Summary
The initial signalling events leading to Helicobacter pylori infection associated changes in motility, cytoskeletal reorganization and elongation of gastric epithelial cells remain poorly understood. Because focal adhesion kinase (FAK) is known to play important roles in regulating actin cytoskeletal organization and cell motility we examined the effect of H. pylori in gastric epithelial cells co-cultured with H. pylori or its isogenic cag pathogenicity island (PAI) or oipA mutants. H. pylori induced FAK phosphorylation at distinct tyrosine residues in a dose- and time-dependent manner. Autophosphorylation of FAK Y397 was followed by phosphorylation of Src Y418 and resulted in phosphorylation of the five remaining FAK tyrosine sites. Phosphorylated FAK and Src activated Erk and induced actin stress fibre formation. FAK knock-down by FAK-siRNA inhibited H. pylori-mediated Erk phosphorylation and abolished stress fibre formation. Infection with oipA mutants reduced phosphorylation of Y397, Y576, Y577, Y861 and Y925, inhibited stress fibre formation and altered cell morphology. cag PAI mutants reduced phosphorylation of only FAK Y407 and had less effect on stress fibre formation than oipA mutants. We propose that activation of FAK and Src are responsible for H. pylori-induced induction of signalling pathways resulting in the changes in cell phenotype important for pathogenesis.
Introduction
Helicobacter pylori infection causes continuing damage to gastric structure and function and is the major cause of gastric malignancy. H. pylori strain-specific factors including the cag pathogenicity island (PAI) and the outer membrane protein, OipA, are known to influence the risk of developing clinical H. pylori-related diseases (Yamaoka et al., 2000; 2002). The cag PAI encodes a type IV secretion system that injects CagA and possibly other bacterial proteins into host cells (Odenbreit et al., 2000; Viala et al., 2004). Injected CagA undergoes tyrosine phosphorylation by Src family kinases (SFK) and has been associated with cytoskeletal changes, cell elongation and motility (Selbach et al., 2002; Moese et al., 2004). The role of other virulence factors has not been studied in detail in relation to signalling molecules involved in regulating cell proliferation, migration and motility.
Multiple signalling pathways are involved in inducing changes in actin filament dynamics and cell adhesion (for review see reference Parsons, 2003). Focal adhesion kinase (FAK) is a cytoplasmic non-receptor tyrosine kinase that serves as a scaffolding protein to recruit the Src homology (SH) 2- and SH3-containing molecules that function in the regulation of cell shape, migration and motility. FAK contains six tyrosine (Y) residues (Y397, Y407, Y576, Y577, Y861 and Y925) and the phosphorylation patterns at these sites differ in response to different stimuli (Schlaepfer et al., 1999). Extracellular stimuli are associated with autophosphorylation of Y397 resulting in a high-affinity binding site for the SH2 domains of SFK and phosphoinositide-3 kinase (PI3K) (Schaller et al., 1994; Guan, 1997). Phosphorylation of Y397 is thought to be required for normal FAK-mediated regulation of cell motility, migration and cell spreading (Schaller et al., 1994; Sieg et al., 1999). The formation of a transient FAK– Src dual kinase signalling complex results in phosphorylation of the remaining five FAK sites and maximal enzymatic activity. However, phosphorylation of Y407 inhibits FAK autophosphorylation and kinase activity and negatively regulates both the enzymatic and biological activities of FAK (Lim et al., 2007). Phosphorylation of Y576 and Y577 enhance FAK kinase activity and are important for adhesion-induced FAK activation and consequent downstream signalling (Mitra et al., 2005). Phosphorylation of Y861 and Y925 produces active binding sites for Grb2 and is also linked to Ras-mitogen-activated protein kinase (MAPK) signalling (Calalb et al., 1996; Schlaepfer et al., 1999).
Activation of FAK and Src result in phosphorylation of downstream targets including extracellular signal regulated kinase (Erk) with are localized at focal adhesion sites which provide cell contact with the extracellular matrix (ECM). Therefore activated FAK and Src provide the structural links between the ECM and polymerized actin filaments involved in focal adhesion, cell shape and motility (Hanks and Polte, 1997; Weisberg et al., 1997; Small et al., 2002). Recent reports have suggested that the cell surface protein, epidermal growth factor receptor (EGFR) is involved in activation of cytoskeletal proteins including FAK, and cellular processes including focal adhesion formation and actin polymerization (Sieg et al., 2000; Toral et al., 2003).
This study evaluated the early events in H. pylori infection leading to alterations in morphology, migration and motility of gastric epithelial cells. We report a novel H. pylori-induced sequence of events in gastric epithelial cells that involved selective phosphorylation of FAK Y397 and Src Y418 followed by complete activation of FAK, resulting in upregulation of Erk and actin stress fibre formation. We propose that this signalling pathway and its functional consequences are important in the H. pylori-induced phenotypic changes such that FAK or Src inhibitors might constitute important therapeutic targets. We further demonstrate that EGFR and Src are involved in H. pylori-induced tyrosine phosphorylation of FAK and suggest that OipA modulates cell surface receptor activation and provide a driving force that initiates these signal transduction pathways.
Results
H. pylori infection induces dose and time-dependent tyrosine phosphorylation of FAK
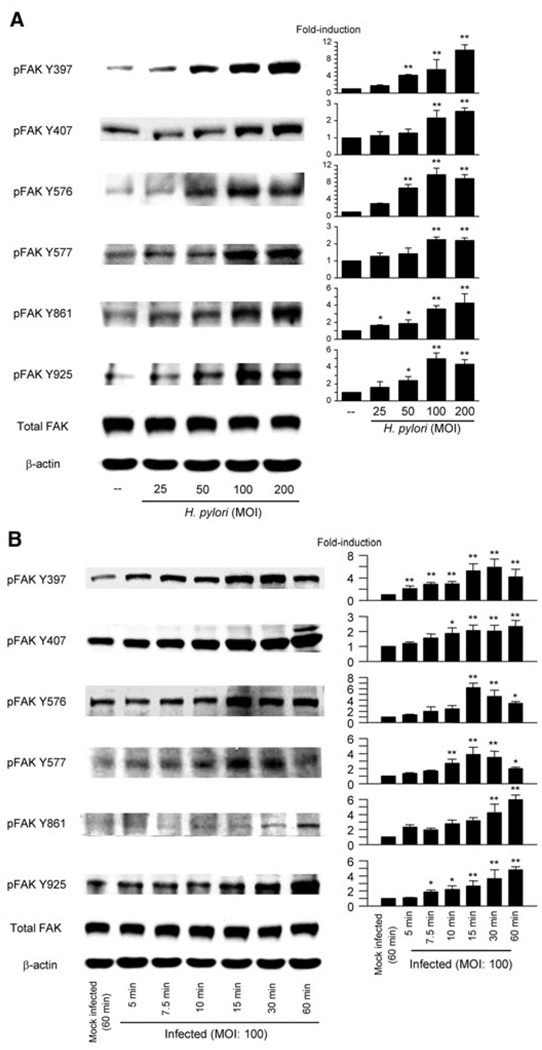
Site-specific phosphorylation of FAK was measured by immunoblot of H. pylori TN2GF4-infected AGS cell lysates. H. pylori infection at a multiplicity of infection (moi) of 100 for 1 h markedly enhanced phosphorylation of all six FAK tyrosine residues (Fig. 1A). Similar patterns were observed using MKN28 and MKN45 cells and different H. pylori strains (data not shown). An moi of 100 was used in all subsequent experiments based on our previous studies showing that cell viability of cells infected with H. pylori at an moi of 100 was unchanged compared with uninfected cells (Choi et al., 2007) and that the potential confounding effects of reduced adherence by the oipA mutants were eliminated (Yamaoka et al., 2004).
Fig. 1. H. pylori infection promotes dose and time-dependent phosphorylation of FAK in AGS cells.
A. Whole-cell lysates from mock-infected cells or from cells infected for 1 h with wild-type H. pylori at moi of 25–200 were analysed by immunoblot using indicated phospho-specific FAK antibodies.
