Abstract
Objective: To investigate the effects of mycotoxin moniliformin (MON) on the metabolism of aggrecan and type II collagen in human chondrocytes in vitro and the relationship between MON and Kashin-Beck disease (KBD). Methods: Human chondrocytes were isolated and cultured on bone matrix gelatin to form an artificial cartilage model in vitro with or without MON toxin. Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The expression of aggrecan and type II collagen in the cartilage was determined using immunocytochemical staining. Results: MON toxin inhibited chondrocyte viability in dose-dependent and time-dependent manners. MON reduced aggrecan and type II collagen syntheses in the tissue-engineered cartilage. MON also increased the expression of matrix metalloproteinase-1 (MMP-1), MMP-13, BC4 epitopes, and CD44 in cartilages. However, the expression of 3B3(−) epitopes in cartilages was inhibited by MON. Selenium partially alleviated the damage of aggrecan induced by MON toxin. Conclusion: MON toxin promoted the catabolism of aggrecan and type II collagen in human chondrocytes.
Keywords: Chondrocytes, Moniliformin, Selenium, Aggrecan, Collagen, Matrix metalloproteinases (MMPs), CD44, Kashin-Beck disease
1. Introduction
Kashin-Beck disease (KBD) is an endemic chronic deformative osteoarthropathy (Cao et al., 2007) affecting a large proportion of population in China. The pathological alterations are observed in articular cartilages of main joints such as knee and elbow joints (Cao et al., 2008). The major pathophysiological changes in KBD patients are chondrocyte necrosis in the hypertrophied layer of the cartilage, with cell cluster formation nearby (Li et al., 2008).
The etiology and pathogenesis of KBD are still unclear. Several hypotheses have been raised, including selenium deficiency, fungal contamination of staple grains, and iodine deficiency (Li et al., 2008). However, it has been confirmed that selenium deficiency alone cannot lead to the occurrence of KBD and it is just one of the risk factors (Mo et al., 1997). In recent 20 years, it was found that the KBD endemic area coincides with the area where the grains contaminated by fungal mycotoxins; therefore, mycotoxin-poisoning hypothesis was established (Yang, 2002).
Mycotoxin moniliformin (MON) is one of the major mycotoxins detected in the contaminated grains in the KBD endemic area. Previous studies have shown that MON caused chondrocyte necrosis, apoptosis (Zhao, 2002), and DNA damage (Cao et al., 1995). Animal model studies have shown that the administration of MON can induce significant catabolic alterations in articular cartilages of the knee joint of mini pig (Xiong et al., 1998). Therefore, MON might be a possible risk factor to KBD occurrence and development (Cao et al., 1995). However, the mechanism of how MON induces the degeneration of articular cartilages is still not clear. In this study, the effects of MON toxin on the metabolism of aggrecan and collagen in human artificial cartilage were investigated.
2. Materials and methods
2.1. Materials
MON toxin was kindly provided by Prof. Jin-sheng Yang (Institute of Pharmacology and Toxicology, Academy of Military Medical Sciences of China) and Prof. Shuang-qing Peng (Institute of Disease Control and Prevention, Academy of Military Medical Sciences of China). Na2SeO3 was purchased from the Fine Chemical Plant (Chongqing, China). Dulbecco’s modified Eagle’s medium/Ham’s nutrient mixture F12 (DMEM/F-12) culture medium and 0.25% (v/v) trypsin were from GIBCO (Carlsbad, CA, USA). Hyaluronidase, collagenase II, and chondroitinase ABC were from Sigma-Aldrich (St. Louis, MO, USA). Matrix metalloproteinase-1 (MMP-1) and MMP-13 monoclonal antibodies and biotinylated goat anti-rabbit IgG conjugated with streptavidin-biotin-peroxidase complex (SABC) were from Jacksonimmuno Co. (West Baltimore Pike, USA). Fetal bovine serum (FBS) was from Biological Products Ltd. (Beijing, China). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), propidium iodide (PI), and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG were from Gold Bridge Co. (Beijing, China). SABC was from Boster Co. (Wuhan, China).
2.2. Sources of the human foetal articular cartilage
Human articular cartilages of the knee and sciatic joints of aborted fetuses were obtained from informed donors who had to stop pregnancy at the fifth month with medicine as approved by the Humane and Ethical Committee, College of Medicine, Xi’an Jiaotong University.
2.3. Chondrocyte isolation and monolayer culture
Chondrocytes were isolated from fetal cartilage as described previously (Li et al., 2008; Chen et al., 2006). Briefly, articular cartilage slides were dissected and chondrocytes were obtained by sequential digestions with hyaluronidase and collagenase type II. Cells were cultured as a monolayer in DMEM/F-12 containing 15% (v/v) FBS and 1% (v/v) penicillin at 37 °C in a humidified atmosphere containing 5% CO2 until confluence. All of the following experiments were performed using the first passage chondrocytes to avoid cell dedifferentiation (Shi et al., 2009).
2.4. Determination of cell viability by MTT assay
Cell viability was determined using MTT assay as described preciously (Chen et al., 2006) with minor modification. Briefly, human fetal chondrocytes were seeded onto a 96-well plate at the density of 5×103 per well. After 24 h of culture in DMEM/F-12 culture medium containing 15% (v/v) FBS and 1% (v/v) penicillin at 37 °C in a humidified atmosphere containing 5% CO2, 0.1–32 μg/ml MON toxin was added for different time periods (1–5 d). Then, 10 μl MTT at a final concentration of 500 μg/ml was added into the culture medium. After 4 h of incubation, the medium containing MTT was aspirated and replaced by dimethyl sulphoxide (DMSO) for 0.5 h. The optical density (OD) was measured using a plate reader at 490 nm (Chen et al., 2006).
2.5. Engineered cartilage preparation
Human fetal chondrocytes were isolated and seeded on the bone matrix gelatin (BMG) scaffolds for 24 h, and continuously cultured for 15 d in the presence or absence of 4 μg/ml MON with or without selenium supplementation at 0.1 μg/ml (Cao et al., 1994). The BMG grafts were prepared as described preciously (Nathanson et al., 1978). BMG scaffolds were sterilized with ethylene oxide and pre-incubated in DMEM/F-12 with 15% (v/v) FBS for 1 h at 37 °C. The BMG scaffolds were pelleted down at the bottom of 15-ml centrifuge tubes (Li X.D. et al., 2006), and 1.0×106 chondrocytes were then seeded on the BMG graft. The reconstructed cartilage was cultured for 15 d in DMEM/F-12 culture medium containing 10% (v/v) FBS and 1% (v/v) penicillin at 37 °C in a humidified atmosphere containing 5% CO2 with medium changing every other day.
