost productivity each day. A death meant that the employer lost that employee’s productivity for the remainder of his or her employment.
Computational Specifics
The ABM was programmed in C++. Simulations were performed at the Pittsburgh Supercomputing Center on Axon, an Intel Xeon–based Infiniband cluster. Each simulation run took on average 45 minutes to complete.
RESULTS
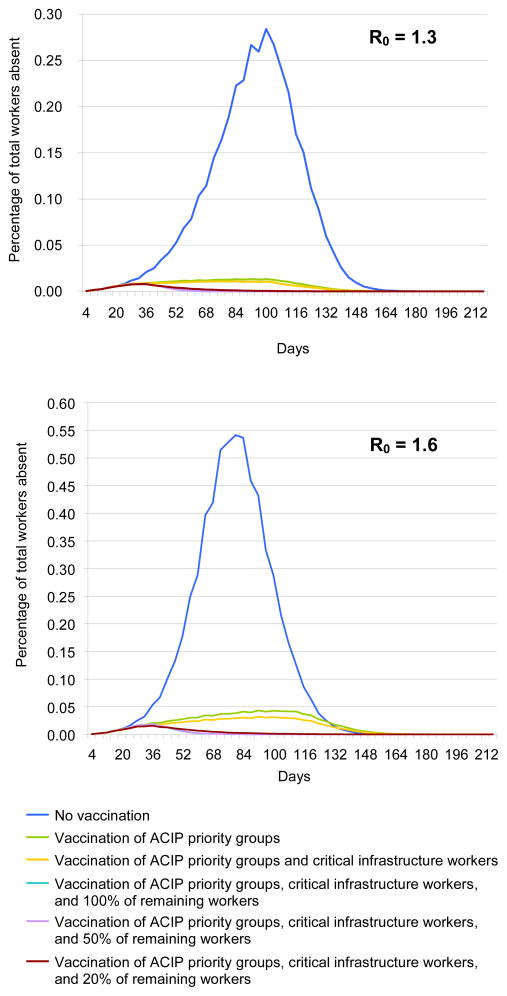
Each presented epidemic curve is the average of 20 simulation runs and 4-day moving average trend lines calculated to smooth out irregular patterns produced by the weekend effect (i.e., students and workers having different weekend contact patterns). The duration of the R0=1.6 epidemic was approximately 120 days long, with a peak around Day 76. The duration of the R0=1.3 epidemic was approximately 150 days long, with a peak around Day 96.
Economic Effects of Unmitigated Epidemics
Simulation runs suggest that an unmitigated Ro = 1.3 epidemic in the DC Metro Region resulted in a total serologic attack rate of 15% (or 1.1 million cases). An average of 579,392 employees (37,003 critical infrastructure workers and 20,516 healthcare workers) were infected with 193,131 (i.e., the fraction of infected who were symptomatic and missed work) missing a mean total of 950,270 days of work (60,689 days for critical infrastructure workers and 33,648 days for healthcare workers), which led to a mean of $118.4 million in productivity losses ($7.6 million for critical infrastructure workers and $4.2 million for healthcare workers). This equates to a mean of 2.0 lost hours or $31.87 in productivity losses per employee in the DC Metro region. So a 100-person firm may have expected to lose 200 hours or $3,187 in productivity over about a 150-day period.
All of these numbers increased for an unmitigated Ro=1.6 epidemic: a total serologic attack rate of 25% (or 1.9 million cases). An average of 948,779 employees (61,451 critical infrastructure workers and 35,910 healthcare workers) were infected with 316,260 missing a mean total of 1,556,107 days of work (100,786 days for critical infrastructure workers and 58,896 days for healthcare workers), which led to a mean of $193.8 million in productivity losses ($12.6 million for critical infrastructure workers and $7.3 million for healthcare workers). This projected to a mean of 3.4 lost hours or $52.19 in productivity losses per employee in the DC Metro region. So a 100-person firm may have expected to lose 340 hours or $5,219 in productivity over about a 120-day period.
Vaccination of Different Employee Groups
Tables 2 and 4 and Figure 2 delineates the effects of progressively vaccinating the following employee groups: ACIP-defined priority groups, which include healthcare workers, critical infrastructure workers, and all remaining workers. While vaccinating ACIP priority groups resulted in the largest savings, vaccinating all remaining workers also captured additional savings. In fact, because vaccination is not 100% effective, vaccinating remaining employees provided additional savings for healthcare workers and critical infrastructure workers by reducing everyone’s chances of infection.