B. Whole-cell lysates from mock-infected cells or from cells infected for 5–60 min with wild-type H. pylori at an moi of 100 were analysed using indicated phospho-specific FAK antibodies. Mock-infected control cells were incubated for 60 min. The density of phospho-specific FAK was normalized to that of total FAK and the levels were expressed as fold increase compared with those of mock-infected control cells. At least three independent co-cultures were performed. Data are presented as average values ± SE. *P < 0.05, **P < 0.01 versus mock-infected control cells.
Time-course experiments showed that significant upregulation of tyrosine phosphorylation of FAK Y397 was observed within 5 min after infection with strain TN2GF4. Upregulation was observed at all six phosphorylation sites within 30 min in AGS cells (Fig. 1B). These findings are in agreement with the previous consensus in other systems that tyrosine phosphorylation of FAK Y397 is an early event (Parsons, 2003). Phosphorylation of Y397, Y576 and Y577 reached maximal levels within 1 h (Fig. 1B) whereas sites Y407, Y861 and Y925 reached maximal levels at 90 min and declined to basal levels 12 h after H. pylori infection (data not shown). Phosphorylation of Y397, Y576 and Y577 also declined to basal levels 3 h post infection (data not shown). Similar patterns were observed using MKN28 and MKN45 cells and using the different H. pylori strains (data not shown). We therefore used AGS cells and strain TN2GF4 in subsequent experiments.
H. pylori-mediated phosphorylation of Src Y418/Y527 and FAK–Src complex formation
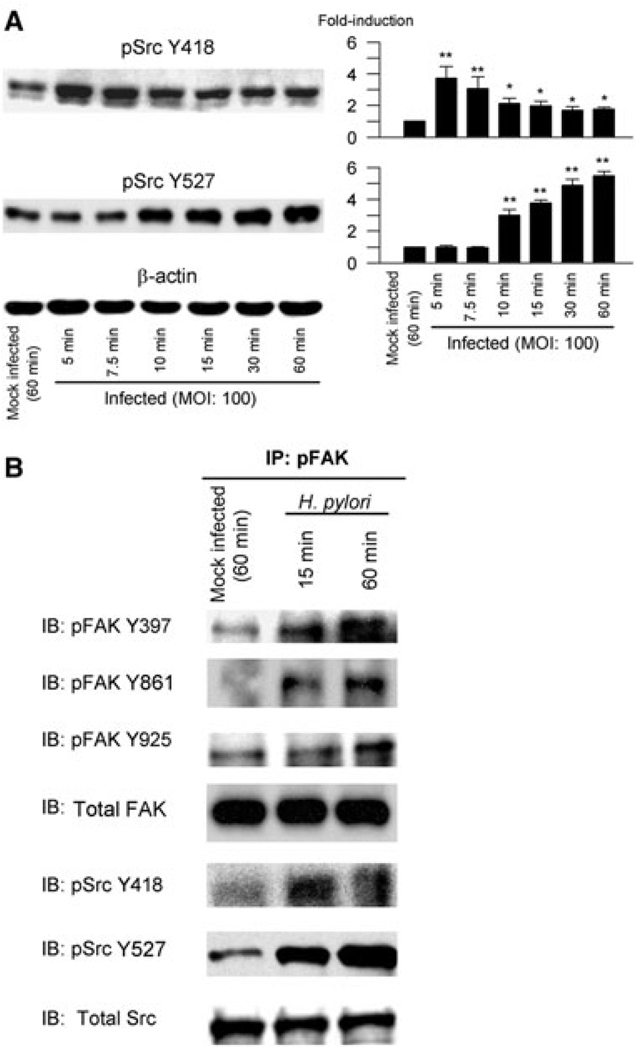
FAK Y397 binds to the SH2 domain of Src to form the Src–FAK signalling complex required for complete FAK activation and for downstream signalling (Thomas et al., 1998; Schlaepfer et al., 1999). Autophosphorylation of Src Y418, which is located in the activation loop of the kinase domain, results in upregulation of Src activity. In contrast, Src Y527 is located in the carboxyl terminus and is a negative regulator of Src (Cooper et al., 1986; Nada et al., 1991). We performed a time-course analysis of activation of Src Y418 and Y527 following H. pylori infection of AGS cells (Fig. 2A). H. pylori induced phosphorylation of Src Y418 as early as 5 min post infection. Phosphorylation of Src Y418 then gradually decreased. In contrast, phosphorylation of Src Y527 was first evident as early as 10 min (Fig. 2A) and remained phosphorylated up to 4 h after infection (data not shown). Total Src levels were unchanged during the observation periods (data not shown) and thus the density of phospho-specific sites was normalized to that of β-actin for semi-quantitative analyses (Fig. 2A).
Fig. 2. H. pylori-mediated activation of Src in AGS cells.
A. Lysates from mock-infected cells or cells infected with wild-type H. pylori at an moi of 100 for 5–60 min were prepared as described in Experimental procedures and were immunoblotted with phospho-specific Src Y418 or Y527 antibodies. Blots were re-probed with β-actin antibody as an internal control. For quantification, the density of phospho-specific sites was normalized to that of β-actin and the levels were expressed as fold increase compared with those of mock-infected control cells. At least three independent co-cultures were performed. Data are presented as average values ± SE. *P < 0.05, **P < 0.01 versus mock-infected control cells.
B. Mock-infected cells or cells infected with wild-type H. pylori for the indicated times were lysed and subjected to immunoprecipitation with anti-FAK antibody. Immune complexes were analysed by immunoblot to detect activated FAK and coprecipitated Src using phospho-specific FAK Y397, Y861, Y925, Src Y418 and Y527 antibodies. The amount of precipitated protein was monitored by stripping and re-probing the same membranes with anti-Src antibodies. At least three independent co-cultures were performed. Phosphorylation was not detected when lysates from uninfected or infected cells were immunoprecipitated with normal rabbit IgG or protein A-Sepharose beads alone (data not shown). Data representative of three separate experiments are presented. IP, immunoprecipitation.
To test whether FAK was associated with Src, whole-cell lysates from mock-infected or H. pylori-infected AGS cells were subjected to immunoprecipitation with anti-FAK antibody followed by immunoblotting with anti-FAK or Src-phosphospecific antibodies (Fig. 2B). These experiments confirmed that H. pylori infection resulted in phosphorylation of FAK Y397, Y861 and Y925 and that FAK was associated with Src. Phosphorylation of Y418 reached maximal levels at 15 min after H. pylori infection and declined after 1 h. In contrast, H. pylori time-dependent induced phosphorylation of Src at Y527 with the levels increasing up to 60 min post infection. The total levels of Src remained unchanged. The association of FAK with Src suggests the formation of FAK–Src signalling complexes likely to be involved in modulating downstream signalling responses.