2.6. Histochemistry and immunohistochemistry
Paraformaldehyde-fixed paraffin-embedded reconstructed cartilages were cut into 6-μm sections and placed on poly-L-lysine-coated glass slides. Sections were stained with hematoxylin-eosin (H & E) and toluidine blue (TB). Immunohistochemical stainings of type II collagen (1:100), MMP-1 (1:1000), MMP-13 (1:1000), and CD44 (1:40) were performed with the SABC method using SABC kits as described preciously (Li et al., 2008) with minor modification.
3B3 is a monoclonal antibody recognizing the mimic epitopes at the non-reducing terminal chondroitin sulphate (CS) chains in proteoglycan such as aggrecan (Mario Geysen, 1985; Caterson et al., 1990; Slater et al., 1995). BC4 is a monoclonal antibody recognizing the neo-epitope generated by MMPs on the core protein of aggrecan (Caterson et al., 2000). The expression of these epitopes was determined by the fluorescent immunostaining. Briefly, after deparaffinization, sections were incubated with 2% (v/v) Triton X-100 to wipe off the endogenous peroxidase for 25 min. The slides had been digested with 0.1% (v/v) chondroitinase ABC at 37 °C for 25 min to expose the antigen (3B3(−) needless), and then incubated with 10% (v/v) normal goat serum to block non-specific binding at room temperature for 10 min. Monoclonal antibodies 3B3(−) and BC4 were then incubated with the sections at the 1:500 and 1:200 dilution in phosphate buffered saline (PBS) at 4 °C for 18 h, followed by the incubation with 1:500 FITC-conjugated secondary antibody for 1 h at room temperature. The cell nucleuses were counterstained with 5 μg/ml PI.
2.7. Statistic analysis
Data are presented as mean±standard error of the mean (SEM) and were checked for the normal distribution and equal variance. Student’s t-test or one-way analysis of variance (ANOVA) was carried out using SPSS 12.0 software. Differences were considered significant at P≤0.05.
3. Results
3.1. Chondrocyte viability decreased by MON toxin
Chondrocyte damage caused by MON toxin was quantified using the MTT reduction assay (Cetin and Bullerman, 2005) (Fig. 1). Different concentrations of MON induced a significant cell proliferation in the first 24 h when compared with the control group. However, there was a significant decrease in the cell viability after 48 h of culture in the presence of high concentrations of MON (2, 4, 8, 16, and 32 μg/ml) when compared with the control group (P≤0.05). After 5 d of culture, 1 μg/ml of MON also induced a significant decrease in the cell viability.
Fig. 1.
Effects of different concentrations of MON on the cell viability of chondrocytes
Human chondrocytes cultured as monolayer were incubated with different concentrations of MON toxin for 5 d. MTT assay was done to determine the cell viability. The cellular viability of control is regarded as 1. Data are the mean of the experiment (n=5). * P≤0.05 when compared with the control group
3.2. H & E staining of the chondrocytes cultured on the BMG
There were 10–20 layers of chondrocytes on the surface of the BMG in the control group (Fig. 2a). However, in the MON group, much fewer chondrocytes were observed on the surface of the BMG and there were some cell necroses (black arrow, Fig. 2b). After selenium addition, there was still cell necrosis (black arrow) but more cell layers were observed when compared with the toxin group (Figs. 2c and 2d).
Fig. 2.




H & E staining of the chondrocytes cultured on BMG
Human chondrocytes were cultured on BMG for 16 d. (a) Control group; (b) MON group; (c) Control+Se group; (d) MON+Se group. Black arrow: cell necrosis
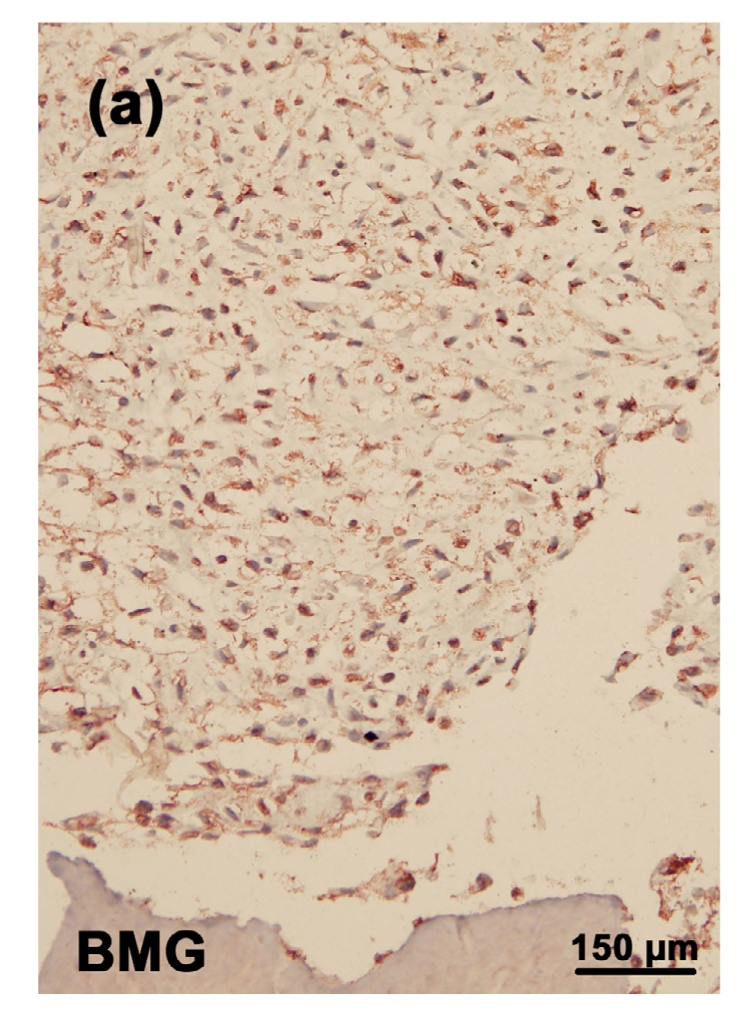
3.3. Expression of aggrecan in the tissue-engineered cartilage
The TB staining in the MON toxin group was the weakest (Fig. 3b) when compared with the control group (Fig. 3a) and control+Se group (Fig. 3c), suggesting a significant decrease in the aggrecan expression induced by the toxin. However, the TB staining in the MON+Se group (Fig. 3d) was stronger than that in the MON group, indicating a protective effect of selenium on the aggrecan expression in the presence of MON.
Fig. 3.


