Abstract
Waterlogging is a major abiotic stress limiting barley (Hordeum vulgare L.) yield and its stability in areas with excessive rainfall. Identification of genomic regions influencing the response of yield and its components to waterlogging stress will enhance our understanding of the genetics of waterlogging tolerance and the development of more tolerant barley cultivars. Quantitative trait loci (QTLs) for grain yield and its components were identified using 156 doubled haploid (DH) lines derived from a cross between the cultivars Yerong (waterlogging-tolerant) and Franklin (waterlogging-sensitive) grown under different conditions (waterlogged and well drained). A total of 31 QTLs were identified for the measured characters from two experiments with two growth environments. The phenotypic variation explained by individual QTLs ranged from 4.74% to 55.34%. Several major QTLs determining kernel weight (KW), grains per spike (GS), spikes per plant (SP), spike length (SL) and grain yield (GY) were detected on the same region of chromosome 2H, indicating close linkage or pleiotropy of the gene(s) controlling these traits. Some different QTLs were identified under waterlogging conditions, and thus different markers may have to be used in selecting cultivars suitable for high rainfall areas.
Keywords: Barley (Hordeum vulgare L.), Waterlogging tolerance, Yield, Quantitative trait locus (QTL)
1. Introduction
Barley (Hordeum vulgare L.) is an important cereal crop, ranking fourth in the world in terms of planted area after only wheat, rice and maize. Barley production is often influenced by abiotic stress caused, for example, by salinity, drought, frost or waterlogging. In southern China, waterlogging occurs frequently at the seedling and tillering stages, causing huge yield losses (Xiao et al., 2007).
Waterlogging tolerance in plants is a complex trait involving many morphological and physiological responses. The phenotypic characters associated directly with waterlogging tolerance are still unclear and controversial, although some researchers have noted that leaf chlorosis after waterlogging is associated with waterlogging tolerance (Hamachi et al., 1990; Wang et al., 1996; Zhou et al., 2007; Li et al., 2008). In recent years, there have been many investigations to determine the morphological, physiological, anatomical and metabolic responses to waterlogging in barley (Garthwaite et al., 2003; Pang et al., 2004; 2006; 2007a; 2007b; Xiao et al., 2005; Zhang et al., 2007). However, little progress has been made in mapping quantitative trait loci (QTLs) controlling waterlogging tolerance in barley (Setter and Waters, 2003). Li et al. (2008) identified 7 QTLs controlling waterlogging tolerance using two barley double haploid (DH) populations based on leaf chlorosis, plant survival and biomass reduction after waterlogging treatment. There have been no reports on QTL analysis of barley waterlogging tolerance based on major agronomic traits, such as plant height and yield components.
In practice, it is very difficult for breeders to control the multiple environmental factors operating in a field experiment over thousands of barley genotypes; thus selection in the natural environment is not very effective. Development of molecular markers associated with barley waterlogging tolerance could effectively avoid environmental effects. QTL analysis has proven to be very useful in identifying the genetic components of variation in important economic traits. A molecular marker closely linked to the target gene or QTL can act as a “tag” which can be used for indirect selection of the gene(s) in a breeding program (Babu et al., 2004). Numerous QTLs have been reported to affect plant height and yield components (Backes et al., 1995; Kjær and Jensen, 1996; Hori et al., 2003; Pillen et al., 2003; Sameri et al., 2006; von Korff et al., 2006; Baghizadeh et al., 2007), but none of them was identified under abiotic stress. To use molecular markers effectively for the selection of waterlogging tolerant barley cultivars, it is crucial to find QTLs controlling agronomic traits and yield components under waterlogged conditions.
The objectives of the current study were: (1) to identify QTLs for important characters such as plant height (PH), grains per spike (GS), spikes per plant (SP), kernel weight (KW), spike length (SL), and grain yield per plot (GY) using a barley DH population grown under different conditions; and (2) to compare QTLs identified from waterlogged trials with those identified from non-waterlogged trials.
2. Materials and methods
2.1. Plant materials
One hundred and fifty-six DH lines derived from a cross between the cultivars Yerong and Franklin were used in this study. Yerong is a waterlogging-tolerant, six-rowed feed barley, while Franklin is a waterlogging-sensitive, two-rowed malting barley (Li et al., 2008).