Effect of inhibition of Src and PI3K on FAK and Src phosphorylation
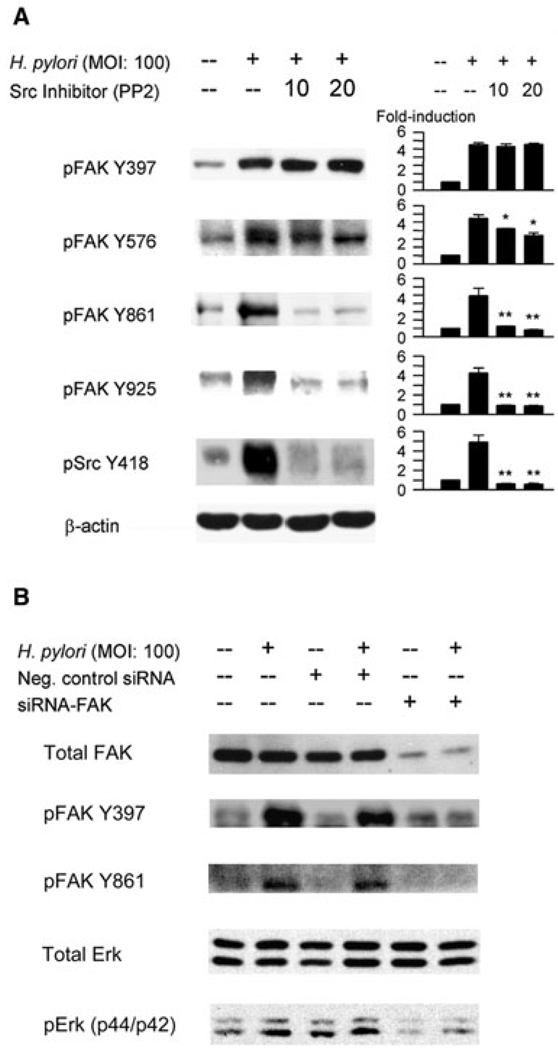
Src is involved in the complete activation of FAK. Binding of FAK to the SH2 domain of Src is associated with activation of PI3K as well as other downstream partners and substrates that together regulate actin and adhesion dynamics and cell migration (Schlaepfer and Hunter, 1996; Guan, 1997; Westhoff et al., 2004; Mitra et al., 2005). To examine the roles of Src and PI3K in H. pylori infection-associated activation of FAK, AGS cells were pretreated for 1 h with chemical inhibitors of the Src tyrosine kinase (PP2) 10 and 20 mM or PI3K inhibitor LY294002 (5 and 10 µM) and then infected with H. pylori. Our preliminary experiments using Akt as a control down-stream mediator of PI3K showed that 5 µM of LY294002 was sufficient to inhibit H. pylori-mediated phosphorylation of PI3K (data not shown). These inhibitors did not affect the total levels of FAK, Src or Erk1/2 (data not shown) and therefore the density of phospho-specific sites was normalized to that of β-actin for semi-quantitative analyses (Fig. 3A). Pre-treatment with PP2 resulted in the dramatic inhibition of phosphorylation of all sites except for FAK Y397. These results show that H. pylori-mediated activation of FAK Y397 is located upstream and independent of Src whereas tyrosine phosphorylation at the other FAK sites requires the involvement of Src. In contrast, administration of LY294002 (5 and 10 µM) prior to infection had no effect on H. pylorimediated activation of all sites for FAK (data not shown), suggesting that PI3K is localized downstream of FAK and is not involved in H. pylori induced tyrosine phosphorylation of FAK in AGS cells.
Fig. 3. Effect of inhibition of Src or downregulation of FAK on H. pylori-mediated phosphorylation of FAK and Erk1/2.
A. AGS cells were pretreated for 1 h with 10 or 20 µM of Src kinase inhibitor PP2 followed by infection with H. pylori for 1 h at an moi of 100. Src Y418 phosphorylation was assessed after 5 min. Whole-cell lysates were subjected to SDS-PAGE and were immunoblotted with indicated phospho-specific antibodies. The same blots were re-probed with β-actin antibody to verify equal loading. At least three independent co-cultures were performed. For quantification, the density of phospho-specific sites was normalized to that of β-actin and the levels were expressed as fold increase compared with those of mock-infected control cells. Data are presented as average values ± SE. *P < 0.05, **P < 0.01 versus H. pylori-infected cells without inhibitors.
B. Equal amounts of total cell lysate from mock-infected AGS cells, cells transfected with plasmid containing negative control sequence, or cells transfected with a plasmid containing siRNA sequence specific for FAK. Seventy-two hours post transfection, cells were infected for 1 h with wild-type H. pylori and proteins were analysed by SDS-PAGE and immunoblot with total FAK, total ERK, phospho-specific FAK Y397, Y861 and Erk1/2 antibodies. Data representative of six independent experiments are presented.
Reduced expression of FAK by siRNA inhibits phosphorylation of Erk1/2
Numerous studies have shown that H. pylori induces Erk1/2 activation in gastric epithelial cells via both cag PAI-dependent and independent mechanisms (Choi et al., 2007; Pillinger et al., 2007). In agreement with our previous studies (Choi et al., 2007), H. pylori induced phosphorylation of Erk1/2 in AGS cells in a time-dependent manner with maximal levels occurring between 60 and 120 min (data not shown). Activation of Erk1/2 is a candidate target for mediating FAK-mediated signalling and actin stress fibre formation. This idea is based on the observations that Erk1/2 activated by extracellular stimuli localizes in newly forming focal adhesions as well by the fact that involvement of FAK is a key step in regulation of kinases responsible for modulation of cell motility and migration (Klemke et al., 1997; Fincham et al., 2000; Sawhney et al., 2006; Chen et al., 2007). We therefore inhibited FAK expression in AGS cells to explore the relationship between FAK, Erk1/2 and actin stress fibre formation. We confirmed that the expression of total FAK was markedly inhibited by FAK-specific siRNA. Cell lysates from AGS cells transfected with FAK siRNA followed by H. pylori infection for 1 h were unable to induce tyrosine phosphorylation of FAK Y397 and Y861 (Fig. 3B). Downregulation of FAK expression by siRNA resulted in dramatic inhibition of H. pylori-induced tyrosine phosphorylation of Erk1/2 (Fig. 3B). These data are consistent with involvement of FAK in the regulation H. pylori-associated Erk1/2 phosphorylation.
Immunofluorescence analyses of FAK activation
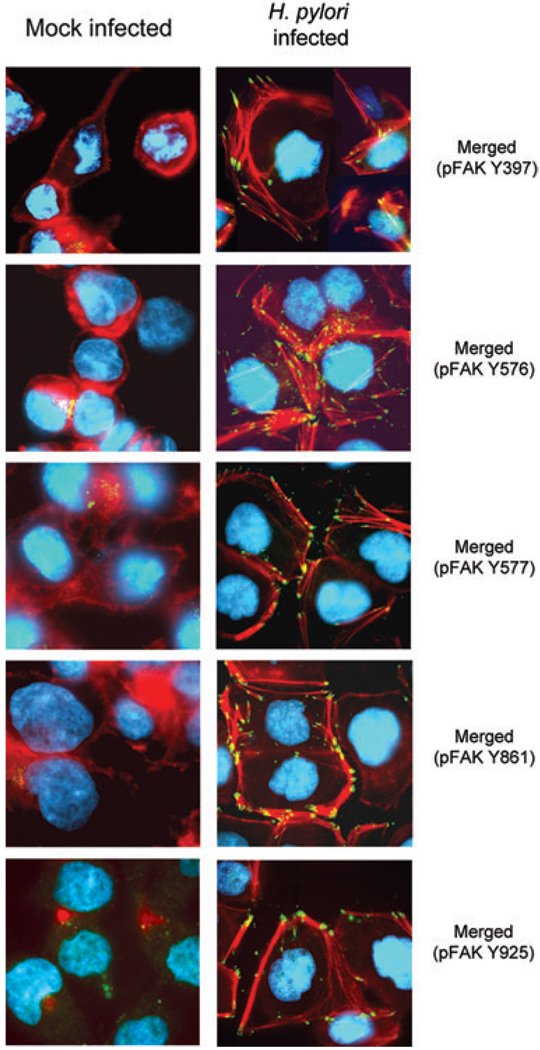
The above experiments showed that H. pylori infection resulted in complete activation of FAK followed by Src induced Erk1/2 phosphorylation. The effect of H. pylori infection on phosphorylation of FAK in focal complexes and their relationship to actin stress fibre formation was examined by immunofluorescence microscopy. Mock-infected AGS cells had low basal levels of FAK with poorly developed actin stress fibres or lamellipodium (Fig. 4; left column). H. pylori infection markedly enhanced phosphorylation of FAK Y397, Y576, Y577, Y861 and Y925; phosphorylated FAK colocalized along the tips of long and enlarged actin filaments (Fig. 4; right column). These bundles of actin filaments (stress fibres) typically were found in the cytoplasm of H. pylori-infected AGS cells, possibly related to phosphorylation of focal adhesion proteins. This cellular stressor resulted in alignment of the actin filaments to form stress fibres and areas of focal adhesion. Phosphorylated FAK was localized at the leading edges of the actin stress fibres. Overall, the results were consistent with interaction between FAK phosphorylation and actin stress fibre formation leading to phenotypic changes in cell morphology resulting from changes in actin cytoskeletal organization and formation of lamellipodia.
Fig. 4.
H. pylori-induced tyrosine phosphorylation of FAK colocalizes with F-actin in AGS cells. AGS cells grown on coverslips were mock infected (left column) or infected for 1 h at an moi of 100 with wild-type H. pylori (right column) and prepared for immunofluorescence to detect actin stress fibre formation and localization of FAK. Fixed cells were incubated with indicated phospho-specific FAK antibodies followed by incubation with FITC-conjugated anti-rabbit IgG secondary antibody (green), Alexa Fluor 594 phalloidin (red), and DAPI (blue) as described in Experimental procedures. Data representative of five independent experiments are presented.