TABLE 2.
Impact of vaccinating different employee groups (R0 = 1.3)a
| Infected (median) | Days lost (median) | Median productivity Lost (millions) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scenario | Days to complete vaccination | Attack rateb (%) | Total cases | n | CIW | HCW | n | CIW | HCW | Total workers | CIW | HCW |
| No vaccination | 15.0 | 1,108,844 | 579,392 | 37,003 | 20,516 | 950,270 | 60,689 | 33,648 | $ 118.37 | $ 7.56 | $ 4.19 | |
| Vaccinating ACIP priority groups only | 60 | 1.2 | 89,653 | 56,321 | 3,450 | 1,185 | 92,373 | 5,658 | 1,943 | $ 11.51 | $ 0.70 | $ 0.24 |
| 90 | 1.5 | 111,268 | 70,082 | 4,225 | 1,516 | 114,942 | 6,930 | 2,486 | $ 14.32 | $ 0.86 | $ 0.31 | |
| 120 | 1.7 | 125,027 | 78,571 | 4,806 | 1,682 | 128,866 | 7,882 | 2,759 | $ 16.05 | $ 0.98 | $ 0.34 | |
| 240 | 2.8 | 205,366 | 127,160 | 7,852 | 2,942 | 208,557 | 12,878 | 4,826 | $ 25.98 | $ 1.60 | $ 0.60 | |
| Vaccinating priority + CIW | 60 | 1.0 | 76,236 | 47,541 | 1,893 | 1,012 | 77,973 | 3,105 | 1,659 | $ 9.71 | $ 0.39 | $ 0.21 |
| 90 | 1.2 | 86,446 | 53,767 | 2,107 | 1,186 | 88,183 | 3,455 | 1,945 | $ 10.98 | $ 0.43 | $ 0.24 | |
| 120 | 1.4 | 101,856 | 63,314 | 2,538 | 1,387 | 103,841 | 4,163 | 2,275 | $ 12.93 | $ 0.52 | $ 0.28 | |
| 240 | 2.3 | 170,083 | 103,968 | 4,456 | 2,529 | 170,520 | 7,309 | 4,148 | $ 21.24 | $ 0.91 | $ 0.52 | |
| Vaccinating all workers | 60 | 0.3 | 18,553 | 9,896 | 475 | 264 | 16,231 | 778 | 432 | $ 2.02 | $ 0.10 | $ 0.05 |
| 90 | 0.4 | 32,472 | 18,198 | 828 | 508 | 29,846 | 1,358 | 834 | $ 3.72 | $ 0.17 | $ 0.10 | |
| 120 | 0.6 | 45,090 | 25,930 | 1,203 | 678 | 42,528 | 1,973 | 1,113 | $ 5.30 | $ 0.25 | $ 0.14 | |
| 240 | 2.2 | 161,017 | 97,535 | 6,085 | 2,391 | 159,969 | 9,979 | 3,922 | $ 19.93 | $ 1.24 | $ 0.49 | |
Assuming 50% vaccination rate for children and nonworking elderly people.
Serologic attack rate. Clinical attack rate is 66.7% of this value
CIW, critical infrastructure workers; HCW, healthcare workers
TABLE 4.