2.2. Plant growth conditions
The field experiment was conducted at the experimental farm on the Huajiachi Campus, Zhejiang University, Hangzhou, China (30°10′ N, 120°12′ E). Two experiments, Exp. 1 and Exp. 2, were conducted in the 2005–2006 and 2006–2007 barley growing seasons, respectively. The field was divided in half by making a ridge, which was encircled by plastic film to a depth of 50 cm to avoid water percolation between the two halves. The seeds of the DH lines and the two parents were sown early in November. Each plot consisted of 1 line with a length of 2 m. There was a gap of 0.25 m between plots. Fifty seeds were sown of each line. Prior to seeding, 70 kg/ha of nitrogen as urea and 180 kg/ha of potassium chloride were applied. Another 70 kg/ha of nitrogen was top-dressed at the 4-leaf stage. The experiments were laid out in a randomized complete-block design with three replications. The waterlogging treatment was imposed at the tillering stage (100 d after sowing) to one half of the field by pouring water into the plots to 2–3 cm above the soil surface and maintaining the water level by applying successive water supplements. After 12 d, the water in the waterlogged plots was drained out and the plants were allowed to grow to maturity. Normal agronomic practices were applied to the other half of the field which was used as the control.
2.3. Determination of yield and yield components
At maturity, PH, GS, SP, KW and GY were measured in Exp. 1. PH, GS, KW and SL were measured in Exp. 2.
2.4. Statistical and QTL analyses
A genetic linkage map was constructed using 496 diversity arrays technology (DArT) markers, 80 amplified fragment length polymorphism (AFLP) markers and 28 microsatellite markers (Wenzl et al., 2006; Li et al., 2008). Population distribution analysis was performed using SPSS 11.0 statistical software. QTL analysis for individual environments was performed using software QTLMAPPER 1.60, which was developed based on a mixed linear model (Wang et al., 1999; 2003), to identify QTLs, with main effects. QTLs were determined using a threshold of P<0.005. A threshold of likelihood of odds (LOD) >2.5 was chosen for claiming a putative QTL. The nomenclature of McCouch et al. (1997) was used to describe the QTLs.
3. Results
3.1. Phenotypic variation
Table 1 shows the mean values of measured traits for the parents and the DH population. In Exp. 1, the KW of both parents was reduced by waterlogging stress while other traits were less affected. In the DH population, the mean values of nearly all the measured traits were reduced as a result of waterlogging. The biggest reduction was found for one of the yield components, SP, which was 24% lower under waterlogged conditions. Similar results were found in Exp. 2 with the waterlogging treatment, showing great effects on most of the traits measured in this experiment.
Table 1.
Phenotypic values of yield and yield components in a DH population and its two parent cultivars, Yerong and Franklin, under control and waterlogged conditions in two experiments
| Exp. 1 |
Exp. 2 |
|||||||||||||||||
| KW (mg) |
GS |
SP |
PH (cm) |
GY (g) |
KW (mg) |
GS |
SL (cm) |
PH (cm) |
||||||||||
| C | W | C | W | C | W | C | W | C | W | C | W | C | W | C | W | C | W | |
| Patient | ||||||||||||||||||
| Yerong | 45.70 | 39.85 | 35.7 | 39.7 | 9.4 | 8.5 | 70.3 | 69.5 | 140.8 | 146.2 | 53.58 | 49.05 | 46.3 | 43.0 | 7.1 | 6.4 | 112 | 92 |