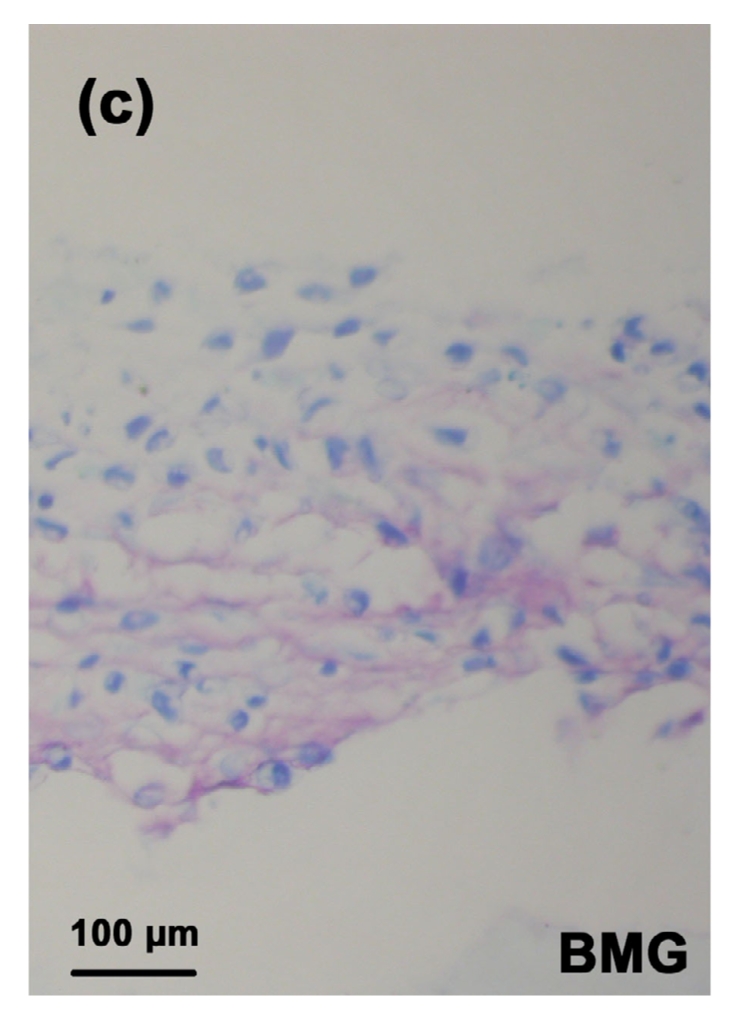

TB staining of the cartilage reconstructed in vitro
Chondrocytes were cultured for 16 d after seeded on BMG. Proteoglycan were stained with purple and the nuclei were stained with blue. (a) Control group; (b) MON group; (c) Control+Se group; (d) MON+Se group
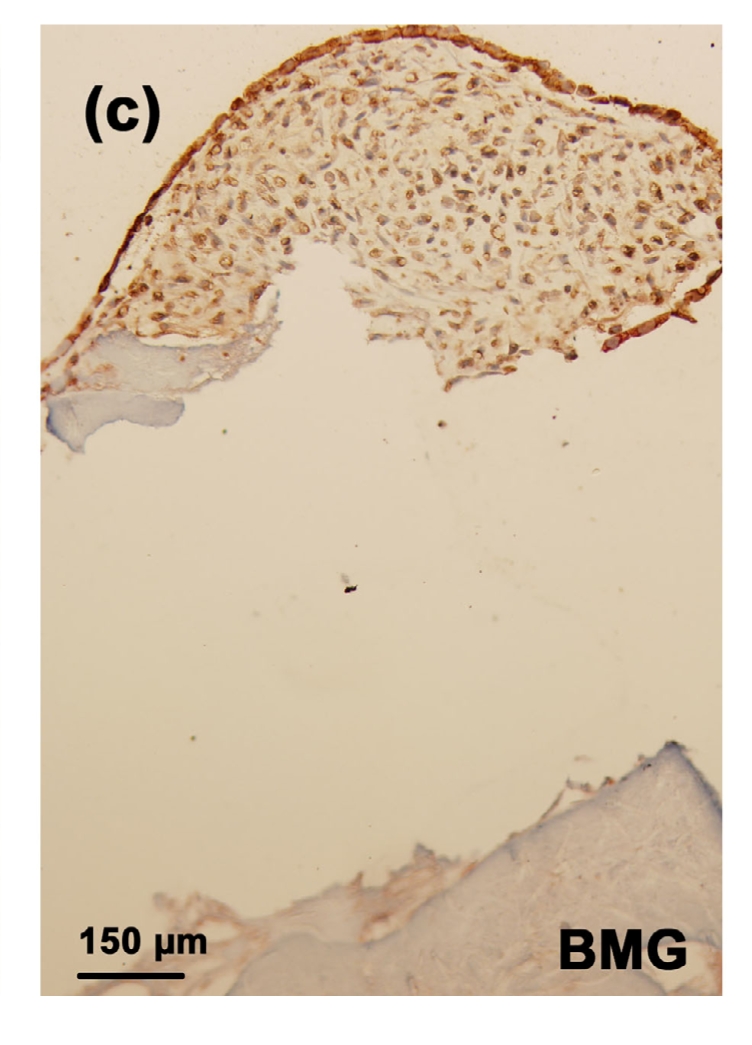
3.4. Immunostaining of type II collagen in the tissue-engineered cartilage
There was a significant decrease in the expression of type II collagen in the toxin group (Fig. 4b). Addition of selenium slightly increased the staining of type II collagen (Fig. 4d). There was no significant difference in the staining of type II collagen between the control (Fig. 4a) and control+Se groups (Fig. 4c).
Fig. 4.