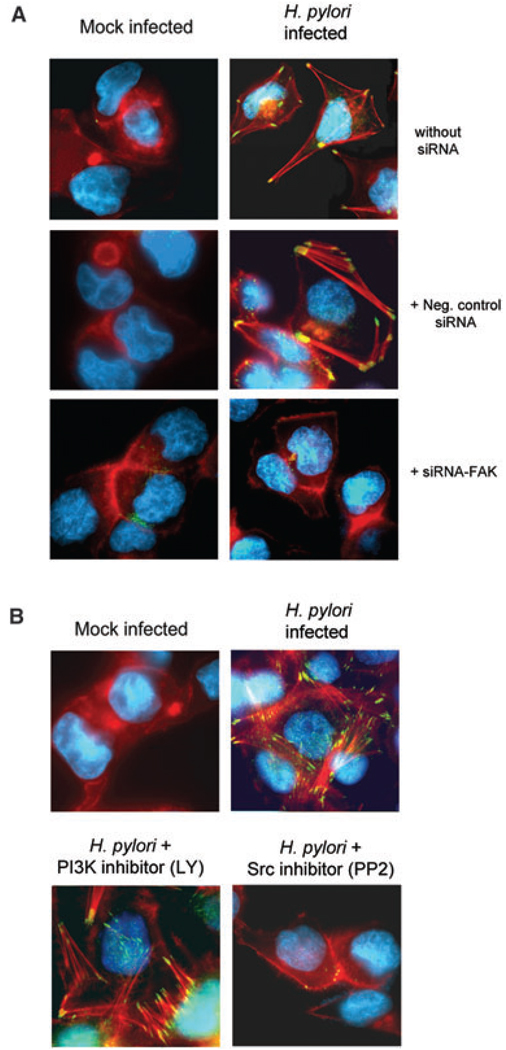
The effects of FAK knock-down by siRNA on phosphorylation at Y397 and its relationship to actin stress fibre formation in AGS cells were examined by immunofluorescence microscopy (Fig. 5A). Non-transfected cells or cells transfected with control siRNA vectors followed by H. pylori infection showed an increase in phosphorylation of FAK Y397 and actin stress fibre formation. Knock-down of FAK by siRNA not only diminished H. pylori-induced FAK Y397 phosphorylation, but also abolished actin stress fibre formation. These data confirm that phosphorylation of FAK is required for H. pylori-induced actin stress fibre formation. Similar results were obtained using FAK Y861 antibody (data not shown). Collectively, these results strongly suggest that phosphorylation of FAK and actin stress fibre formations are key early events in H. pylori-associated phenotypic changes.
Fig. 5. Effects of inhibition of FAK and Src on actin fibre formation.
A. AGS cells were plated on coverslips and were left untreated, transfected with negative control siRNA plasmid or with FAK siRNA plasmid, and then mock infected or infected with H. pylori for 1 h. Cells were fixed and incubated with phospho-specific FAK Y397 antibody followed by incubation with FITC-conjugated anti-rabbit IgG secondary antibody (green), Alexa Fluor 594 phalloidin (red) and DAPI (blue). Data representative of five independent experiments are presented.
B. AGS cells were grown on coverslips and were mock-treated, pretreated for 1 h with 10 mM of Src kinase inhibitor PP2 or 5 µM of PI3K inhibitor LY294002 (LY), followed by infection for 1 h with H. pylori at an moi of 100. Cells were fixed and incubated with FAK Y861 antibody followed by incubation with FITC-conjugated anti-rabbit IgG, Alexa Flour 594 for F-actin and DAPI for nuclear staining. Data representative of five independent experiments are presented.
To confirm that activation of Src is involved in changes in cell morphology, AGS cells were treated for 1 h with the Src tyrosine kinase inhibitor PP2 or PI3K inhibitor LY294002 the and then infected with H. pylori at an moi of 100. Staining with anti-FAK Y861 antibody confirmed involvement of Src, but not PI3K (Fig. 5B) and was consistent with the immunoblot data (Fig. 3A). These results are also consistent with the notion that Src plays an important role in transmitting downstream signals that affect cytoskeletal reorganization and other phenotypic changes in H. pylori-infected cells.
Effect of H. pylori virulence factors on phosphorylation of FAK and Src
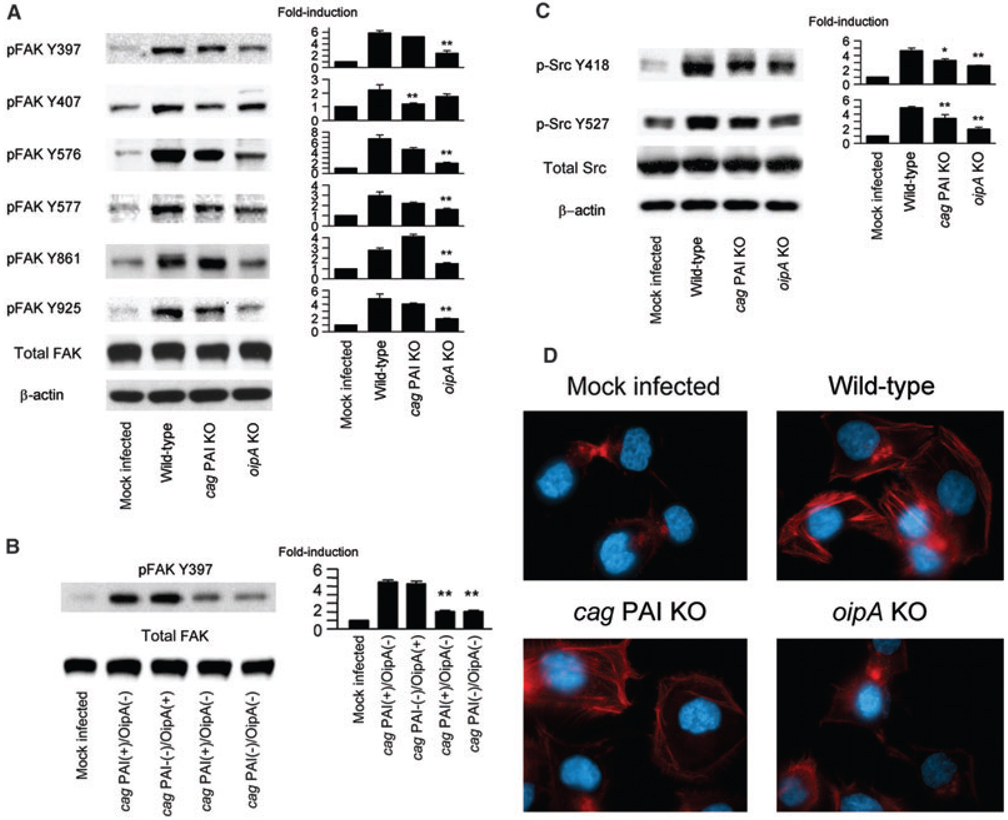
A number of cellular signalling pathways are known to be affected by the cag PAI and OipA (Yamaoka et al., 2004). Therefore, we used wild-type H. pylori TN2GF4 and its cag PAI totally deleted and oipA mutants to examine the effects of these virulence factors on phosphorylation of FAK and Src activation in AGS cells (Fig. 6A). The oipA mutants were associated with significantly reduced phosphorylation of FAK Y397, Y576, Y577, Y861 and Y925 compared with wild-type H. pylori. In contrast, the cag PAI mutants were only associated with reduced phosphorylation of FAK Y407. OipA is an outer membrane protein and to examine whether other outer membrane proteins had similar effects, we evaluated isogenic mutants of the outer membrane proteins BabA, AlpAB, HorG and HorK. In contrast to the oipA mutants, none of these mutants reduced phosphorylation of FAK Y397 (data not shown). These results suggest that OipA likely is the major factor involved in inducing tyrosine phosphorylation of FAK. Interestingly, the cag PAI mutant synergized phosphorylation of FAK Y861. Phosphorylation levels of FAK Y397, Y576, Y577 and Y925 in AGS cells infected with the double oipA/cag PAI mutants were similar to those seen with the oipA mutant alone, levels of FAK Y407 were similar to those seen with the cag PAI mutants, and levels of FAK Y861 were similar to those in cells infected with the parental strain TN2GF4 (data not shown). These data suggest the function of OipA and cag PAI in activation of FAK are independent of one another. These results were confirmed using isogenic mutants for the cag PAI or oipA from strains ATCC43504 and 26695 as parental strains (data not shown). In addition, we also examined 14 clinical isolates (four with cag PAI-positive/OipA-positive, three with cag PAI-positive/OipA-negative, three with cag PAI-negative/OipA-positive and four with cag PAI-negative/ OipA-negative strains). Phosphorylation levels of FAK Y397 was significantly higher in AGS cells infected with either cag PAI-positive/OipA-positive or cag PAI-negative/OipA-positive strains than those with either cag PAI-positive/OipA-negative or cag PAI-negative/Oip-Anegative strains (Fig. 6B), confirming that cag PAI is not involved in phosphorylation of FAK Y397.