| Franklin | 34.56 | 33.65 | 15.7 | 14.9 | 14.2 | 14.1 | 66.3 | 69.8 | 29.4 | 27.9 | 36.49 | 33.61 | 25.6 | 24.3 | 9.1 | 7.7 | 109 | 81 |
| DH population | ||||||||||||||||||
| Two-row line mean | 40.91 | 40.78 | 22 | 19.7 | 13.3 | 9.6 | 73.0 | 69.7 | 84.3 | 67.6 | 49.21 | 47.97 | 24.5 | 23.2 | 8.1 | 7.5 | 100 | 82 |
| Six-row line mean | 33.21 | 31.68 | 37.6 | 36.5 | 12.2 | 9.2 | 70.1 | 70.4 | 109.1 | 95.5 | 35.22 | 34.55 | 40.7 | 36.8 | 6.9 | 5.9 | 101 | 77 |
| All lines | ||||||||||||||||||
| Mean | 35.8 | 34.8 | 31.9 | 30.4 | 12.6 | 9.3 | 71.1 | 70.1 | 99.5 | 85.9 | 40.5 | 40.0 | 34.8 | 31.6 | 7.4 | 6.6 | 100.8 | 78.8 |
| Range | 16.0–57.4 | 20.3–59.7 | 13.6–57.7 | 5.9–59.9 | 3.8–22.4 | 1.5–15.3 | 58.0–90.8 | 61.1–76.6 | 26.0–220.1 | 7.2–204.0 | 25.9–68.1 | 22.0–64.6 | 15.3–57.0 | 16.8–54.4 | 3.9–11.4 | 3.1–11.6 | 84–120 | 57–99 |
| Skewness | 0.45 | 0.58 | 0.25 | 0.39 | 0.42 | 0.05 | 0.26 | −0.52 | 0.42 | 0.52 | 0.66 | 0.39 | 0.26 | 0.45 | 0.48 | 0.61 | 0.04 | −0.13 |
KW: kernel weight; GS: grains per spike; SP: spikes per plant; PH: plant height; SL: spike length; GY: grain yield; C: control; W: waterlogging
The two parents differed greatly in the traits measured in the two experiments. In general, the six-rowed barley, Yerong, had higher KW and yield, more GS, higher PH, but shorter SL and less SP than the two-rowed barley, Franklin. In the DH lines, all phenotypic traits showed a continuous normal distribution with skew values ranging from −0.52 to 0.66 (Table 1), indicating that these characters were quantitatively inherited. In addition, transgressive segregation in both directions was observed for all characters under both control and waterlogging treatments. Unlike the parents, the average KW of two-rowed DH lines was much higher than that of six-rowed DH lines, being 7.7 g higher in Exp. 1 and 13.99 g higher in Exp. 2. In Exp. 1, the average GY of two-rowed DH lines showed greater reduction (24.7%) due to waterlogging than that of six-rowed DH lines (14.2%) (Table 1).
3.2. QTL analysis
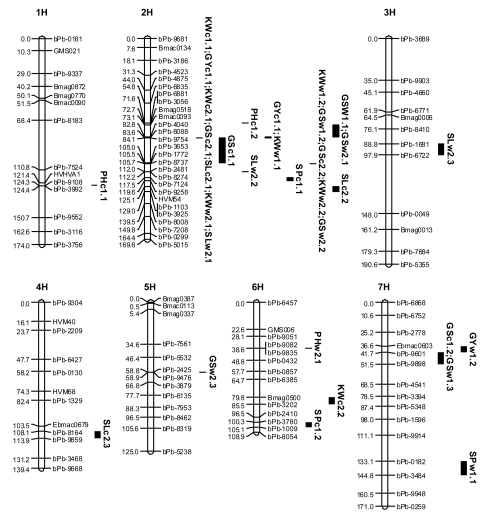
A total of 32 QTLs were identified for the measured characters from the two years and the two growth environments. Some of the QTLs identified from different environments were at the same position (Table 2). QTLs were mapped on all seven chromosomes with the majority being on 2H (Fig. 1). In the genetic map of this population, 1H and 2H were each separated into two groups with the gap between the two groups being more than 20 cM.
Table 2.
QTLs for agronomic traits detected in a DH population derived from Yerong×Franklin and grown in different environments
| Traits | QTLa | Chr.b | Marker interval | LODc | Add.d | R2 (%)e |
| Exp. 1-control | ||||||
| Kernel weight | KWc1.1 | 2H | bPb-6088-bPb-5440 | 2.79 | −1.92 | 6.54 |
| Grains per spike | GSc1.1 | 2H | bPb-9754-bPb-3653 | 13.22 | 7.71 | 50.86 |
| GSc1.2 | 7H | bPb-9601-bPb-9898 | 6.17 | 2.84 | 6.91 | |
| Spikes per plant | SPc1.1 | 2H | bPb-7124-bPb-9258 | 5.31 | −1.04 | 10.91 |
| SPc1.2 | 6H | bPb-1009-bPb-7323 | 5.12 | −1.02 | 10.49 | |
| Plant height | PHc1.1 | 1H | bPb-9108-bPb-3992 | 2.91 | 1.37 | 5.61 |
| PHc1.2 | 2H | bPb-6881-bPb-3056 | 6.47 | −2.04 | 12.40 | |
| Grain yield | GYc1.1 | 2H | bPb-4040-bPb-6088 | 6.94 | 15.52 | 15.94 |
| Exp. 1-waterlogging | ||||||
