Abstract
SPAK/STK39 is a mammalian protein kinase involved in the regulation of inorganic ion transport mechanisms known to modulate GABAergic neurotransmission in the both central and the peripheral nervous systems. We have previously shown that disruption of the gene encoding SPAK by homologous recombination in mouse embryonic stem cells results in viable mice that lack expression of the kinase [16]. With the exception of reduced fertility, these mice do not exhibit an overt adverse phenotype. In the present study, we examine the neurological phenotype of these mice by subjecting them to an array of behavioral tests. We show that SPAK knockout mice displayed a higher nociceptive threshold than their wild-type counterparts on the hot plate and tail flick assays. SPAK knockout mice also exhibited a strong locomotor phenotype evidenced by significant deficits on the rotarod and decreased activity in open field tests. In contrast, balance and proprioception was not affected. Finally, they demonstrated an increased anxiety-like phenotype, spending significantly longer periods of time in the dark area of the light/dark box and increased thigmotaxis in the open field chamber. These results suggest that the kinase plays an important role in CNS function, consistent with SPAK regulating ion transport mechanisms directly involved in inhibitory neurotransmission.
Keywords: dorsal root ganglion, spinal cord, Na-K-2Cl cotransporters, K-Cl cotransporter
Introduction
Sensory afferent fiber neurons, which have their cell bodies located in dorsal root ganglia, abundantly express NKCC1, a Na-K-2Cl cotransporter which functions as a secondary active transport mechanism and accumulates Cl− above equilibrium [1, 41]. Upon GABA release, afferent fiber terminals depolarize, leading to inhibition of incoming action potentials. How this primary afferent depolarization results in synaptic inhibition is still not completely understood. However, loss of NKCC1 results in a redistribution of Cl− and absence of depolarizing GABA responses [41]. As a result, NKCC1 knockout mice exhibit increased response latency to nociceptive stimuli [41; 22]. In agreement with a role for the cotransporters in mediating pain signals, NKCC1 inhibitors have been shown to have anti-nociceptive activity [17; 34]. A recent study demonstrated that peripheral nerve injury (axonotomy) results in increased NKCC1 activity [33]. This enhanced activity was not due to increase NKCC1 expression, but rather due to increased phosphorylation of the cotransporter.
Both NKCC1 and KCC2, a K-Cl cotransporter that actively extrudes Cl− from central neurons, are expressed in spinal cord [26]. Spinal cord dorsal horn neurons maintain relatively high level of KCC2 [24] and, as a consequence, have low [Cl−]i. Postsynaptic inhibition of spinal cord dorsal horn neurons via KCC2 may be critical for controlling the flow of sensory information from the periphery through the spinal cord to the brain. Indeed, several studies have shown that decreased KCC2 expression in the spinal cord is associated with increased pain perception [3; 23; 28; 20; 25; 47].
SPAK (Ste20-related Proline Alanine rich Kinase) and OSR1 (Oxidative-Stress Response 1) are mammalian protein kinases involved in the regulation of inorganic ion transport mechanisms known to modulate GABAergic neurotransmission in the both the central and peripheral nervous systems. In previous work, we identified SPAK and OSR1 as regulators of the Na-K-2Cl cotransporters NKCC1 [32; 14; 13]. The kinases share high homology in both their catalytic and regulatory domains. Although they belong to a large group of mammalian kinases related to the yeast Ste20p protein, their regulatory domain is unique, indicating a unique function [9; 6]. We have recently examined the expression of the two Ste20 kinases in dorsal root ganglion neurons and found that both kinases are expressed in similar amounts and that each kinase participates equally in the regulation of the cotransporter [16]. As SPAK knockout mice are viable, we were able to analyze NKCC1 activity in the absence of SPAK function and observed that the cotransporter function was reduced by half. A similar study of OSR1 function in native DRG neurons was not possible as the OSR1 knockout mouse is embryonic lethal. SPAK is also expressed in gray and white matter of the spinal cord and brain [31]. SPAK not only controls NKCC1 function, but also affects KCC2 function in a reciprocal fashion [14]. The regulatory and expression pattern of SPAK suggests that it influences intracellular Cl− concentration and thus the amplitude/direction of the GABA response. The present study is the first to characterize the impact of SPAK deletion on general behavioral function.