Impact of vaccinating different employee groups (R0 = 1.6)a
| Infected (median) | Days lost (median) | Median productivity lost (millions) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scenario | Days to Complete Vaccination | Attack rateb(%) | Total Cases | n | CIW | HCW | n | CIW | HCW | Total workers | CIW | HCW |
| No vaccination | 25.5 | 1,891,405 | 948,779 | 61,451 | 35,910 | 1,556,107 | 100,786 | 58,896 | $ 193.83 | $ 12.55 | $ 7.34 | |
| Vaccinating ACIP priority groups only | 60 | 3.8 | 279,485 | 173,345 | 10,634 | 4,356 | 284,306 | 17,441 | 7,144 | $ 35.41 | $ 2.17 | $ 0.89 |
| 90 | 4.2 | 311,365 | 193,152 | 11,863 | 4,814 | 316,792 | 19,457 | 7,896 | $ 39.46 | $ 2.42 | $ 0.98 | |
| 120 | 4.6 | 344,045 | 212,469 | 13,158 | 5,376 | 348,474 | 21,580 | 8,817 | $ 43.41 | $ 2.69 | $ 1.10 | |
| 240 | 7.4 | 549,296 | 330,939 | 20,521 | 9,089 | 542,778 | 33,656 | 14,908 | $ 67.61 | $ 4.19 | $ 1.86 | |
| Vaccinating priority + CIW | 60 | 2.8 | 209,242 | 128,515 | 4,982 | 3,389 | 210,779 | 8,171 | 5,559 | $ 26.25 | $ 1.02 | $ 0.69 |
| 90 | 3.4 | 255,555 | 156,094 | 6,088 | 4,225 | 256,012 | 9,985 | 6,930 | $ 31.89 | $ 1.24 | $ 0.86 | |
| 120 | 3.8 | 278,376 | 169,685 | 6,747 | 4,589 | 278,303 | 11,066 | 7,527 | $ 34.67 | $ 1.38 | $ 0.94 | |
| 240 | 6.5 | 481,942 | 286,026 | 12,274 | 8,424 | 469,116 | 20,131 | 13,816 | $ 58.43 | $ 2.51 | $ 1.72 | |
| Vaccinating all workers | 60 | 0.5 | 35,593 | 18,381 | 898 | 659 | 30,147 | 1,473 | 1,081 | $ 3.76 | $ 0.18 | $ 0.13 |
| 90 | 0.9 | 63,856 | 34,563 | 1,590 | 1,246 | 56,687 | 2,608 | 2,044 | $ 7.06 | $ 0.32 | $ 0.25 | |
| 120 | 1.5 | 109,050 | 60,769 | 2,696 | 2,097 | 99,668 | 4,422 | 3,439 | $ 12.41 | $ 0.55 | $ 0.43 | |
| 240 | 5.7 | 425,401 | 245,945 | 10,892 | 7,768 | 403,378 | 17,864 | 12,740 | $ 50.24 | $ 2.23 | $ 1.59 | |
Assuming 50% vaccination rate for children and nonworking elderly people.
Serologic attack rate. Clinical attack rate is 66.7% of this value.
CIW, critical infrastructure workers; HCW, healthcare workers
Figure 2.
Daily absenteeism for DC Metropolitan Region ACIP,
Employee Vaccination Timing
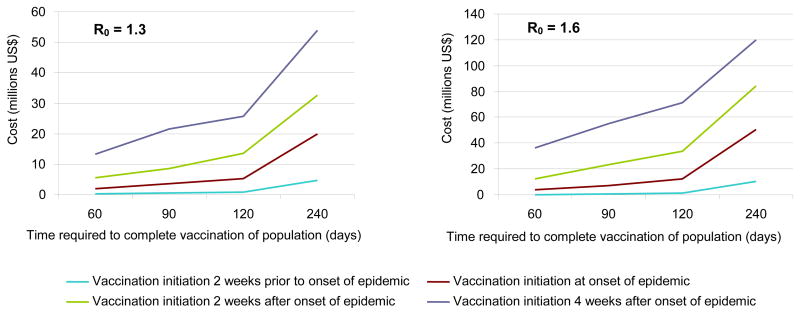
The timing of vaccination played a crucial role in epidemic mitigation. Initiating employee vaccination 2 weeks prior to the epidemic start nearly completely quelled the epidemic, even when the vaccination of the population took 240 days to complete (0.5% total serologic attack rate). Figure 3 shows the substantial impact in terms of number of infected of delaying vaccination, a cost that increased as it took longer to vaccinate the population.
Figure 3.
Impact when varying vaccination timing
Employee Vaccination Coverage and Compliance
Employee compliance with vaccination affected the epidemic to a much greater degree at low compliance levels. Once 20% compliance was achieved, additional increases in compliance did not provide as much benefit. (Tables 3 and 5)
TABLE 3.