Fig. 6. Isogenic mutants of H. pylori virulence factors downregulate site-specific phosphorylation of FAK and Src.
A–C. Cells were mock infected or infected with wild-type H. pylori, cag PAI mutants or oipA mutants, and whole-cell lysates were subjected to immunoblot analyses using (A) phospho-specific FAK or (C) phospho-specific Src. The same blots were re-probed with anti-FAK and/or anti-β-actin antibody for internal controls. Cells were also mock infected or infected with 14 clinical isolates (four with cag PAI-positive/OipA-positive, three with cag PAI-positive/OipA-negative, three with cag PAI-negative/OipA-positive and four with cag PAI-negative/OipA-negative strains) and whole-cell lysates were subjected to immunoblot analyses using phospho-specific FAK Y397 (C). At least three independent co-cultures were performed. For quantification, the density of phospho-specific sites was normalized to that of (A, B) total FAK or (C) or β-actin, and the levels are expressed as fold increase compared with those of mock-infected control cells. Data are presented as average values ± SE. *P < 0.05, **P < 0.01 versus wild-type H. pylori-infected cells (A, C) or cag PAI-positive/OipA-positive isolates infected cells (B).
D. Mock-infected AGS cells or AGS cells infected with wild-type H. pylori, cag PAI mutants or with oipA mutants were stained with F-actin antibodies (red) and DAPI (blue) for nuclear staining.
Both cag PAI and oipA mutants were associated with a reduction in phosphorylation of Src Y418/Y527 compared with wild-type H. pylori, (Fig. 6C) suggesting that both virulence factors modulate Src activation; however, the effects of OipA were greater than those of the cag PAI. The fact that phosphorylation of FAK Y397 was independent of cag PAI suggests that the cag PAI activates Src Y418/Y527 via an FAK Y397 independent pathway. Total Src levels were unchanged irrespective of the mutation of the cag PAI or oipA (data not shown).
Immunofluorescence staining was performed to confirm the effects of these virulence factors on actin stress fibre formation and cell morphology (Fig. 6D). AGS cells infected with wild-type H. pylori showed formation of actin stress fibres. However, actin stress fibre formation was rarely observed when the oipA mutants were used, confirming the OipA dependency of stress fibre formation and phenotypic changes. In contrast, actin stress fibres were evident in cells infected with the cag PAI mutant although fibre formation was less extensive compared with cells infected with wild-type H. pylori. Similar patterns were observed using isogenic mutants for the cag PAI or oipA from strains ATCC43504 and 26695 as parental strains (data not shown).
Involvement of EGFR in H. pylori-induced phosphorylation of FAK and Erk1/2
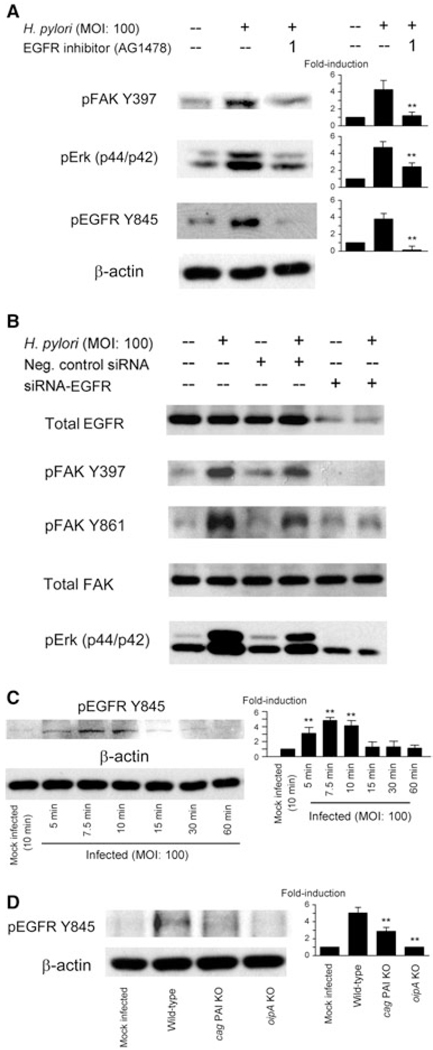
It has been reported that EGFR is overexpressed in various types of tumours and is involved in cell migration, invasion and cell survival signalling. There is also evidence to suggest FAK may be involved in EGF-induced EGFR signalling as inhibiting FAK expression also inhibits EGF or EGFR-dependent cell migration in invasive cancer cells (Jones et al., 2001). Therefore, we examined the role of EGFR in H. pylori-mediated signalling using AG1478, an inhibitor of EGFR (Levitzki and Gazit, 1995). Pre-treatment of AGS cells with AG1478 did not influence the levels of total FAK or Erk1/2 (data not shown); however, it significantly reduced H. pylori-induced FAK Y397 and Erk1/2 phosphorylation (Fig. 7A) consistent with the hypothesis that tyrosine phosphorylation of cell surface receptor EGFR and subsequent activation of FAK and Erk1/2 are involved in H. pylori-mediated downstream signalling. These data were confirmed by using EGFR-specific siRNA which showed reduced phosphorylation of FAK Y397, Y861 and Erk1/2 from lysates of cells transfected with EGFR siRNA followed by H. pylori infection for 1 h (Fig. 7B).
Fig. 7. Effects of EGFR inhibitions on H. pylori-induced activation of FAK and Erk1/2.
A. AGS cells were pretreated for 1 h with 1 µM EGFR inhibitor AG1478 followed by infection with H. pylori for 1 h at an moi of 100. Whole-cell lysates were subjected to SDS-PAGE and immunoblotted with indicated phospho-specific antibodies. The same blots were re-probed with anti-β-actin antibody to verify equal loading (data not shown). At least three independent co-cultures were performed. For quantification, the density of phospho-specific sites was normalized to that of β-actin, and the levels are expressed as fold increase compared with those of mock-treated control cells. Data are presented as average values ± SE. *P < 0.05, **P < 0.01 versus H. pylori-infected cells without inhibitors.
B. Equal amounts of total cell lysate from mock-infected AGS cells, cells transfected with plasmid containing negative control sequence, or cells transfected with a plasmid containing siRNA sequence specific for ERFR. Seventy-two hours post transfection, cells were infected for 1 h with wild-type H. pylori and proteins were analysed by SDS-PAGE and immunoblot with total EGFR, total FAK, phospho-specific FAK Y397 and Y861 antibodies. Data representative of three independent experiments are presented.
C. Whole-cell lysates from mock-infected cells or from cells infected for 5–60 min with wild-type H. pylori at an moi of 100 were analysed using phospho-EGFR Y845 antibody. Cell lysates from cells that were mock infected for 10 min were used as controls. At least three independent co-cultures were performed and semi-quantification was performed as described in (A). Data are presented as average values ± SE. *P < 0.05, **P < 0.01 versus mock-infected control cells.
D. Cells were mock infected or infected with wild-type H. pylori, cag PAI mutants or oipA mutants for 10 min and whole-cell lysates were subjected to immunoblot analyses using EGFR Y845 antibody. At least three independent co-cultures were performed and semi-quantification was performed as described in (A). Data are presented as average values ± SE. **P < 0.01 versus wild-type H. pylori-infected cells.