Immunostaining of type II collagen in the cartilage reconstructed in vitro
Chondrocytes were cultured for 16 d before the immunostaining. The type II collagen was deep brownish red and the nuclei were blue. (a) Control group; (b) MON group; (c) Control+Se group; (d) MON+Se group
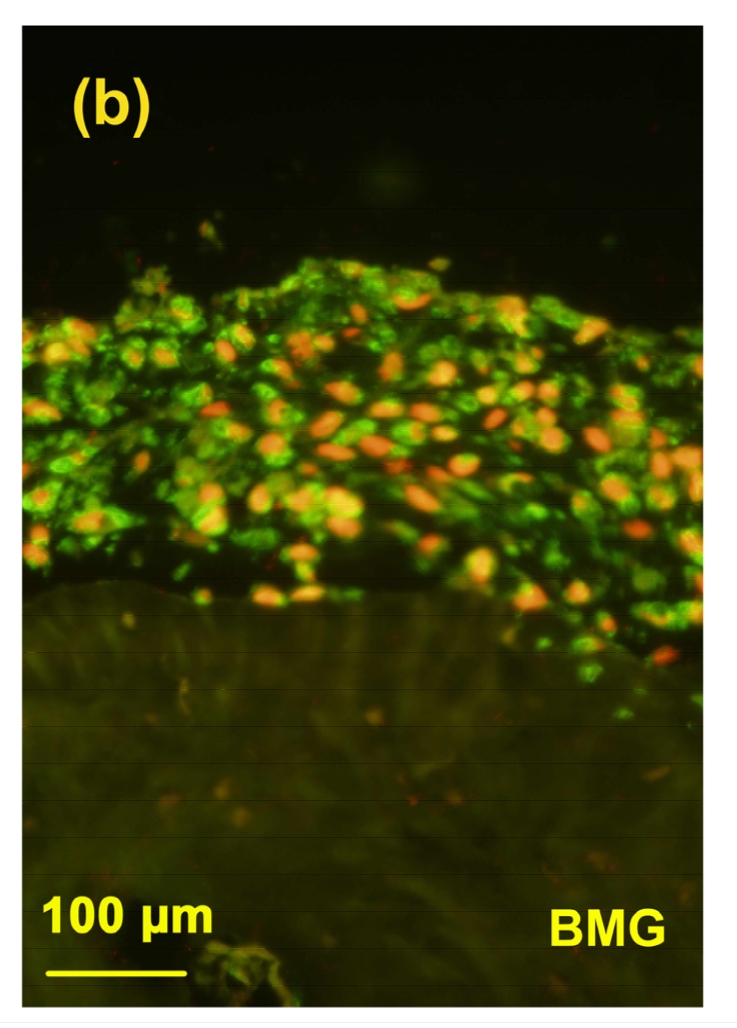
3.5. Immunostaining of 3B3(−)
In this study, immunolocation with monoclonal antibody 3B3(−) was performed without chondroitinase ABC pre-digestion. Under this condition, antibody 3B3(−) was used to identify a “native” mimotope that occurs at the non-reducing terminal of CS chains; therefore it is a marker for the newly synthesized aggrecan in cartilage (National KBD Surveillance Group, 2001; Moreno, 2009). In the toxin group, very little positive staining of 3B3(−) epitope was observed (Fig. 5b). After adding selenium, there was more 3B3(−) epitope expression in the cartilage (Fig. 5d), although the staining of 3B3(−) in the control (Fig. 5a) and control+Se groups (Fig. 5c) was much higher than that in MON and MON+Se groups.
Fig. 5.


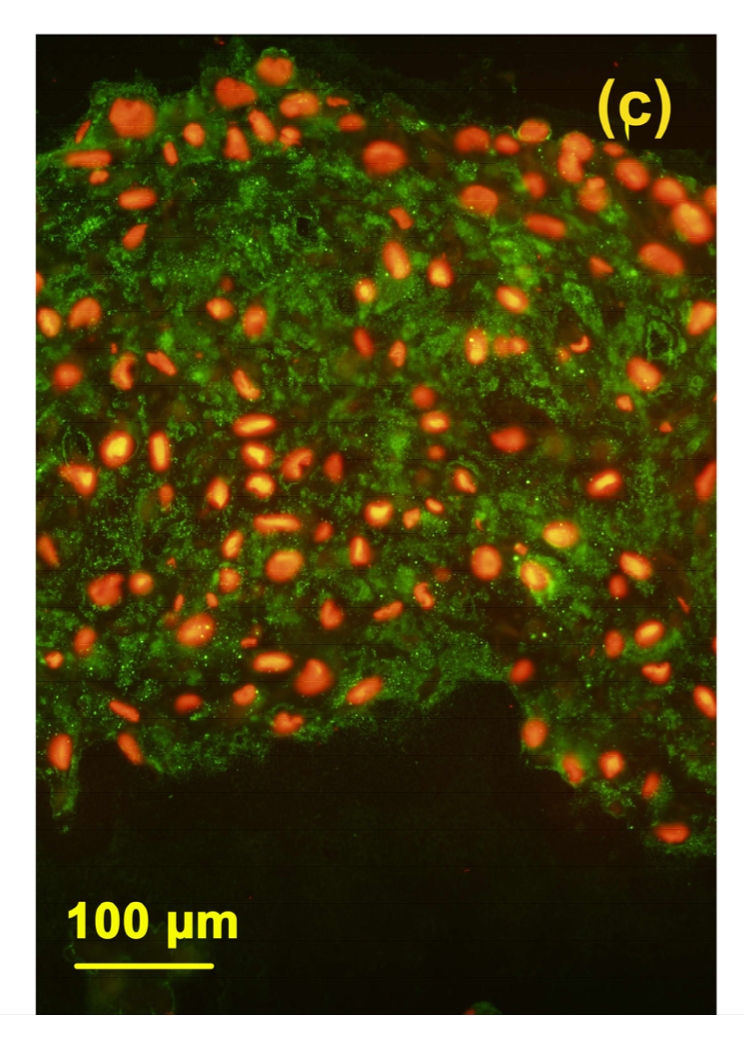
Immunostaining of 3B3(−) in the cartilage reconstructed in vitro
Chondrocytes were cultured for 16 d before the immunostaining. The epitopes of 3B3(−) were green and the nuclei were red. (a) Control group; (b) MON group; (c) Control+Se group; (d) MON+Se group
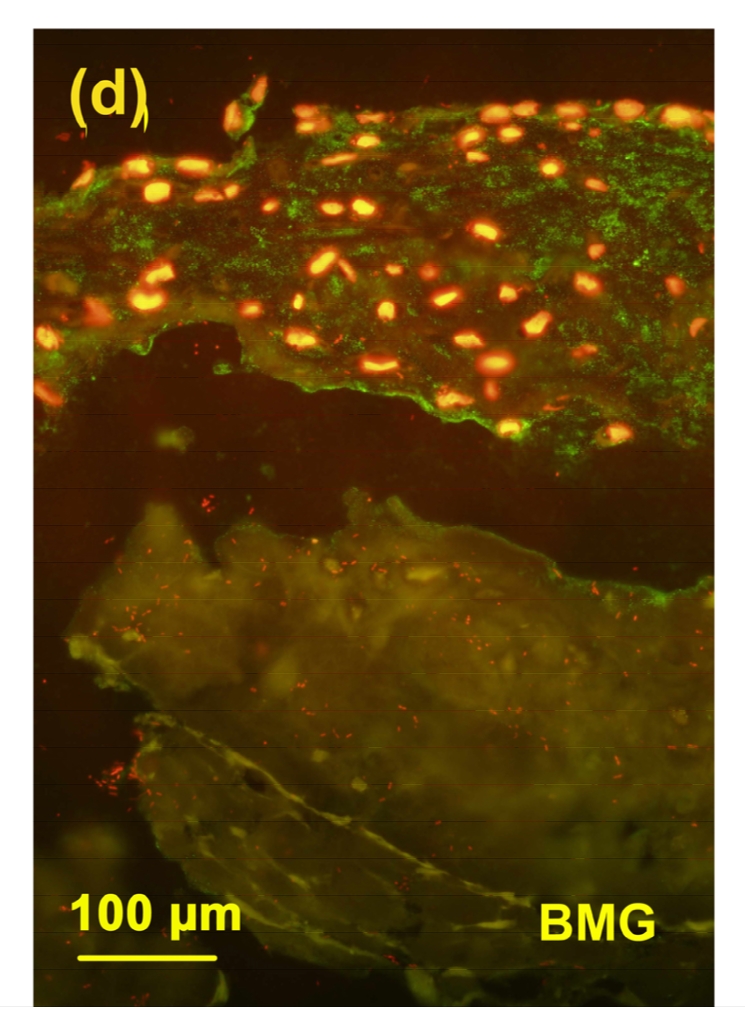
3.6. Immunostaining of the BC4 epitopes
Different from the 3B3(−), there was an intensive staining of BC4 epitopes in the cartilage from toxin group (Fig. 6b) when compared with the control group (Fig. 6a). The staining of BC4 in the MON+Se group (Fig. 6d) was weaker than that in the MON group. There was no significant difference in the staining of BC4 between the control and control+Se groups (Fig. 6c).