Varying employee vaccination coverage and days to complete vaccination (R0 = 1.3)a
| Median infected | Median Days Lost | Median Productivity Lost (millions) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Employee vaccine coverage (%) | Days to complete vaccination | Attack rateb(%) | Total | n | CIW | HCW | n | CIW | HCW | Workers | CIW | HCW |
| No vaccine | 15.0 | 1,108,844 | 579,392 | 37,003 | 20,516 | 950,270 | 60,689 | 33,648 | $ 118.37 | $ 7.56 | $ 4.19 | |
| 100 | 60 | 0.3 | 18,553 | 9,896 | 475 | 264 | 16,231 | 778 | 432 | $ 2.02 | $ 0.10 | $ 0.05 |
| 50 | 60 | 0.2 | 18,415 | 9,980 | 470 | 236 | 16,369 | 772 | 387 | $ 2.04 | $ 0.10 | $ 0.05 |
| 20 | 60 | 0.3 | 24,066 | 13,532 | 590 | 325 | 22,195 | 968 | 534 | $ 2.76 | $ 0.12 | $ 0.07 |
| 0 | 60 | 1.2 | 89,653 | 56,321 | 3,450 | 1,185 | 92,373 | 5,658 | 1,943 | $ 11.51 | $ 0.70 | $ 0.24 |
| 100 | 90 | 0.3 | 18,553 | 18,198 | 828 | 508 | 29,846 | 1,358 | 834 | $ 3.72 | $ 0.17 | $ 0.10 |
| 50 | 90 | 0.4 | 30,740 | 17,271 | 792 | 481 | 28,327 | 1,299 | 788 | $ 3.53 | $ 0.16 | $ 0.10 |
| 20 | 90 | 0.5 | 36,492 | 20,890 | 949 | 533 | 34,262 | 1,556 | 875 | $ 4.27 | $ 0.19 | $ 0.11 |
| 0 | 90 | 1.5 | 111,268 | 70,082 | 4,225 | 1,516 | 114,942 | 6,930 | 2,486 | $ 14.32 | $ 0.86 | $ 0.31 |
| 100 | 120 | 0.6 | 45,090 | 25,930 | 1,203 | 678 | 42,528 | 1,973 | 1,113 | $ 5.30 | $ 0.25 | $ 0.14 |
| 50 | 120 | 0.6 | 46,009 | 26,405 | 1,185 | 627 | 43,307 | 1,944 | 1,028 | $ 5.39 | $ 0.24 | $ 0.13 |
| 20 | 120 | 0.7 | 55,539 | 32,596 | 1,453 | 862 | 53,462 | 2,384 | 1,414 | $ 6.66 | $ 0.30 | $ 0.18 |
| 0 | 120 | 1.7 | 125,027 | 78,571 | 4,806 | 1,682 | 128,866 | 7,882 | 2,759 | $ 16.05 | $ 0.98 | $ 0.34 |
| 100 | 240 | 2.2 | 161,017 | 97,535 | 6,085 | 2,391 | 159,969 | 9,979 | 3,922 | $ 19.93 | $ 1.24 | $ 0.49 |
| 50 | 240 | 1.9 | 144,577 | 86,374 | 3,861 | 2,181 | 141,664 | 6,333 | 3,578 | $ 17.65 | $ 0.79 | $ 0.45 |
| 20 | 240 | 2.1 | 154,238 | 92,207 | 4,111 | 2,356 | 151,230 | 6,742 | 3,863 | $ 18.84 | $ 0.84 | $ 0.48 |
| 0 | 240 | 2.8 | 205,366 | 127,160 | 7,852 | 2,942 | 208,557 | 12,878 | 4,826 | $ 25.98 | $ 1.60 | $ 0.60 |
Assuming 50% vaccination rate for children and nonworking elderly people.
Serologic attack rate. Clinical attack rate is 66.7% of this value.
CIW, critical infrastructure workers; HCW, healthcare workers
TABLE 5.