| Kernel weight | KWw1.1 | 2H | bPb-4040-bPb-6088 | 4.48 | −2.13 | 6.82 |
| KWw1.2 | 2H | bPb-1772-bPb-8737 | 7.34 | −3.31 | 16.59 | |
| Grains per spike | GSw1.1 | 2H | Bmac0093-bPb-4040 | 11.53 | 7.20 | 35.35 |
| GSw1.2 | 2H | bPb-1772-bPb-8737 | 6.29 | 4.22 | 12.18 | |
| GSw1.3 | 7H | bPb-9601-bPb-9898 | 7.35 | 3.64 | 9.06 | |
| Spikes per plant | SPw1.1 | 7H | bPb-0182-bPb-3484 | 3.31 | −0.65 | 8.13 |
| Plant height | − | − | − | − | − | − |
| Grain yield | GYw1.1 | 2H | bPb-6088-bPb-5440 | 2.85 | 8.90 | 4.74 |
| GYw1.2 | 7H | Ebmac0603-bPb-9601 | 7.45 | 22.53 | 30.43 | |
| Exp. 2-control | ||||||
| Kernel weight | KWc2.1 | 2H | bPb-6088-bPb-5440 | 11.56 | −3.78 | 17.77 |
| KWc2.2 | 6H | Bmag0500-bPb-3202 | 4.46 | −5.85 | 6.00 | |
| Grains per spike | GSc2.1 | 2H | bPb-6088-bPb-5440 | 4.09 | 3.20 | 9.40 |
| GSc2.2 | 2H | bPb-1772-bPb-8737 | 7.22 | 4.56 | 19.13 | |
| Spike length | SLc2.1 | 2H | bPb-6088-bPb-5440 | 7.76 | −0.45 | 12.97 |
| SLc2.2 | 2H | HVM54-bPb-1103 | 7.05 | 0.41 | 10.97 | |
| SLc2.3 | 4H | bPb-8164-bPb-9859 | 3.64 | −0.29 | 5.50 | |
| Plant height | − | − | − | − | − | − |
| Exp. 2-waterlogging | ||||||
| Kernel weight | KWw2.1 | 2H | bPb-6088-bPb-5440 | 9.10 | −4.56 | 27.35 |
| KWw2.2 | 2H | bPb-1772-bPb-8737 | 3.94 | −2.26 | 6.78 | |
| Grains per spike | GSw2.1 | 2H | Bmac0093-bPb-4040 | 5.63 | 7.15 | 55.34 |
| GSw2.2 | 2H | bPb-1772-bPb-8737 | 4.76 | 2.76 | 8.22 | |
| GSw2.3 | 5H | bPb-2425-bPb-9476 | 6.18 | −3.04 | 10.02 | |
| Spike length | SLw2.1 | 2H | bPb-6088-bPb-5440 | 11.16 | −0.53 | 17.44 |
| SLw2.2 | 2H | bPb-2481-bPb-8274 | 8.74 | −0.46 | 13.05 | |
| SLw2.3 | 3H | bPb-1681-bPb-6722 | 7.74 | −0.48 | 14.37 | |
| Plant height | PHw2.1 | 6H | bPb-9082-bPb-9835 | 5.52 | 2.63 | 9.74 |
Individual QTLs are designated with the abbreviation of the trait, treatment, experiment number and QTL number
Chromosome number
Maximum-likelihood LOD score for the QTL calculated by QTLMAPPER 1.60
Additive effect
The positive or negative value indicates that allele from Yerong or Franklin increases the trait score, respectively
Variation explained by the putative QTL
Fig. 1.
QTLs identified for different traits in the DH population derived from Yerong×Franklin. Only SSR markers and a few DArT markers (prefix bPb-) are shown in the map. For detailed map, please refer to Li et al. (2008)
The QTLs controlling KW were located on chromosome 2H. In Exp. 1, only one QTL was found on chromosome 2H in the control trial, and only 6.54% of the phenotypic variation was determined by this QTL. Under waterlogged conditions, two QTLs were identified. One of them was in a similar position to that found in the control trial and another one showed greater effect. Similar QTLs were found inExp. 2. One more minor QTL was found on chromosome 6H (Table 2 and Fig. 1). For all the QTLs, the alleles from Franklin increased KW.
The QTLs controlling GS were located mainly on chromosome 2H with Yerong alleles contributing to more grains. Some minor QTLs were also identified from different experiments, all determining less than 10% of the genetic variation. In both the control and waterlogged conditions of Exp. 1, a minor QTL was located on chromosome 7H with the Franklin allele also contributing a positive effect. The minor QTL identified in the waterlogged trial of Exp. 2 was located on chromosome 5H and the Franklin allele contributed a negative effect.