We also examined H. pylori-mediated activation of EGFR in AGS cells using Y845 phosphospecific antibody. H. pylori induced phosphorylation of EGFR Y845 in a time-dependent manner with the maximum effect being observed 7.5 min after H. pylori infection (Fig. 7C). Importantly, the oipA mutants abolished the H. pylori-associated phosphorylation of EGFR Y845 at 10 min (Fig. 7D). Of interest, the cag PAI mutants were also associated with a reduction of phosphorylation of EGFR Y845. Total EGFR levels were unchanged during the observation periods irrespective of the mutation of the cag PAI or oipA (data not shown). Thus, these experiments did not identify whether there is a threshold level or critical level of phosphorylation or whether EGFR is directly involved in activating phosphorylation of FAK Y397. EGFR has several phosphorylation sites in addition to Y845 and additional studies will be required to provide a detailed understanding of the proposed relationship between H. pylori infection and activation of EGFR.
Discussion
The interaction of FAK and Src produces a molecular switch resulting in tyrosine phosphorylation of the remaining FAK sites and determines the fate of downstream signalling events (for review see reference Parsons, 2003). Recent studies in non-gastric cells have shown that Y397 is required for FAK-mediated regulation of cell motility, migration and cell-spreading signals (Schaller et al., 1994; Sieg et al., 1999). Using gastric epithelial cells, we show that H. pylori infection is associated with rapid and transient phosphorylation of FAK Y397, followed by phosphorylation of Src Y418 and Y527 as well as activation of the five remaining FAK tyrosine phosphorylation sites. We show that OipA is responsible for most of the FAK phosphorylation and propose that OipA-induced activation of FAK Y397 is an early event and possibly a prerequisite for complete activation of FAK and the subsequent intracellular signalling that results in actin stress fibre formation. Our data are consistent with activation of FAK being a key regulator of the H. pylori-induced morphological changes of gastric epithelial cells. This observation also is consistent with recent reports of a pool of active FAK at focal adhesion sites localized at distal tips of cell extensions following H. pylori infection (Tsutsumi et al., 2006).
Although there is agreement that OipA is involved in attachment of the bacterium to host cells (Yamaoka et al., 2004; Dossumbekova et al., 2006), its other functions are still unclear as many of the same candidate functions have also been linked with the cag PAI (Yamaoka et al., 2000; 2002). The current study using both OipA-negative isogenic mutants and clinical isolates clearly showed a novel role for OipA in the initiation of site-specific activation of FAK. OipA appears to be involved in the steps leading to phosphorylation of FAK at Y397, Y576, Y577, Y861 and Y925. Tyrosine phosphorylation of Src and FAK are key elements in cell motility and invasiveness; elevated levels of FAK and Src have also been observed in various epithelial tumours (Owens et al., 1995; Slack et al., 2001; Playford and Schaller, 2004; Avizienyte and Frame, 2005) and site-specific tyrosine phosphorylation of FAK has been linked to cell adhesion, invasive ovarian tumours, expression of gastrin releasing peptide and tumour differentiation in colon cancer (Aronsohn et al., 2003; Matkowskyj et al., 2003; Moon et al., 2003; Avizienyte and Frame, 2005; Grisaru-Granovsky et al., 2005). These data suggest a role for OipA in gastric carcinogenesis through its involvement with FAK and its known effects on cell motility and invasiveness. Together these observations suggest that inhibition of OipA may lead to site-specific inhibition of FAK tyrosine phosphorylation and provide a therapeutic strategy for inhibiting the FAK–Src signalling complex and its various downstream signalling pathways.
The molecular mechanisms by which OipA interacts with host cells remains unclear. However, because OipA is an outer membrane protein involved in attachment of the organism to host cells, it seems likely that phosphorylation of FAK Y397 may be involved in the interaction between OipA and cell surface receptors responsible for the transmitting the downstream signals leading to activation of FAK. H. pylori-induced phosphorylation of EGFR Y845 and inhibition of FAK and Erk1/2 phosphorylation by inhibitions of EGFR function suggest that OipA might associate either directly or indirectly with EGFR to modulate FAK and downstream signals leading to cytoskeletal changes and cell motility. Experiments are underway to test this hypothesis.
Although the cag PAI has been associated with marked changes in cell morphology, cell migration and cell motility (Churin et al., 2001; Su et al., 2003; Moese et al., 2004), we clearly show that the cag PAI is not involved in the initial phosphorylation of the FAK Y397 or of other sites except Y407. Our results both agree and differ somewhat from those recently reported (Kwok et al., 2007). Kwon et al. reported that wild-type H. pylori induce phosphorylation of FAK Y397 (the only phosphorylation site examined) followed by phosphorylation of Src Y418 which are in agreement with our results. They also reported that cagL mutants of parental strain P12 did not induce phosphorylation of FAK Y397. CagL is a component of the cag PAI type IV secretion system. We did not assess selective CagL deletion but instead deleted the entire cag PAI. Our results were confirmed with multiple strains including three parental strains and their cag PAI totally deleted mutants and importantly 14 clinical isolates with different cag PAI/OipA function. These results confirmed that the cag PAI is not involved in the initial phosphorylation of the FAK Y397. The reason for the different results is unclear and they might be explained by strain differences and further studies will be needed to explore outcomes related to elimination of the cag PAI compared with elimination of only cagL.
Our results also differ from studies in which epithelial cells were transfected with plasmids expressing the CagA protein (Tsutsumi et al., 2006). In those experiments, ectopic expression of CagA was associated with a reduction in FAK activation (Tsutsumi et al., 2006). We used live H. pylori and thus CagA was naturally injected into epithelial cells by the bacterial type IV secretion system. In contrast to the transfection experiments, activation of FAK was one of the earliest events noted in the natural infection. Some of the differences in outcome between experiments in which CagA was transfected and the natural injection of CagA include the possible injection of other proteins (e.g. peptidoglycan) into host cells (Viala et al., 2004) and the fact that injection would tend to focus events near the cell surface. Most importantly, transfection also would eliminate identification of other critical bacterial–host cell interactions (e.g. the OipA-associated activation of FAK). Our results clearly confirm that OipA, and not the cag PAI or CagA, plays an important role in overall FAK activation. These conclusions are also supported by recent reports showing that H. pylori-induced activation of actin stress fibre formation was independent of cagA, cagE or VacA status and by evidence that during the initial stage of H. pylori infection, the motility of AGS cells are independent of the presence of the cag PAI (Su et al., 2003; Moese et al., 2004).
Finally, we note that our experiments were performed in vitro. Preliminary studies using immunohistochemistry of human gastric mucosal biopsy specimens showed phosphorylated FAK is present in the cytoplasm of epithelial cells in infected mucosa (see Fig. S1). Further in vitro–in vivo correlation studies are warranted.
Experimental procedures
Reagents
Site- and phospho-specific affinity-purified polyclonal antibodies for FAK (Y397, Y407, Y576, Y577, and Y861), Src Y418 (in human c-Src), Erk1/2 (p44/p42 MAPK) (T202/Y204), polyclonal purified anti-FAK and monoclonal antipaxillin were purchased from Biosource (Camarillo, CA). Site- and phospho-specific antibodies to FAK Y925, EGFR Y845 and Src Y527, mouse anti-Src monoclonal antibody, affinity-purified horseradish peroxidase (HRP)-conjugated goat-anti-rabbit IgG (H and L) or horse-antimouse IgG and the LumiGLO® chemiluminescent substrate detection system were obtained from Cell Signaling Technology, (Beverly, MA). Mouse anti-β-actin monoclonal antibody and FITC-conjugated anti-rabbit IgG were purchased from Sigma-Aldrich (St Louis, MO). Selective F-actin probe Alexa Fluor 594 phalloidin, 4′,6-diamidino-2-phenylindole (DAPI), and SlowFade Antifade kit were obtained from Molecular Probes (Eugene, OR). All chemical inhibitors were obtained from Calbiochem (San Diego, CA). The mammalian FAK siRNA and EGFR siRNA/siAB™ Assay Kits were purchased from Upstate Cell Signaling Solution (Lake Placid, NY). Immunoprecipitation reagents were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell culture
The human gastric epithelial cancer cell lines AGS (American Type Culture Collection, Manassas, VA), MKN28 and MKN45 (Riken Bank, Tsukuba, Japan) were grown at 37°C and 5% CO2 in RPMI 1640 medium supplemented with penicillin, streptomycin and 10% FBS. Cells were seeded at a density of 1 × 105 cells per well in six-well plates, 1 × 105 cells in 10 cm dishes, or were grown on glass coverslips in 10% FBS. Cells were serum-starved overnight before experiments. Cells at 80% confluence were left untreated in RPMI 1640 medium or were co-cultured with H. pylori for specified times and specified moi as described in the legends for all figures.