Varying employee vaccination coverage and days to complete vaccination (R0 = 1.6)
| Median infected | Median days lost | Median productivity lost (millions) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Employee vaccine coverage (%) | Days to complete vaccination | Attack rate(%) | Total | Workers | CIW | HCW | Workers | CIW | HCW | Workers | CIW | HCW |
| No Vaccine | 25.5 | 1,891,405 | 948,779 | 61,451 | 35,910 | 1,556,107 | 100,786 | 58,896 | $ 193.83 | $ 12.55 | $ 7.34 | |
| 100 | 60 | 0.5 | 35,593 | 13,532 | 590 | 325 | 22,195 | 968 | 534 | $ 2.76 | $ 0.12 | $ 0.07 |
| 50 | 60 | 0.6 | 40,854 | 18,381 | 898 | 659 | 30,147 | 1,473 | 1,081 | $ 3.76 | $ 0.18 | $ 0.13 |
| 20 | 60 | 0.7 | 50,149 | 21,297 | 989 | 817 | 34,930 | 1,621 | 1,339 | $ 4.35 | $ 0.20 | $ 0.17 |
| 0 | 60 | 2.8 | 209,242 | 128,515 | 4,982 | 3,389 | 210,779 | 8,171 | 5,559 | $ 26.25 | $ 1.02 | $ 0.69 |
| 100 | 90 | 0.5 | 35,593 | 34,563 | 1,590 | 1,246 | 56,687 | 2,608 | 2,044 | $ 7.06 | $ 0.32 | $ 0.25 |
| 50 | 90 | 0.9 | 64,857 | 35,268 | 1,584 | 1,259 | 57,844 | 2,597 | 2,065 | $ 7.21 | $ 0.32 | $ 0.26 |
| 20 | 90 | 1.1 | 81,812 | 45,545 | 2,021 | 1,550 | 74,698 | 3,315 | 2,543 | $ 9.30 | $ 0.41 | $ 0.32 |
| 0 | 90 | 3.4 | 255,555 | 156,094 | 6,088 | 4,225 | 256,012 | 9,985 | 6,930 | $ 31.89 | $ 1.24 | $ 0.86 |
| 100 | 120 | 1.5 | 109,050 | 60,769 | 2,696 | 2,097 | 99,668 | 4,422 | 3,439 | $ 12.41 | $ 0.55 | $ 0.43 |
| 50 | 120 | 1.5 | 109,114 | 60,922 | 2,658 | 2,062 | 99,919 | 4,359 | 3,382 | $ 12.45 | $ 0.54 | $ 0.42 |
| 20 | 120 | 1.8 | 131,213 | 74,230 | 3,228 | 2,471 | 121,746 | 5,295 | 4,052 | $ 15.16 | $ 0.66 | $ 0.50 |
| 0 | 120 | 3.8 | 278,376 | 169,685 | 6,747 | 4,589 | 278,303 | 11,066 | 7,527 | $ 34.67 | $ 1.38 | $ 0.94 |
| 100 | 240 | 5.7 | 425,401 | 245,945 | 10,892 | 7,768 | 403,378 | 17,864 | 12,740 | $ 50.24 | $ 2.23 | $ 1.59 |
| 50 | 240 | 5.8 | 430,682 | 249,466 | 11,066 | 7,820 | 409,153 | 18,150 | 12,825 | $ 50.96 | $ 2.26 | $ 1.60 |
| 20 | 240 | 6.0 | 447,061 | 258,537 | 11,370 | 8,157 | 424,031 | 18,647 | 13,378 | $ 52.82 | $ 2.32 | $ 1.67 |
| 0 | 240 | 6.5 | 481,942 | 286,026 | 12,274 | 8,424 | 469,116 | 20,131 | 13,816 | $ 58.43 | $ 2.51 | $ 1.72 |
CIW, critical infrastructure workers; HCW, healthcare workers
Speed of Vaccine Administration
As Tables 2 and 3 and Figure 3 demonstrate, the speed of vaccination also was important in mitigation. The benefits of vaccinating each employee group decreased as the rate of vaccination decreased. Taking 240 days to vaccinate everyone instead of 60 days cost employers over $20 million in addition. Even stretching the vaccination campaign from 60 to 90 days yielded almost $2 million in additional losses. The longer it took to vaccinate the population, the less important employee vaccination compliance became. Low vaccination rates meant that much of the vaccination during the early parts of the epidemic was, when the impact on the epidemic was greatest, were for compliant employees.
Prioritizing Firm Size
Even though a vast majority of the workplaces in the DC Metro region had fewer than 100 employees, focusing on vaccinating larger firms with more than 100 employees was just as effective in mitigating the epidemic as trying to vaccinate employees in all of the workplaces.
DISCUSSION
The current study demonstrates how actions regarding vaccination may have significant effects during an epidemic or pandemic. Earlier vaccination, increased compliance, and swifter vaccine administration can all blunt the overall population attack rate and save employers’ productivity. While vaccinating priority groups (including healthcare workers) and critical infrastructure workers can help mitigate the epidemic, neglecting other employees can miss potential gains in influenza mitigation. In fact, vaccinating nonpriority workers could help healthcare workers and critical infrastructure workers. These findings suggest that investing in mechanisms to accelerate vaccine administration, enhance compliance, and expedite vaccine availability among businesses could pay dividends to society and employers. Workplace vaccine education, awareness, and administration programs may be able to address each of these parameters.