Some QTLs were identified for SP from different growth conditions in Exp. 1. Under the control conditions, two QTLs for this trait were located on chromosomes 2H and 6H, respectively, while under waterlogged conditions, only one QTL was found, located on chromosome 7H. For all the QTLs identified, the Franklin allele contributed a negative effect, reducing the number of SP.
SL was measured only in Exp. 2. Three QTLs were found for this trait in both the control and waterlogged trials. Of the two QTLs on chromosome 2H, one was in the same position (84 cM) in both treatments. The other was at 140 cM in the control, with the Yerong allele being positive, and at a slightly different position (124 cM) under waterlogging, with the Yerong allele being negative. The third QTL was located on chromosome 4H with the Franklin allele being negative in the control and on chromosome 3H with the Yerong allele being negative under waterlogged conditions.
There was no obvious difference in PH between the two parents. No QTL was identified in Exp. 1 under waterlogging or in Exp. 2 in the control environment. In the control treatment of Exp. 1, two QTLs were found for PH. One was located on chromosome 1H at the interval of bPb-9108-bPb-3992 and one in chromosome 2H flanked by bPb-6881-bPb-3056. Under the waterlogging treatment in Exp. 2, the QTL on chromosome 6H was located between the two markers bPb-9082 and bPb-9835.
For GY, one QTL on chromosome 2H was identified in both the control and waterlogging treatments in Exp. 1. Another QTL on chromosome 7H was identified for GY under waterlogged conditions, which determined 15% of yield variation.
4. Discussion
The two parents differed considerably in most measured characters when grown in the different environments. The mean values of the population were close to the mid-parental values for all characters in both experiments (Table 1). Although the phenotypic distributions of the DH population were normal, transgressive segregation was observed in both directions for all characters, indicating that neither of the parents carried all the positive or all the negative alleles. The significant variation and the normal distribution of all characters measured in this study suggest the suitability of this population for QTL analysis.
In the past decade, with the development of molecular marker technologies, QTL analysis has been widely used to detect yield and related characters and a great number of QTLs have been mapped on all 7 chromosomes of barley using different genetic populations (Hayes et al., 1993; Backes et al., 1995; Thomas et al., 1995; Kjær and Jensen, 1996; Tinker et al., 1996; Bezant et al., 1997; Powell et al., 1997; Mather et al., 1997; Yin et al., 1999; Zhu et al., 1999; Marquez-Cedillo et al., 2001; Baum et al., 2003; Pillen et al., 2003; Li et al., 2005; 2006; Sameri et al., 2006). However, no QTLs for agronomic traits and yield components have been mapped in barley under waterlogging stress to dissect the genetic mechanism of waterlogging tolerance. In the present study, many QTLs were identified for some important agronomic traits and yield components under different growing conditions, i.e., normal and waterlogged. The majority of the QTLs were on chromosome 2H (Fig. 1) in the region where the gene controlling row-type is located (Li et al., 2008). Not surprisingly, the QTLs controlling GS and KW were co-located with the QTL for row-type, with the six-rowed barley cultivar Yerong contributing a positive effect for GS and the two-rowed barley cultivar Franklin contributing a positive effect on KW. Some QTLs controlled more than one trait. The QTL located on chromosome 2H at the position of around 84 cM was responsible for KW, GS, SL and GY. The results indicated that QTLs in the same region of a chromosome controlling different traits could be closely linked or pleiotropic. One or more QTLs were also found on the rest of the chromosomes including one on 3H and one on 4H for SL, one on 5H for GS, two on 6H for SP and KW, respectively, and four on 7H for GS, SP and GY, respectively.
Barley shows wide genetic diversity for waterlogging tolerance (Takeda and Fukuyama, 1986; Qiu and Ke, 1991; Fufa and Assefa, 1995; Setter et al., 1999; Pang et al., 2004; 2006; 2007a; 2007b; Zhou et al., 2007; Li et al., 2008). The identification of QTL for yield and related characters under waterlogging stress will be of great interest to plant breeders in developing barley cultivars with waterlogging tolerance. In this study, some QTLs were found to be affected by the growth conditions. The QTL for GS on chromosome 7H was found only in Exp. 1. A QTL for GS on chromosome 5H was found only under waterlogged conditions in Exp. 2. For PH, two QTLs were identified in the control treatment of Exp. 1, while under waterlogged conditions, the differences in PH between different DH lines were very small (Table 1) and no QTLs were identified. In contrast, a QTL for PH was found under waterlogging but not in the control. For SP, two QTLs on chromosomes 2H and 6H, respectively, were found in the control treatment while only one on chromosome 7H was found under waterlogged conditions.