H. pylori
Three functional oipA-positive/cag PAI-positive H. pylori strains (TN2GF4, ATCC43504 and 26695), their isogenic oipA mutants and cag PAI totally deleted mutants, and oipA and cag PAI double mutant of strain TN2GF4 have been described (Yamaoka et al., 2000; Kudo et al., 2005). We also used isogenic babA mutant, alpAB mutant, horG mutant and horK mutant (Yamaoka et al., 2000; Lu et al., 2007). H. pylori TN2GF4 was isolated from a Japanese gastric ulcer patient and causes gastric cancer in Mongolian gerbils (Watanabe et al., 1998). In some experiments, we also used clinical isolates obtained from Colombian patients with non-ulcer dyspepsia. H. pylori were cultured on brain heart infusion agar plates containing 7% horse blood for 24–36 h at 37°C under microaerophilic conditions. The bacteria were collected and suspended in phosphate-buffered saline (PBS) and the density was estimated by spectrophotometry (A625) and by microscopic observation.
Immunoblotting and immunoprecipitation
AGS cells were co-cultured with H. pylori at the specified moi or were co-cultured at an moi of 100 for specified times as indicated in the figure legends. Protein extraction and immunoblots were performed using standard techniques. For semi-quantitative analysis, X-ray films were scanned and quantified using Image J 1.36 software (http://rsbweb.nih.gov/ij/) from the National Institutes of Health.
For immunoprecipitation analysis, equivalent amounts of protein from control or infected samples were incubated with antibodies for 2 h at 4°C and then collected with Protein A Sepharose 4 Fast Flow or Protein G Sepharose 4 Fast Flow (Amersham Biosciences, Piscataway, NJ). The precipitates were washed three times with lysis buffer and once with PBS, and then were subjected to 8% SDS-PAGE, transferred to immobilon membranes, and then probed with specific antibodies as described in the text.
Immunofluorescence
After co-culture with H. pylori, AGS cells were fixed in 3.7% formaldehyde in PBS (pH 7.6) for 10 min at room temperature, washed with PBS, and then permeabilized by soaking coverslips in 0.1% Triton X-100 in PBS for 5 min. Non-specific reactivity was blocked with 5% normal goat serum for 30 min at room temperature. Polymerized actin and phosphorylated proteins were visualized by incubating the cells with phospho-specific antibodies (10–30 µg ml−1) in a humidified chamber overnight at 4°C followed by incubation with FITC-conjugated anti-rabbit IgG secondary antibody and selective F-actin probe Alexa Fluor 594 phalloidin. Nucleic acid was counterstained with DAPI (300 nM). The coverslips were mounted on slides using the SlowFade Antifade kit (Molecular Probes, Eugene, OR) to prevent rapid photo-bleaching as recommended by the manufacturer. The images were acquired using filters appropriate for FITC, Alexa Fluor 594 phalloidin and DAPI.
Fluorescence microscopy
H. pylori-induced tyrosine phosphorylation and F-actin stress fibre formation was visualized by fluorescence microscopy (Olympus, America Melville, NY, USA). Images were captured at 1000× magnification or as indicated in the figure legends. Representative images of each sample were taken in triplicate. Images were obtained before substantial photo-bleaching occurred, and uninfected or H. pylori-infected cells subjected to identical culture, fixation, staining and microscopy conditions allowed meaningful comparisons between samples.
siRNA transfection and silencing FAK or EGFR protein expression
AGS cells (1 × 105) plated in six-well plates (~80% confluent) were transfected with FAK-specific siRNAs or non-specific siRNA (pKD-NegCon-v1) or transfected similarly with EGFR-specific siRNA or non-specific negative control siRNAs (100 pmol) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Cells were cultured for 1–4 days after transfection. Cells were collected and suspended in serum-free medium overnight before infection with H. pylori for 1 h at an moi of 100.
Statistical analyses
All experiments were performed at least three times. Statistical analysis was performed by Mann–Whitney Rank Sum test or non-paired t-test depending on the data set using SigmaStat 3.01 (SPSS, Chicago, IL). A P-value of less than 0.05 was accepted as statistically significant.
Supplementary Material
Acknowledgements
This work was supported in part by grants from the National Institutes of Health DK62813 (Y.Y.), a Public Health Service grant (DK56338) which funds the Texas Gulf Coast Digestive Diseases Center and the Office of Research and Development Medical Research Service Department of Veterans Affairs. The authors thank Dr Momoyo Asahi (Fukui Prefectural University, Fukui, Japan) for providing prudent comments.
Footnotes
This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/abs/10.1111/j.1462-5822.2007.01104.x
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Aronsohn MS, Brown HM, Hauptman G, Kornberg LJ. Expression of focal adhesion kinase and phosphorylated focal adhesion kinase in squamous cell carcinoma of the larynx. Laryngoscope. 2003;113:1944–1948. doi: 10.1097/00005537-200311000-00017. [DOI] [PubMed] [Google Scholar]
- Avizienyte E, Frame MC. Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr Opin Cell Biol. 2005;17:542–547. doi: 10.1016/j.ceb.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Calalb MB, Zhang X, Polte TR, Hanks SK. Focal adhesion kinase tyrosine-861 is a major site of phosphorylation by Src. Biochem Biophys Res Commun. 1996;228:662–668. doi: 10.1006/bbrc.1996.1714. [DOI] [PubMed] [Google Scholar]
- Chen H, Bai J, Ye J, Liu Z, Chen R, Mao W, et al. JWA as a functional molecule to regulate cancer cells migration via MAPK cascades and F-actin cytoskeleton. Cell Signal. 2007;19:1315–1327. doi: 10.1016/j.cellsig.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Choi IJ, Fujimoto S, Yamauchi K, Graham DY, Yamaoka Y. Helicobacter pylori environmental interactions: effect of acidic conditions on H. pylori-induced gastric mucosal interleukin-8 production. Cell Microbiol. 2007;9:2457–2469. doi: 10.1111/j.1462-5822.2007.00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churin Y, Kardalinou E, Meyer TF, Naumann M. Pathogenicity island-dependent activation of Rho GTPases Rac1 and Cdc42 in Helicobacter pylori infection. Mol Microbiol. 2001;40:815–823. doi: 10.1046/j.1365-2958.2001.02443.x. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Gould KL, Cartwright CA, Hunter T. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science. 1986;231:1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- Dossumbekova A, Prinz C, Mages J, Lang R, Kusters JG, Van Vliet AH, et al. Helicobacter pylori HopH (OipA) and bacterial pathogenicity: genetic and functional genomic analysis of hopH gene polymorphisms. J Infect Dis. 2006;194:1346–1355. doi: 10.1086/508426. [DOI] [PubMed] [Google Scholar]
- Fincham VJ, James M, Frame MC, Winder SJ. Active ERK/MAP kinase is targeted to newly forming cell-matrix adhesions by integrin engagement and v-Src. EMBO J. 2000;19:2911–2923. doi: 10.1093/emboj/19.12.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisaru-Granovsky S, Salah Z, Maoz M, Pruss D, Beller U, Bar-Shavit R. Differential expression of protease activated receptor 1 (Par1) and pY397FAK in benign and malignant human ovarian tissue samples. Int J Cancer. 2005;113:372–378. doi: 10.1002/ijc.20607. [DOI] [PubMed] [Google Scholar]
- Guan JL. Role of focal adhesion kinase in integrin signaling. Int J Biochem Cell Biol. 1997;29:1085–1096. doi: 10.1016/s1357-2725(97)00051-4. [DOI] [PubMed] [Google Scholar]
- Hanks SK, Polte TR. Signaling through focal adhesion kinase. Bioessays. 1997;19:137–145. doi: 10.1002/bies.950190208. [DOI] [PubMed] [Google Scholar]