The results emphasize the importance of large businesses in influenza transmission and vaccination plans. Even though a majority of employees belong to smaller firms (less than 10 employees), focusing vaccination efforts on just large firms could achieve the same results as trying to catch all of the businesses. This is potentially important since aiming to provide vaccine coverage to the myriad of small businesses can be a logistic challenge. Providing onsite vaccination to each small business may be difficult and unfeasible. Some small businesses may not have enough employees to stay open while their employees visit provider offices or public health clinics to get vaccinated. Many small businesses may not even have health insurance or adequate access to health care. The higher degree of social mixing that occurs in large firms appears to outweigh the sheer combined number of people in small businesses.
Another finding is that vaccination compliance rates do not need to be very high to mitigate the epidemic. Vaccinating only 20% of employees could still yield substantial benefits. In fact, there is little to gain from pushing employee vaccination compliance up to 50% or 100%. This has several implications. While employers may want to encourage or offer vaccination, they do not have to ensure that every individual in their workplaces get vaccinated. Additionally, achieving vaccination rates among working adults commensurate with seasonal influenza vaccination rates could be highly effective in quelling the epidemic.
Projecting the current results to the entire U.S. reveals the following:
An unmitigated R0=1.3 epidemic would result in approximately a total of 45.5 million infected individuals and 23.8 million infected workers generating 39 million lost days of work and 4.9 billion in productivity losses. Vaccinating ACIP priority groups can save $4.4 billion in productivity. Vaccinating critical infrastructure workers can save an additional $70 million and the remainder of workers another $310 million.
An unmitigated R0=1.6 epidemic would result in approximately a total of 77.7 million infected individuals and 39.0 million infected workers generating 63.9 million lost days of work and $8.0 billion in productivity losses. Vaccinating ACIP priority groups can potentially save in $6.5 billion in productivity. Vaccinating critical infrastructure workers may save an additional $376 million in productivity and the remainder of workers another $924 million.
Our study demonstrates the potential impact that an influenza epidemic may have. An influenza virus strain does not have to have high virulence (i.e., high hospitalization and fatality rates) to have a large effect on society. One should not overlook the potential tremendous impact of missed days from work. A seemingly “harmless” influenza strain that causes on average only 3–5 days of missed work for only half of the people who are symptomatic can generate large losses for employers.
Limitations
All computer models are simplifications of reality and can never account for every possible factor or interaction. The model did not distinguish high-risk individuals, who may have much worse outcomes and therefore miss longer periods of work, from healthy individuals. An influenza pandemic and the resulting circumstances may not necessarily conform to the data and assumptions that the current model drew from referenced sources or previously published models and is limited to the DC metropolitan area. The model did not account for workdays missed by parents who have to take care of ill children. The model also did not capture decreased worker productivity associated with presenteeism.
In many ways, the current results may underestimate the economic effects of an epidemic. Losing large proportions of the labor force to illness may have significant negative externalities not captured by the model. Productivity losses do not necessarily scale linearly as assumed by the current model. Some firms may not be able to operate at all when certain crucial personnel are missing. Operational disruptions from labor force loss may have reverberating effects throughout society (e.g., losing a delivery service company will affect agriculture, manufacturing, health care). Additionally, some employers (e.g., self-insured employers as well as other employers who ultimately pay for costs borne by their contracted health plans) may have to bear the healthcare costs of their employees as well.
Conclusion
An unmitigated epidemic could result in substantial productivity losses (a mean of $112.6 million for the DC area for a 15% serologic attack rate and $193.8 million for a 25% serologic attack rate), even with the relatively low estimated mortality impact of H1N1. While vaccinating ACIP priority groups resulted in the largest savings, vaccinating remaining workers also captured additional savings. In fact, vaccinating remaining employees may provide additional savings for healthcare workers and critical infrastructure workers by reducing everyone’s chances of infection. Timely employee vaccination may play a crucial role in epidemic mitigation. While employee vaccination compliance affected the epidemic, once 20% compliance is achieved, additional increases in compliance provide limited additional benefit. Even though a vast majority of the workers are employed by small businesses, focusing on vaccinating larger firms may be just as effective in epidemic mitigation as trying to vaccinate employees in all of the workplaces.