The use of marker assisted selection (MAS) in combination with traditional field selection could significantly enhance barley breeding for waterlogging tolerance. However, as shown in this experiment, some different markers should be used for selecting cultivars which have superior agronomic traits and yield components when grown in high rainfall areas. As the genetics and expression of the characters related to waterlogging tolerance are quite complex, more studies are need involving more populations and environments.
Footnotes
Project supported by the Hi-Tech Research and Development Program (863) of China (No. 2006AA10Z1C3), the Ministry of Education and the State Administration of Foreign Experts Affairs (111 Project) of China (No. B06014), and the Zhejiang Provincial Department of Education Project (No. 20070214), China
References
- 1.Babu R, Nair SK, Prasanna BM, Gupta HS. Integrating marker-assisted selection in crop breeding-prospects and challenges. Curr Sci. 2004;87(5):607–619. [Google Scholar]
- 2.Backes G, Graner A, Foroughi-Wehr B, Fischbeck G, Wenzel G, Jahoor A. Localization of quantitative trait loci (QTL) for agronomic important characters by the use of an RFLP map in barley (Hordeum vulgare L.) Theor Appl Genet. 1995;90(2):294–302. doi: 10.1007/BF00222217. [DOI] [PubMed] [Google Scholar]
- 3.Baghizadeh A, Taleei AR, Naghavi MR. QTL analysis for some agronomic traits in barley (Hordeum vulgare L.) Int J Agric Biol. 2007;9:372–374. [Google Scholar]
- 4.Baum M, Grando S, Backes G, Jahoor A, Sabbagh A, Ceccarelli S. QTLs for agronomic traits in the Mediterranean environment identified in recombinant inbred lines of the cross ‘Arta’×H. spontaneum 41-1. Theor Appl Genet. 2003;107(7):1215–1225. doi: 10.1007/s00122-003-1357-2. [DOI] [PubMed] [Google Scholar]
- 5.Bezant J, Laurie D, Pratchett N, Chojecki J, Kearsey M. Mapping QTLs controlling yield and yield components in a spring barley (Hordeum vulgare L.) cross using marker regression. Mol Breed. 1997;3(1):29–38. doi: 10.1023/A:1009648220852. [DOI] [Google Scholar]
- 6.Fufa F, Assefa A. Response of barley to waterlogging: improved varieties versus local cultivars. IAR Newsl Agric Res. 1995;10:6–7. [Google Scholar]
- 7.Garthwaite AJ, von Bothmer R, Colmer TD. Diversity in root aeration traits associated with waterlogging tolerance in the genus Hordeum . Funct Plant Biol. 2003;30(8):875–889. doi: 10.1071/FP03058. [DOI] [PubMed] [Google Scholar]
- 8.Hamachi Y, Yoshino M, Furusho M, Yoshida T. Index of screening for wet endurance in malting barley. Jpn J Breed. 1990;40:361–366. [Google Scholar]
- 9.Hayes PM, Liu BH, Knapp SJ, Chen F, Jones B, Blake T, Franckowiak J, Rasmusson D, Sorrells M, Ullrich SE, et al. Quantitative trait locus effects and environmental interaction in a sample of North American barley germplasm. Theor Appl Genet. 1993;87(3):392–401. doi: 10.1007/BF01184929. [DOI] [PubMed] [Google Scholar]
- 10.Hori K, Kobayashi T, Shimizu A, Sato K, Takeda K, Kawasaki S. Efficient construction of high-density linkage map and its application to QTL analysis in barley. Theor Appl Genet. 2003;107(5):806–813. doi: 10.1007/s00122-003-1342-9. [DOI] [PubMed] [Google Scholar]
- 11.Kjær B, Jensen J. Quantitative trait loci for grain yield and yield components in a cross between a sixrowed and a two-rowed barley. Euphytica. 1996;90(1):39–48. [Google Scholar]