- Jones G, Machado J, Jr, Merlo A. Loss of focal adhesion kinase (FAK) inhibits epidermal growth factor receptor-dependent migration and induces aggregation of nh(2)-terminal FAK in the nuclei of apoptotic glioblastoma cells. Cancer Res. 2001;61:4978–4981. [PubMed] [Google Scholar]
- Klemke RL, Cai S, Giannini AL, Gallagher PJ, de Lanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Lu H, Wu JY, Graham DY, Casola A, Yamaoka Y. Regulation of RANTES promoter activation in gastric epithelial cells infected with Helicobacter pylori. Infect Immun. 2005;73:7602–7612. doi: 10.1128/IAI.73.11.7602-7612.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok T, Zabler D, Urman S, Rohde M, Hartig R, Wessler S, et al. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature. 2007;449:862–866. doi: 10.1038/nature06187. [DOI] [PubMed] [Google Scholar]
- Levitzki A, Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- Lim Y, Park H, Jeon J, Han I, Kim J, Jho EH, et al. Focal adhesion kinase is negatively regulated by phosphorylation at tyrosine 407. J Biol Chem. 2007;282:10398–10404. doi: 10.1074/jbc.M609302200. [DOI] [PubMed] [Google Scholar]
- Lu H, Wu JY, Beswick EJ, Ohno T, Odenbreit S, Haas R, et al. Functional and intracellular signaling differences associated with the Helicobacter pylori AlpAB adhesin from Western and East Asian strains. J Biol Chem. 2007;282:6242–6254. doi: 10.1074/jbc.M611178200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matkowskyj KA, Keller K, Glover S, Kornberg L, Tran-Son-Tay R, Benya RV. Expression of GRP and its receptor in well-differentiated colon cancer cells correlates with the presence of focal adhesion kinase phosphorylated at tyrosines 397 and 407. J Histochem Cytochem. 2003;51:1041–1048. doi: 10.1177/002215540305100807. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- Moese S, Selbach M, Kwok T, Brinkmann V, Konig W, Meyer TF, et al. Helicobacter pylori induces AGS cell motility and elongation via independent signaling pathways. Infect Immun. 2004;72:3646–3649. doi: 10.1128/IAI.72.6.3646-3649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon HS, Park WI, Choi EA, Chung HW, Kim SC. The expression and tyrosine phosphorylation of E-cadherin/catenin adhesion complex, and focal adhesion kinase in invasive cervical carcinomas. Int J Gynecol Cancer. 2003;13:640–646. doi: 10.1046/j.1525-1438.2003.13396.x. [DOI] [PubMed] [Google Scholar]
- Nada S, Okada M, MacAuley A, Cooper JA, Nakagawa H. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature. 1991;351:69–72. doi: 10.1038/351069a0. [DOI] [PubMed] [Google Scholar]
- Odenbreit S, Puls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- Owens LV, Xu L, Craven RJ, Dent GA, Weiner TM, Kornberg L, et al. Overexpression of the focal adhesion kinase (p125FAK) in invasive human tumors. Cancer Res. 1995;55:2752–2755. [PubMed] [Google Scholar]
- Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Pillinger MH, Marjanovic N, Kim SY, Lee YC, Scher JU, Roper J, et al. Helicobacter pylori stimulates gastric epithelial cell MMP-1 secretion via CagA-dependent and -independent ERK activation. J Biol Chem. 2007;282:18722–18731. doi: 10.1074/jbc.M703022200. [DOI] [PubMed] [Google Scholar]
- Playford MP, Schaller MD. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23:7928–7946. doi: 10.1038/sj.onc.1208080. [DOI] [PubMed] [Google Scholar]
- Sawhney RS, Cookson MM, Omar Y, Hauser J, Brattain MG. Integrin alpha2-mediated ERK and calpain activation play a critical role in cell adhesion and motility via focal adhesion kinase signaling: identification of a novel signaling pathway. J Biol Chem. 2006;281:8497–8510. doi: 10.1074/jbc.M600787200. [DOI] [PubMed] [Google Scholar]
- Schaller MD, Hildebrand JD, Shannon JD, Fox JW, Vines RR, Parsons JT. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Hunter T. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol Cell Biol. 1996;16:5623–5633. doi: 10.1128/mcb.16.10.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Hanks SK, van der Hunter T, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- Selbach M, Moese S, Hauck CR, Meyer TF, Backert S. Src is the kinase of the Helicobacter pylori CagA protein in vitro and in vivo. J Biol Chem. 2002;277:6775–6778. doi: 10.1074/jbc.C100754200. [DOI] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Schlaepfer DD. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112(Part 16):2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, et al. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- Slack JK, Adams RB, Rovin JD, Bissonette EA, Stoker CE, Parsons JT. Alterations in the focal adhesion kinase/Src signal transduction pathway correlate with increased migratory capacity of prostate carcinoma cells. Oncogene. 2001;20:1152–1163. doi: 10.1038/sj.onc.1204208. [DOI] [PubMed] [Google Scholar]
- Small JV, Stradal T, Vignal E, Rottner K. The lamellipodium: where motility begins. Trends Cell Biol. 2002;12:112–120. doi: 10.1016/s0962-8924(01)02237-1. [DOI] [PubMed] [Google Scholar]
- Su B, Ceponis PJ, Sherman PM. Cytoskeletal rearrangements in gastric epithelial cells in response to Helicobacter pylori infection. J Med Microbiol. 2003;52:861–867. doi: 10.1099/jmm.0.05229-0. [DOI] [PubMed] [Google Scholar]
- Thomas JW, Ellis B, Boerner RJ, Knight WB, White GC, Schaller MD. SH2- and SH3-mediated interactions between focal adhesion kinase and Src. J Biol Chem. 1998;273:577–583. doi: 10.1074/jbc.273.1.577. [DOI] [PubMed] [Google Scholar]
- Toral C, Solano-Agama MC, Luna J, Romano MC, Mendoza-Garrido ME. Epidermal growth factor induces an increase in cell adhesion and an arrangement of actin skeleton in stress fibres in pituitary cultured cells from infantile rats but not adult rats. J Cell Physiol. 2003;195:80–91. doi: 10.1002/jcp.10231. [DOI] [PubMed] [Google Scholar]
- Tsutsumi R, Takahashi A, Azuma T, Higashi H, Hatakeyama M. Focal adhesion kinase is a substrate and downstream effector of SHP-2 complexed with Helicobacter pylori CagA. Mol Cell Biol. 2006;26:261–276. doi: 10.1128/MCB.26.1.261-276.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol. 2004;5:1166–1174. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Tada M, Nagai H, Sasaki S, Nakao M. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology. 1998;115:642–648. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]
- Weisberg E, Sattler M, Ewaniuk DS, Salgia R. Role of focal adhesion proteins in signal transduction and oncogenesis. Crit Rev Oncog. 1997;8:343–358. doi: 10.1615/critrevoncog.v8.i4.40. [DOI] [PubMed] [Google Scholar]
- Westhoff MA, Serrels B, Fincham VJ, Frame MC, Carragher NO. SRC-mediated phosphorylation of focal adhesion kinase couples actin and adhesion dynamics to survival signaling. Mol Cell Biol. 2004;24:8113–8133. doi: 10.1128/MCB.24.18.8113-8133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka Y, Kwon DH, Graham DY. A M(r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc Natl Acad Sci USA. 2000;97:7533–7538. doi: 10.1073/pnas.130079797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka Y, Kikuchi S, El-Zimaity HM, Gutierrez O, Osato MS, Graham DY. Importance of Helicobacter pylori oipA in clinical presentation, gastric inflammation, and mucosal interleukin 8 production. Gastroenterology. 2002;123:414–424. doi: 10.1053/gast.2002.34781. [DOI] [PubMed] [Google Scholar]
- Yamaoka Y, Kudo T, Lu H, Casola A, Brasier AR, Graham DY. Role of interferon-stimulated responsive element-like element in interleukin-8 promoter in Helicobacter pylori infection. Gastroenterology. 2004;126:1030–1043. doi: 10.1053/j.gastro.2003.12.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.