Acknowledgments
This study was supported by the National Institute General Medical Sciences Models of Infectious Agent Study (MIDAS) grant 1U54GM088491-0109. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
RKZ consults for MedImmune to analyze a worksite vaccination trial. No other authors reported financial disclosures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bureau of Labor Statistics. Occupational employment statistics. U.S. Department of Labor. Washington, DC: U.S. Department of Labor; 2008. [updated 2008; cited September 1, 2009]; Available from: http://www.bls.gov/oes/current/oes_nat.htm. [Google Scholar]
- 2.Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature. 2006 Jul 27;442(7101):448–52. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halloran ME, Ferguson NM, Eubank S, Longini IM, Jr, Cummings DA, Lewis B, et al. Modeling targeted layered containment of an influenza pandemic in the U.S. Proc Natl Acad Sci U S A. 2008 Mar 25;105(12):4639–44. doi: 10.1073/pnas.0706849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wheaton W, Cajka J, Chsteen B, Wagener D, Cooley P, Ganapathi L, et al. Synthesized population databases: A U.S. geospatial database for agent-based models. RTI Press; Jan, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Use of influenza A (H1N1) 2009 monovalent vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009 Aug 28;58(RR 10):1–8. [PubMed] [Google Scholar]
- 6.Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009 Jul 31;58(RR8):1–52. [PubMed] [Google Scholar]
- 7.National Health Interview Survey (NHIS), National Center for Health Statistics (NCHS), CDC. 2006–2008 [updated 2006–2008; cited 2009 August 1]; Available from: http://www.cdc.gov/nchs/nhis.htm.
- 8.Beckman RJ, Baggerly K, McKay M. Creating synthetic baseline populations. Transportation Research Part A: Policy and Practice. 1996 January 14;30(6):415–29. [Google Scholar]
- 9.Wheaton W, Cajka J, Chasteen B, Wagener D, Cooley P, Ganapathi L, et al. In: A U.S. geospatial database for agent-based models. Park RT, editor. NC: RTI International: RTI Press; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Age Groups and Sex: 2000. 2000 [updated 2000; cited 2009–19 October]; Available from: http://factfinder.census.gov/servlet/QTTable?_bm=y&-qr_name=DEC_2000_SF1_U_QTP1&-geo_id=01000US&-ds_name=DEC_2000_SF1_U&-_lang=en&-format=&-currentselections=DEC_2000_SF1_U_QTP1&-CONTEXT=qt.
- 11.Cauchemez S, Valleron AJ, Boelle PY, Flahault A, Ferguson NM. Estimating the impact of school closure on influenza transmission from Sentinel data. Nature. 2008 Apr 10;452(7188):750–4. doi: 10.1038/nature06732. [DOI] [PubMed] [Google Scholar]
- 12.Halloran E, Ferguson N, Eubank S, Longini IJ, Cummings D, Lewis B, et al. Modeling targeted layered containment of an influenza pandemic in the U.S. PNAS. 2008 March 25;105(12):4639–44. doi: 10.1073/pnas.0706849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eubank S, Guclu H, Anil Kumar V, et al. Modeling disease outbreaks in realistic urban social networks. Nature. 2004;429:180–4. doi: 10.1038/nature02541. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson N, Cummings D, Cauchemez S, et al. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature. 2005 September 08;437:209–14. doi: 10.1038/nature04017. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson N, Cummings D, Fraser C, Cajka J, Cooley P, Burke D. Strategies for mitigating an influenza pandemic. Nature. 2006 July 27;442:448–52. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Germann T, Kadau K, Longini IJ, Macken C. Mitigation strategies for pandemic influenza in the U.S. PNAS. 2006 April 11; doi: 10.1073/pnas.0601266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longini I, Halloran E, Nizam A, Yang Y. Containing pandemic influenza with antiviral agents. Am J Epidemiol. 2004;159:623–33. doi: 10.1093/aje/kwh092. [DOI] [PubMed] [Google Scholar]