Fig. 6.


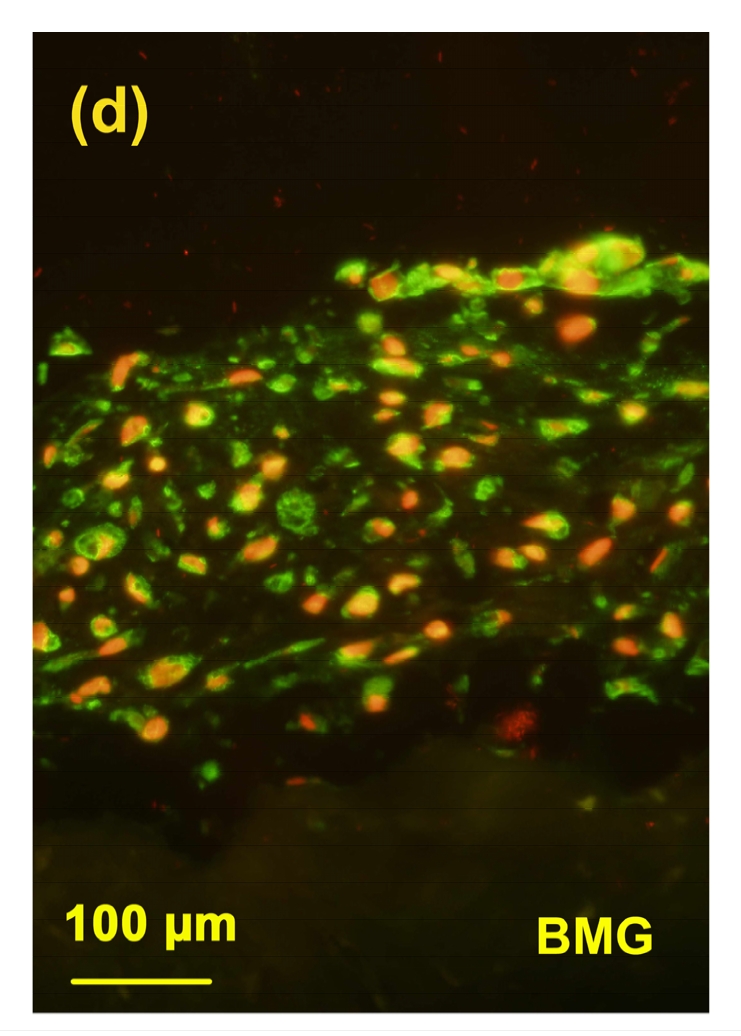
Immunostaining of BC4 epitopes in the cartilage reconstructed in vitro
Chondrocytes were cultured for 16 d after seeded on BMG. The epitopes of BC4 were green and the nuclei were red. (a) Control group; (b) MON group; (c) Control+Se group; (d) MON+Se group
3.7. Immunostaining of MMP-1 and MMP-13 in the tissue-engineered cartilages
The strongest stainings of MMP-1 and MMP-13 were observed in the toxin group (Figs. 7b and 8b). There were significant decreases in MMP-1 and MMP-13 stainings in the MON+Se group (Figs. 7d and 8d) when compared with the toxin group. There were no or very weak MMP-1 and MMP-13 stainings in the control (Figs. 7a and 8a) or control+Se group (Figs. 7c and 8c).
Fig. 7.




Immunostaining of MMP-1 in the cartilage reconstructed in vitro
Chondrocytes were cultured for 16 d before the immunostaining. MMP-1 was deep brownish red and the nuclei were blue. (a) Control group; (b) MON group; (c) Control+Se group; (d) MON+Se group
Fig. 8.


Immunostaining of MMP13 in the cartilage reconstructed in vitro
Chondrocytes were cultured for 16 d before the immunostaining. MMP-13 was deep brownish red and the nuclei were blue. (a) Control group; (b) MON group; (c) Control+Se group; (d) MON+Se group
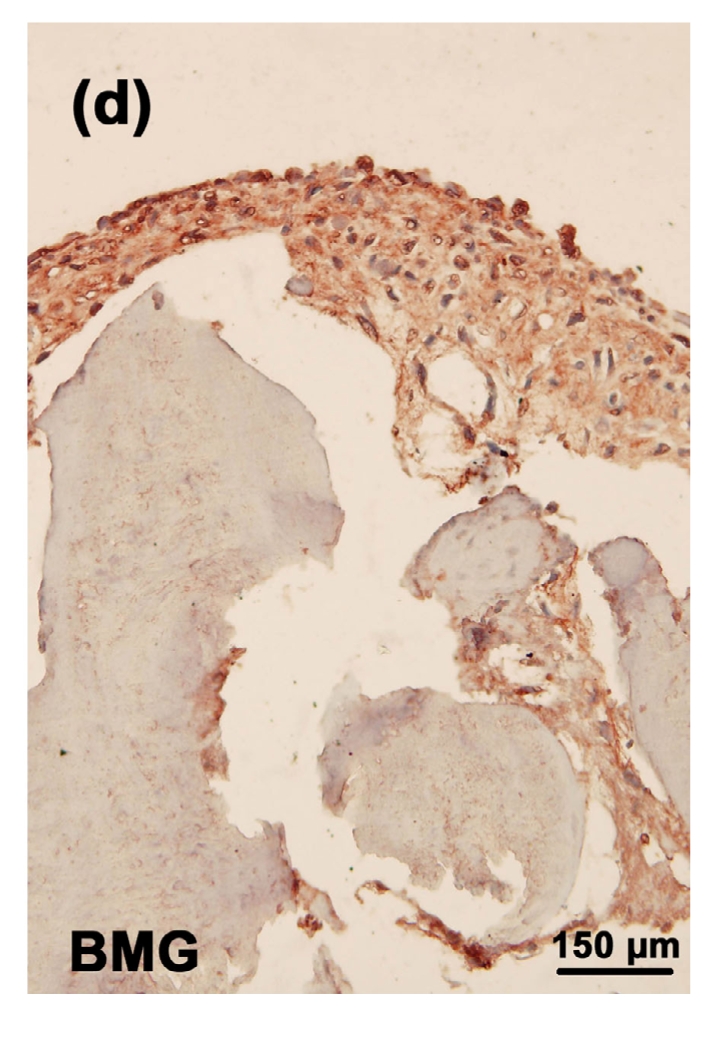
3.8. Immunostaining of CD44 in the tissue-engineered cartilages
The strongest staining of CD44 was observed in the toxin group (Fig. 9b). In the MON+Se group, there was a significant decrease in the staining of CD44 (Fig. 9d) when compared with the toxin group. There was very weak CD44 staining in both control (Fig. 9a) and control+Se groups (Fig. 9c).