- 12.Li HB, Vaillancourt R, Mendham NJ, Zhou MX. Comparative mapping of quantitative trait loci associated with waterlogging tolerance in barley (Hordeum vulgare L.) BMC Genomics. 2008;9(1):401. doi: 10.1186/1471-2164-9-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li JZ, Huang XQ, Heinrich F, Ganal MW, Röder MS. Analysis of quantitative trait loci for yield, yield components and malting quality in a BC3-DH population of spring barley. Theor Appl Genet. 2005;110(2):356–363. doi: 10.1007/s00122-004-1847-x. [DOI] [PubMed] [Google Scholar]
- 14.Li JZ, Huang XQ, Heinrichs F, Ganal MW, Röder MS. Analysis of QTLs for yield components, agronomic traits, and disease resistance in an advanced backcross population of spring barley. Genome. 2006;49(5):454–466. doi: 10.1139/G05-128. [DOI] [PubMed] [Google Scholar]
- 15.Marquez-Cedillo LA, Hayes PM, Kleinhofs A, Legge WG, Rossnagel BG, Sato K, Ullrich SE, Wesenberg DM. QTL analysis of agronomic traits in barley based on the doubled-haploid progeny of two elite North American varieties representing different germplasm groups. Theor Appl Genet. 2001;103(4):625–637. doi: 10.1007/PL00002919. [DOI] [Google Scholar]
- 16.Mather D, Tinker NA, LaBerge DE, Edney M, Jones BL, Rossnagel BG, Legge WG, Briggs KG, Irvine RB, Falk DE, et al. Regions of the genome that affect grain and malt quality in a North American two-row barley cross. Crop Sci. 1997;37(2):544–554. [Google Scholar]
- 17.McCouch SR, Cho YG, Yano M, Paul E, Blinstrub M. Report on QTL nomenclature. Rice Genet Newsl. 1997;14:11–13. [Google Scholar]
- 18.Pang JY, Zhou MX, Mendham N, Shabala S. Growth and physiological responses of six barley genotypes to waterlogging and subsequent recovery. Aust J Agric Res. 2004;55(8):895–906. doi: 10.1071/AR03097. [DOI] [Google Scholar]
- 19.Pang JY, Mendham N, Zhou MX, Newman I, Shabala S. Microelectrode ion and O2 flux measurements reveal differential sensitivity of barley root tissues to hypoxia. Plant Cell Environ. 2006;29(6):1107–1121. doi: 10.1111/j.1365-3040.2005.01486.x. [DOI] [PubMed] [Google Scholar]
- 20.Pang J, Ross J, Zhou MX, Mendham N, Shabala S. Amelioration of detrimental effects of waterlogging by foliar nutrient sprays in barley. Funct Plant Biol. 2007;34(3):221–227. doi: 10.1071/FP06158. [DOI] [PubMed] [Google Scholar]
- 21.Pang JY, Cuin T, Shabala S, Zhou MX, Mendham NJ, Shabala S. Effect of secondary metabolites associated with anaerobic soil conditions on ion fluxes and electrophysiology in barley roots. Plant Physiol. 2007;145(1):266–276. doi: 10.1104/pp.107.102624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pillen K, Zacharias A, Léon J. Advanced backcross QTL analysis in barley (Hordeum vulgare L.) Theor Appl Genet. 2003;107(2):340–352. doi: 10.1007/s00122-003-1253-9. [DOI] [PubMed] [Google Scholar]
- 23.Powell W, Thomas WTB, Baird E, Lawrence P, Booth A, Harrower B, McNicol JW, Waugh R. Analysis of quantitative traits in barley by the use of amplified fragment length polymorphisms. Heredity. 1997;79(1):48–59. doi: 10.1038/hdy.1997.122. [DOI] [Google Scholar]
- 24.Qiu JD, Ke Y. Study of determination of wet tolerance of 4572 barley germplasm resources. Acta Agric Shanghai. 1991;7(4):27–32. (in Chinese) [Google Scholar]
- 25.Sameri M, Takeda K, Komatsuda T. Quantitative trait loci controlling agronomic traits in recombinant inbred lines from a cross of oriental- and occidental-type barley cultivars. Breed Sci. 2006;56(3):243–252. doi: 10.1270/jsbbs.56.243. [DOI] [Google Scholar]
- 26.Setter TL, Waters I. Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant Soil. 2003;253(1):1–34. doi: 10.1023/A:1024573305997. [DOI] [Google Scholar]
- 27.Setter TL, Burgess P, Waters I, et al. Genetic Diversity of Barley and Wheat for Waterlogging Tolerance in Western Australia; Proceedings of the 9th Australian Barley Technical Symposium; Melbourne: Australian Barley Technical Symposium Inc; 1999. [Google Scholar]