- 18.Longini IJ, Nizam A, Xu S, et al. Containing pandemic influenza at the source. Science. 2005 August 12;309:1083–7. doi: 10.1126/science.1115717. [DOI] [PubMed] [Google Scholar]
- 19.ACIP Presentation Slides: Special July 2009 Meeting. CDC; 2009. [updated 2009; cited 2009 August 1, 2009]; Available from: http://www.cdc.gov/vaccines/recs/acip/slides-july09-flu.htm. [Google Scholar]
- 20.Rothberg M, Rose D. Vaccination versus treatment of influenza in working adults: A cost-effectiveness analysis. Am J Med. 2005 Jan;118(1):68–77. doi: 10.1016/j.amjmed.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Sugimoto JD, Halloran ME, Basta NE, Chao DL, Matrajt L, et al. The Transmissibility and Control of Pandemic Influenza A (H1N1) Virus. Science. 2009 Sep 10; doi: 10.1126/science.1177373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fraser C, Donnelly CA, Cauchemez S, Hanage WP, Van Kerkhove MD, Hollingsworth TD, et al. Pandemic potential of a strain of influenza A (H1N1): early findings. Science. 2009 Jun 19;324(5934):1557–61. doi: 10.1126/science.1176062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weycker D, Edelsberg J, Halloran M, Longini I, Ciuryla V, Oster G. Population-wide benefits of routine vaccination of children against influenza. Vaccine. 2005;23(10):1284–93. doi: 10.1016/j.vaccine.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 24.ACIP Presentation Slides: Special July 2009 Meeting. 2009 [updated 2009; cited]; Available from: http://www.cdc.gov/vaccines/recs/acip/slides-july09-flu.htm.
- 25.Novel H1N1 Flu: Facts and Figures. Atlanta, GA: 2009. [updated 2009; cited 2009–19 October]; Available from: http://www.cdc.gov/H1N1FLU/surveillanceqa.htm. [Google Scholar]
- 26.Adirim T. In: Security H, editor. 2009-H1N1 Vaccine and Critical Infrastructure Key Resources Priority Groups; ACIP Meeting; July 29, 2009; 2009. [Google Scholar]
- 27.Greenberg ME, Lai MH, Hartel GF, Wichems CH, Gittleson C, Bennet J, et al. Response after One Dose of a Monovalent Influenza A (H1N1) 2009 Vaccine—Preliminary Report. N Engl J Med. 2009 Sep 10; doi: 10.1056/NEJMoa0907413. [DOI] [PubMed] [Google Scholar]
- 28.Basta NE, Halloran ME, Matrajt L, Longini IM., Jr Estimating influenza vaccine efficacy from challenge and community-based study data. Am J Epidemiol. 2008 Dec 15;168(12):1343–52. doi: 10.1093/aje/kwn259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jefferson TO, Rivetti D, Di Pietrantonj C, Rivetti A, Demicheli V. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2007;(2):CD001269. doi: 10.1002/14651858.CD001269.pub3. [DOI] [PubMed] [Google Scholar]
- 30.Rivetti D, Jefferson T, Thomas R, Rudin M, Rivetti A, Di Pietrantonj C, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2006;3:CD004876. doi: 10.1002/14651858.CD004876.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Smith S, Demicheli V, Di Pietrantonj C, Harnden AR, Jefferson T, Matheson NJ, et al. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2006;(1):CD004879. doi: 10.1002/14651858.CD004879.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Levit K, Reuters Thomson, SETR, Ryan K, Reuters Thomson, Elixhauser A., AHRQ . HCUP Facts and Figures, 2007: Statistics on Hospital-based Care in the U.S. Rockville, MD: Agency for Healthcare Research and Quality; 2008. Contract No.: Document Number|. [Google Scholar]
- 33.Wilmoth JR, Shkolnikov V. Human Mortality Database. University of California; Berkeley (U.S.): Max Planck Institute for Demographic Research; Germany: 2008. [updated 2008; cited 2008 January 21, 2008]; Available from: www.mortality.org or www.humanmortality.de. [Google Scholar]
- 34.Discounting and the Treatment of Uncertainty in Natural Resource Damage Assessment. Silver Spring, MD: NOAA (National Oceanic and Atmospheric Administration); 1999. Technical Paper 99-1. Contract No.: Document Number|. [Google Scholar]
- 35.Sloan FA. Valuing health care: costs, benefits, and effectiveness of pharmaceuticals and medical technologies. New York: Cambridge University Press; 1995. [Google Scholar]