Fig. 9.


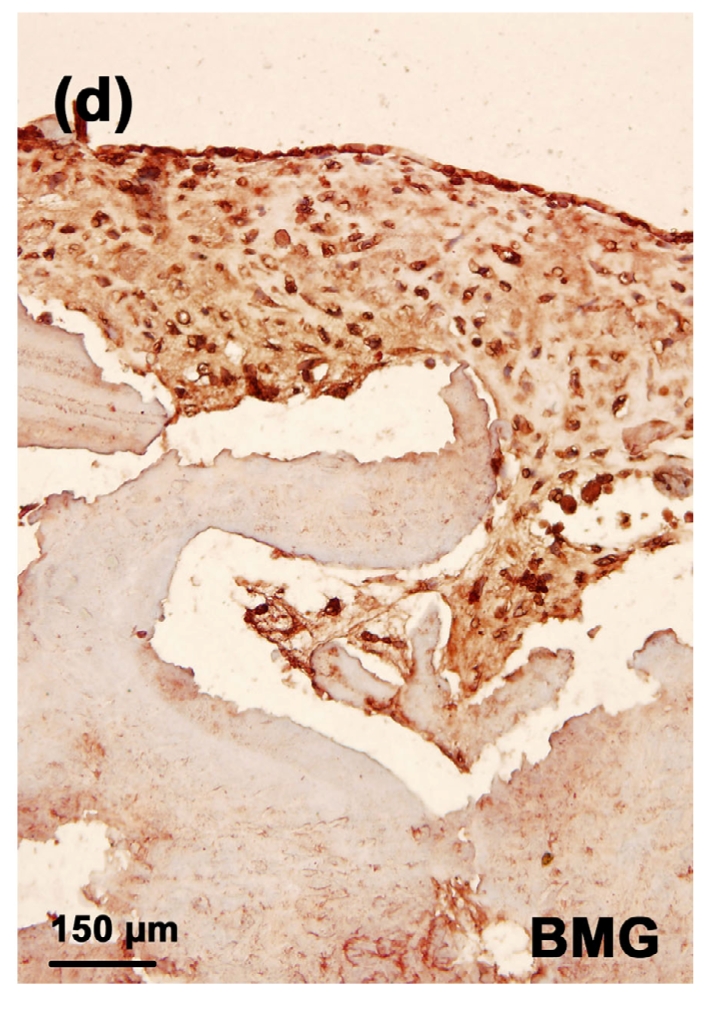
Immunostaining of CD44 in the cartilage reconstructed in vitro
Chondrocytes were cultured for 16 d before the immunostaining. CD44 was deep brownish red and the nuclei were blue. (a) Control group; (b) MON group; (c) Control+Se group; (d) MON+Se group
4. Discussion
MON toxin is one of the mycotoxins widely detected in the contaminated food crops in the KBD areas. Previous studies have shown that MON affects cellular metabolism and cell division and inhibits oxidative decarboxylation system in chondrocytes (Cao et al., 1995). In this study MON toxin significantly decreased cell viability of human chondrocytes cultured in vitro in dose- and time-dependent manners, suggesting its potential damage effect on chondrocytes. It is interesting that, in the first 24 h, the presence of MON caused an apparent increase in cell viability due likely to cell proliferation. We chose to add MON at 4.0 μg/ml to chondrocytes grown in BMG cartilage graft culture because this concentration led to less than 50% cell death after 5 d of culture and this change was consistent with chronic effect in KBD.
Selenium is an essential element for human, animals, and some species of microorganisms, for it is an integral part of the enzyme glutathione peroxidase (GSH-Px). It can protect organisms against oxidative damage by reducing lipoperoxides and hydrogen peroxide (Tan et al., 2002). It is found that there is a significantly lower selenium level in the grains in KBD areas when compared with KBD-free areas (Mo et al., 1997), suggesting a close relationship between the selenium and KBD occurrence (Tan et al., 2002; Stadtman, 1980; Li S.J. et al., 2006). According to a previous study (Cao et al., 1994), 0.1 μg/ml is an average concentration of selenium in human body. Therefore, this concentration of selenium was used as a potential protective factor to antagonize the chondrocyte damage induced by MON toxin.
Articular cartilage contains a large number of matrix macromolecules such as proteoglycan and type II collagen, which give it elastic and strong tensile properties to resist mechanical loading (Chan and Wu, 2006). Under normal conditions, there is a balance between the catabolism and anabolism in the extracellular matrix (ECM) in articular cartilage. However, some etiologies such as interleukin-1 (IL-1) may disturb this balance and accelerate the catabolism of ECM, leading to the loss of ECM components from the cartilage, severely impairing its functionality and causing pain and disability. Previous studies have shown that, similar to osteoarthritis, the continuous loss of aggrecan is one of the principal pathological processes in the development of KBD (Gelse et al., 2003; Aigner and Stöve, 2003). In this study, it was found that MON significantly inhibited the syntheses of aggrecan and type II collagen, suggesting the pro-catabolism effect of MON on the metabolism in articular cartilage. This is consistent with the previous study showing that MON toxin induced the degradation of proteoglycan and type II collagen in the articular cartilage of mini pig (Xiong et al., 1998).
Aggrecan is the major proteoglycan present in articular cartilages. It contains a core protein with many CS and keratan sulfate glycosaminoglycan chains attached to it (Knudson and Knudson, 2001). Because the negative charge of the glycosaminoglycans, aggrecan can absorb a large amount of water, and thereby endows the cartilage with its intrinsic capacity to bear load and resist compression. One of the central pathophysiological features contributing to cartilage erosion during the progress of a degenerative joint disease is the catabolism and loss of aggrecan (Roughley et al., 2006). In this study, the TB staining of the cartilage from the MON group was much weaker than that in the control and control+Se groups, suggesting the loss of the aggrecan induced by MON toxin. This was further confirmed by the expression of 3B3(−) epitopes in cartilages. 3B3(−) epitope is a disaccharide unit localizing at the non-reducing terminal of the CS chain (Hayes et al., 2008). Therefore it is a biomarker for the newly synthesized aggrecan in the articular cartilage. Previous studies have shown 3B3(−) epitopes are not present in the extracellular matrix of healthy adult articular cartilages and increase in CS chains in patients with osteoarthritis (OA) (Lin et al., 2004; Khan et al., 2008). In this study, the expression of 3B3(−) epitopes in the MON group was the weakest, indicating that MON can inhibit the anabolism of aggrecan in articular cartilages. Interestingly, addition of selenium significantly increased the expression of 3B3(−) in cartilages, suggesting a protective role of selenium against the pro-catabolism effect of MON on the metabolism of aggrecan.