- 28.Takeda K, Fukuyama T. Variation and geographical distribution of varieties for flooding tolerance in barley seeds. Barley Genet Newsl. 1986;16:28–29. [Google Scholar]
- 29.Thomas WTB, Powell W, Waugh R, Chalmers KJ, Barua UM, Jack P, Lea V, Forster BP, Swanston JS, Ellis RP, et al. Detection of quantitative trait loci for agronomic, yield, grain, and disease characters in spring barley (Hordeum vulgare L.) Theor Appl Genet. 1995;91(6-7):1037–1047. doi: 10.1007/BF00223917. [DOI] [PubMed] [Google Scholar]
- 30.Tinker NA, Mather DE, Rossnagel BG, Kasha KJ, Kleinhofs A, Hayes PM, Falk DE, Ferguson T, Shugar LP, Legge WG, et al. Regions of the genome that affect agronomic performance in two-row barley. Crop Sci. 1996;36:1053–1062. [Google Scholar]
- 31.von Korff M, Wang H, Leon J, Pillen K. AB-QTL analysis in spring barley: II. Detection of favourable exotic alleles for agronomic traits introgressed from wild barley (H. vulgare ssp. spontaneum) Theor Appl Genet. 2006;112(7):1221–1231. doi: 10.1007/s00122-006-0223-4. [DOI] [PubMed] [Google Scholar]
- 32.Wang DL, Zhu J, Li ZK, Paterson AH. Mapping QTLs with epistatic effects and QTL×environment interactions by mixed linear model approaches. Theor Appl Genet. 1999;99(7-8):1255–1264. doi: 10.1007/s001220051331. [DOI] [Google Scholar]
- 33.Wang DL, Zhu J, Li ZK, et al. QTLMapper Version 1.6: A Computer Software for Mapping Quantitative Trait Loci (QTLs) with Additive Effects, Epistatic Effects and QTL×Environment Interactions. 2003. Available from http://ibi.zju.edu.cn/software/qtlmapper/index.htm. [accessed on Oct. 12, 2008]
- 34.Wang SG, He LR, Li ZW, Zeng JG, Chai YR, Hou L. A comparative study on the resistance of barley and wheat to waterlogging. Acta Agron Sin. 1996;22(2):228–232. (in Chinese) [Google Scholar]
- 35.Wenzl P, Li H, Carling J, Zhou M, Raman H, Paul E, Hearnden P, Maier C, Xia L, Caig V, et al. A high-density consensus map of barley linking DArT markers to SSR, RFLP and STS loci and agricultural traits. BMC Genomics. 2006;7(1):206. doi: 10.1186/1471-2164-7-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao Y, Wei K, Chen J, Zhou M, Zhang G. Effects of waterlogging on photosynthesis and antioxidant enzyme activities of six barley genotypes with different waterlogging tolerance. Agric Sci China. 2005;4(4):310–316. [Google Scholar]
- 37.Xiao Y, Wei K, Chen J, Zhou M, Zhang G. Genotypic difference in growth inhibition and yield loss in barley under waterlogging stress. J Zhejiang Univ Sci (Agric & Life Sci) 2007;33(5):525–532. [Google Scholar]
- 38.Yin X, Stam P, Johan Dourleijn C, Kropff MJ. AFLP mapping of quantitative trait loci for yield determining physiological characters in spring barley. Theor Appl Genet. 1999;99(1-2):244–253. doi: 10.1007/s001220051230. [DOI] [Google Scholar]
- 39.Zhang G, Tanakamaru K, Abe J, Morita S. Influence of waterlogging on some anti-oxidative enzymatic activities of two barley genotypes differing in anoxia tolerance. Acta Physiol Plant. 2007;29(2):171–176. doi: 10.1007/s11738-006-0022-1. [DOI] [Google Scholar]
- 40.Zhou MX, Li HB, Mendham NJ. Combining ability of waterlogging tolerance in barley. Crop Sci. 2007;47(1):278–284. doi: 10.2135/cropsci2006.02.0065. [DOI] [Google Scholar]
- 41.Zhu H, Briceno G, Dovel R, Hayes PM, Liu BH, Liu CT, Ullrich SE. Molecular breeding for grain yield in barley: an evaluation of QTL effects in a spring barley cross. Theor Appl Genet. 1999;98(5):772–779. doi: 10.1007/s001220051134. [DOI] [Google Scholar]