To further address the mechanism how MON promotes the catabolism of aggrecan and type II collagen in the articular cartilage, the expression of ECM degrading enzymes such as MMP-1 and MMP-13 was investigated (Sumer et al., 2007). MMPs are a family of proteolytic enzymes that play an important role in ECM components such as type II collagen degradation. Previous studies have shown that in the relevant injury site of the articular cartilage, there were high levels of MMPs (Stanton and Fosang, 2002; Ra and Parks, 2007). Moreover, inhibition of MMP-13 can suppress type II collagen cleavage in articular cartilages (Tadashi et al., 2006). Therefore, MMP-1 and MMP-13 could be biomarkers for the cartilage degeneration. In this study, MON significantly increased the expression of MMP-13 and MMP-1, suggesting their pro-catabolism in the engineered cartilage. This may partially explain the decreased type II collagen levels in the toxin group as described above.
Beside collagen fibres, MMPs are also involved in the degradation of aggrecan, another important ECM component in the articular cartilage. Previous studies have shown that MMPs are involved in the C-terminal catabolism of aggrecan and generation of CS deficient aggrecan monomers. MMP-1 and MMP-13 were all capable of truncating aggrecan within at least two cleavage sites on the core protein (Little et al., 2002). During this process, several neo-epitopes are generated and thereby can be used as biomarkers for the aggrecan catabolism in the ECM. BC4 is a neo-epitope, which localizes at the C-terminal of the cleavage point within the interglobular domain (IGD) of aggrecan in cartilages (Caterson et al., 2000). In this study, it was found that MON significantly increased the expression of BC4 epitopes in the engineered cartilage, illustrating a significant increase in the aggrecan degradation induced by MON. In general, MON toxin prompts the expression of MMPs in chondrocytes, which may accelerate the degradation of type II collagen and aggrecan in the ECM, eventually inducing a degeneration of engineered cartilages.
One of the main functions of CD44 is to maintain the aggregation of aggrecan around the chondrocytes through the binding with hyaluronan. Therefore, CD44 acts as a bridge for the communication between the ECM and chondrocytes (Warren et al., 2002). In this study, MON toxin significantly increased CD44 expression in human articular cartilages. This may be a sign of the attempting of chondrocytes to repair the damage induced by MON toxin on aggrecan, which is consistent with the expression of 3B3(−) as described above. More importantly, this result is consistent with the increased CD44 expression in the articular cartilages of KBD patients (Cao et al., 2008), suggesting the potential relationship between MON and KBD occurrence.
In summary, we clearly demonstrated that MON toxin increased expression of matrix catabolic enzymes such as MMP-1 and MMP-13, but decreased syntheses of ECM components such as aggrecan and type II collage, thereby accelerating the catabolism of ECM in articular cartilages. This imbalance of ECM remodeling may change the mechanical feature of articular cartilages and induce the loss of their functions, eventually leading to cartilage degradation. The effects of MON toxin were partially antagonized by selenium, suggesting a potential protective effect of selenium during KBD development. An improved understanding of these biochemical and molecular biological changes may provide new insights into the mechanisms that regulate the pathology of chondrocytes in KBD.
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 30872187, 30471499, and 30170831), the Ministry of Education of China (No. Key 03152), and the Science Foundation of Shaanxi Province of China (No. 2004KW-20)
References
- 1.Aigner T, Stöve J. Collagens–major component of the physiological cartilage matrix, major target of cartilage degeneration, major tool in cartilage repair. Adv Drug Del Rev. 2003;55(12):1569–1593. doi: 10.1016/j.addr.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Cao JL, Mo XY, Wang CY. Effects of selenium and zinc on the growth metabolism of cultured chondrocytes. Chin J Endemiol. 1994;14(10):628–630. (in Chinese) [Google Scholar]
- 3.Cao JL, Zheng B, Zhang SY, Mo DX. The experimental study of moniliformin effects on the chondrocytes. Endemic Dis Bull. 1995;10(4):5–7. (in Chinese) [Google Scholar]
- 4.Cao JL, Zhang A, Yang B, Zhang ZT, Fu Q, Hughes CE, Caterson B. The effect of fungal moniliformin toxin and selenium supplementation on cartilage metabolism in vitro. Osteoarthritis and Cartilage. 2007;15(Suppl. 3):C108. doi: 10.1016/S1063-4584(07)61815-9. [DOI] [Google Scholar]
- 5.Cao JL, Li S, Shi Z, Yue Y, Sun J, Chen J, Fu Q, Hughes CE, Caterson B. Articular cartilage metabolism in patients with Kashin-Beck disease: an endemic osteoarthropathy in China. Osteoarthritis and Cartilage. 2008;16(6):680–688. doi: 10.1016/j.joca.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Caterson B, Mahmoodian F, Sorrel JM, Hardingham TE, Bayliss MT, Carney SL, Ratcliffe A, Muir H. Modulation of native chondroitin sulfate structure in tissue development and in disease. Cell Sci. 1990;97(3):411–417. doi: 10.1242/jcs.97.3.411. [DOI] [PubMed] [Google Scholar]
- 7.Caterson B, Carl RF, Clare EH, Chris BL. Mechanisms involved in cartilage proteoglycan catabolism. Matr Biol. 2000;19(4):333–344. doi: 10.1016/S0945-053X(00)00078-0. [DOI] [PubMed] [Google Scholar]
- 8.Cetin Y, Bullerman LB. Cytotoxicity of fusarium mycotoxins to mammalian cell cultures as determined by the MTT bioassay. Food Chem Toxicol. 2005;43(5):755–764. doi: 10.1016/j.fct.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Chan P, Wu C. A peptide fragment of type II collagen induces the OA phenotype. Matr Biol. 2006;25(Supp. 1):S44. doi: 10.1016/j.matbio.2006.08.123. [DOI] [Google Scholar]
- 10.Chen JH, Chu YL, Cao JL, Yang ZT, Guo X, Wang ZL. T-2 toxin induces apoptosis, and selenium partly blocks, T-2 toxin induced apoptosis in chondrocytes through modulation of the Bax/Bcl-2 ratio. Food Chem Toxicol. 2006;44(4):567–573. doi: 10.1016/j.fct.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Gelse K, Poschl E, Aigner T. Collagens–structure, function, and biosynthesis. Adv Drug Del Rev. 2003;55(12):1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Hayes AJ, Hughes CE, Caterson B. Antibodies and immunohistochemistry in extracellular matrix research. Methods. 2008;45(1):10–21. doi: 10.1016/j.ymeth.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Khan IM, Gilbert SJ, Caterson B, Sandell LJ, Archer CW. Oxidative stress induces expression of osteoarthritis markers procollagen IIA and 3B3(−) in adult bovine articular cartilage. Osteoarthritis and Cartilage. 2008;16(6):698–707. doi: 10.1016/j.joca.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Knudson CB, Knudson W. Cartilage proteoglycans. Sem Cell Dev Biol. 2001;12(2):69–78. doi: 10.1006/scdb.2000.0243. [DOI] [PubMed] [Google Scholar]
- 15.Li SJ, Yang LS, Li YH, Wang WL, Xirao RD. Relationship between the content of selenium in grains and the Kaschin-Beck disease in Tibet China. Chin J Endemiol. 2006;25(6):673–674. (in Chinese) [Google Scholar]
- 16.Li SY, Cao JL, Shi ZL, Chen JH, Zhang ZT, Hughes CE, Caterson B. Promotion of the articular cartilage proteoglycan degradation by T-2 toxin and selenium protective effect. J Zhejiang Univ-Sci B. 2008;9(1):22–33. doi: 10.1631/jzus.B071322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li XD, Li J, Balian G, Laurencin CT, Anderson DG. Demineralized bone matrix gelatinas scaffold for osteochondral tissue engineering. Biomaterials. 2006;27(11):2426–2433. doi: 10.1016/j.biomaterials.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 18.Lin PM, Chen CTC, Torzilli PA. Increased stromelysin-1 (MMP-3), proteoglycan degradation (3B3- and 7D4) and collagen damage in cyclically load-injured articular cartilage. Osteoarthritis and Cartilage. 2004;12(6):485–496. doi: 10.1016/j.joca.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Little CB, Hughes CE, Curtis CL, Janusz MJ, Bohne R, Wang-Weigand S, Taiwo YO, Mitchell PG, Otterness IG, Flannery GR, et al. Matrix metalloproteinases are involved in C-terminal and interglobular domain processing of cartilage aggrecan in late stage cartilage degradation. Matr Biol. 2002;21(3):271–288. doi: 10.1016/S0945-053X(02)00004-5. [DOI] [PubMed] [Google Scholar]
- 20.Mario Geysen H. Antigen-antibody interactions at the molecular level: adventures in peptide synthesis. J Immunol Today. 1985;6(12):364–369. doi: 10.1016/0167-5699(85)90096-9. [DOI] [PubMed] [Google Scholar]
- 21.Mo DX, Ding DX, Wang ZL, Zhang J, Bai C. Twenty-year research on selenium related to Kashin-Beck Disease. Xi’an Med Univ. 1997;9(1):79–89. (in Chinese) [Google Scholar]
- 22.Moreno RR. Comprehensive Handbook of Iodine. Brussels, Belgium: Nutritional, Biochemical, Pathological and Therapeutic Aspects; 2009. Iodine, Selenium Deficiency and Kashin-Beck Disease; pp. 685–700. [Google Scholar]
- 23.Nathanson MA, Robert Hilfer S, Searls RL. Formation of cartilage by non-chondrogenic cell types. Dev Biol. 1978;64(1):99–117. doi: 10.1016/0012-1606(78)90063-5. [DOI] [PubMed] [Google Scholar]
- 24.National KBD Surveillance Group. The monitoring report of KBD prevalence rate of the whole country in 2001. Chin J Endemiol. 2001;20(5):350–353. (in Chinese) [Google Scholar]
- 25.Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matr Biol. 2007;26(8):587–596. doi: 10.1016/j.matbio.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roughley P, Hoemann C, Rosiers ED, Mwale F, Antoniou J, Alini M. The potential of chitosan-based gels containing intervertebral disc cells for nucleus pulposus supplementation. Biomaterials. 2006;27(3):388–396. doi: 10.1016/j.biomaterials.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 27.Shi ZL, Cao JL, Chen JH, Li SY, Zhang ZT, Yang B, Peng SQ. Butenolide induced cytotoxicity by disturbing the prooxidant-antioxidant balance, and antioxidants partly quenchin human chondrocytes. Toxicol in Vitro. 2009;23(1):99–104. doi: 10.1016/j.tiv.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Slater RR, Michael T, Bayliss MT, Lachiewicz PF, Visco DM, Caterson B. Monoclonal antibodies that detect biochemical markers of arthritis in humans. Arthritis Rheum. 1995;38(5):655–659. doi: 10.1002/art.1780380513. [DOI] [PubMed] [Google Scholar]
- 29.Stadtman LC. Biological functions of selenium. Trends Biochem Sci. 1980;5(8):203–206. doi: 10.1016/S0968-0004(80)80008-9. [DOI] [Google Scholar]
- 30.Stanton H, Fosang AJ. Matrix metalloproteinases are active following guanidine hydrochloride extraction of cartilage: generation of DIPEN neoepitope during dialysis. Int Soc Matr Biol. 2002;21(5):425–428. doi: 10.1016/S0945-053X(02)00035-5. [DOI] [PubMed] [Google Scholar]
- 31.Sumer EU, Sondergaard BC, Rousseau JC, Delmas PD, Fosang AJ, Karsdal MA, Christiansen C, Qvist P. MMP and non-MMP-mediated release of aggrecan and its fragments from articular cartilage: a comparative study of three different aggrecan and glycosaminoglycan assays. Osteoarthritis and Cartilage. 2007;15(5):594–595. doi: 10.1016/j.joca.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Tadashi Y, Elena T, Kunitaka O, Peter JR, William W, Aisha M, Mirela I, Isabelle P, Robin PA. Peptides of type II collagen can induce the cleavage of type II collagen and aggrecan in articular cartilage. Int Soc Matr Biol. 2006;25:419–429. doi: 10.1016/j.matbio.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Tan JA, Zhu WY, Wang WY, Li RB, Hou SF, Wang DH, Yang LS. Selenium in soil and endemic diseases in China. Sci Total Environ. 2002;284(1-3):227–235. doi: 10.1016/S0048-9697(01)00889-0. [DOI] [PubMed] [Google Scholar]
- 34.Warren K, Geraldine C, Cheryl BK. CD44-mediated uptake and degradation of hyaluronan. Int Soc Matr Biol. 2002;21:15–23. doi: 10.1016/s0945-053x(01)00186-x. [DOI] [PubMed] [Google Scholar]
- 35.Xiong YM, Mo DX, Li SC, Guo X, Zhang SY, Wang ZL, Bi HY, Xu JR. The experimental study of moniliformin and selenium effects on articular cartilage of Chinese experimental mini pig. Endemic Dis Bull. 1998;3(2):1–3. (in Chinese) [Google Scholar]
- 36.Yang JB. Mycotoxins and human diseases. Chin J Endemiol. 2002;l21(4):314–317. (in Chinese) [Google Scholar]
- 37.Zhao XJ. Research progress moniliformin. Progress in Veterinary Medicine. 2002;23(4):19–22. [Google Scholar]