Material and Methods
Animals
Cohorts of 10 3-4 month old SPAK knockout mice [16] and 11 wild-type mice of identical background were subjected to a battery of behavioral tests. The generation of SPAK knockout mice has been described in detail previously [16]. Briefly, the SPAK gene was disrupted by duplicating exon 6 and inserting tyrosinase, neomycin resistance, and 5′ hprt genes between the two exons. Animals carrying the mutant allele were identified by PCR genotyping of tail DNA. After two matings with C57BL6 mice, heterozygous mice were crossed to obtain homozygous animals. As no PCR strategy could differentiate heterozygous from homozygous animals, we mated each offspring with C57BL6 animals and assessed the number of mutant and wild-type animals. Animals generating 100% heterozygous offsprings were mated together and a homozygote line was thereby created. Verification of homozygosity was done by Western blot analysis. Wild-type mice of the same background (88%C57BL6, 12%129SvEvTac) were generated by breeding the wild-type offspring of the SPAK heterozygous crosses. The animals were transferred to the animal facility within the Vanderbilt Neurobehavioral Laboratory 2 weeks prior to testing and housed in groups of two-four per cage with the exception of two singly-housed wild-type males. Food and water were available ad libitum. The colony room was maintained on a 12:12 hr light/dark cycle, with lights on at 6 A.M. All procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee. Before each test, the animals were transferred to each behavioral unit and allowed to habituate for at least one hour. Behavioral studies were carried out in the light phase between 1200 and 1600 h. The order of tests were tail flick assay, hot plate assay, accelerating rotarod, wire hang, open field, light-dark box, acoustic startle response, forced swim, tail suspension, and righting reflex. Twelve mice (6 knockouts and 6 wild-types) were tested on the entire behavioral battery. In addition, 4 knockouts and 5 wild-types mice from independent breeding were tested for nociception, open field, locomotor, and anxiety tests.
Tail flick assay
The mice were gently restrained by hand with a towel and the distal ~2 cm of the tail was inserted into a thermostatically controlled water bath. The latency to withdraw or flick the tail was recorded using a stop watch. First, the mice were tested at 38°C. Then, the tests performed 3 times at two different temperatures (46°C and 52°C) with at least one hour interval between trials.
Hot plate assay
SPAK KO and wild-type mice were individually placed on a hot plate maintained at 52°C (Hotplate Analgesia Meter, Columbus Instruments, Columbus, OH). A plastic cylinder 15 cm in diameter and 20 cm high confined the mouse to the surface of the hot plate. The time necessary for the mouse to respond to the thermal stimulus, i.e., hind paw fluttering, licking, or jumping was measured with a stop watch. After the first response or the cut-off time of 15 seconds, mice were immediately removed from the hot plate and returned to the home cage. The assay was repeated 3 times with one hour interval.
Beam test
Each mouse was placed on the lower end of a round plastic beam of 2 cm outer diameter and 80 cm length (covered with synthetic material). The beam was placed at a 30 degree angle to increase the motivation of the mouse to move upwards towards the platform. The time taken by the mouse to travel to the platform was recorded. Limbs during beam locomotion were closely examined to identify slippage events.
Accelerating rotarod
A neuromotor coordination task was performed using an accelerating rotating cylinder (model 47600: Ugo Basile, S.R. Biological Research Apparatus, Comerio, Italy). The cylinder was 3 cm in diameter and was covered with scored plastic. Mice were confined to a 4 cm long section of the cylinder by gray Plexiglas dividers. Four mice were placed on the cylinder at once. The rotation rate of the cylinder increased over a 6 min period from 4 to 40 rpm. The latency of each mouse to fall off the rotating cylinder was automatically recorded by the device. Mice that remained on the rotarod during the 360 sec trial period were removed and given a score of 360 sec. The test was performed three trials daily for 3 consecutive days, with an intertrial interval of at least 30 min.
Wire hang
Neuromuscular strength was tested by placing the mice on a wire suspended 50 cm above the surface. The latency for the mouse to fall onto the bedding was recorded with a 60 sec cut-off time.
Open field
Exploratory and locomotor activities were tested using an open-field activity chamber surrounded by a Plexglas enclosure within sound-attenuating cubicles (ENV-022MD-027; Med Associates, Inc., St. Albans, VT). The mice were placed in the center of the open-field and their activity was monitored for 60 min. The test was performed at ambient room temperature (25°C), moderate light (60 lux), and background noise (80 dB). Distance traveled and time spend in the periphery (thigmotaxis) of the chamber were quantified by the number of beam crossings.
Light-dark box exploration
The relative anxiety status of each mouse was tested using the same open-field activity chamber containing a dark-box insert. The dark box insert is opaque to visible light and designed to cover half the area of the open field activity chamber. Mice were released at the center of the light compartment, and allowed to freely explore for a period of 10 min. The test was performed at ambient room temperature (25°C), moderate light (60 lux) and background white noise (80 dB). Travel distance, line-crossing behavior, and time spend in each section of the chamber were quantified by software processing output from the device.
Acoustic startle response
Hearing was tested in both wild-type and knockout mice using the acoustic startle response (Med Associates Inc. model: MALAGUARD S.A.C. ENV-021SX, serial number: 229). Mice were gently restrained in transparent round acrylic animal holders with a 3.2 cm diameter (Med Associates, Inc. model: ENV-263A). A train of 100 single acoustic stimuli of 120 dB in 15 sec intervals, was produced and startle movements were recorded and analyzed using Startle-Reflex software (version 5).
Righting tests
Two types of righting tests were performed: the air righting reflex and the contact righting test. For the air righting reflex, animals were held supine and dropped from a height of approximately 45 cm onto a soft bedding surface. Whether or not the animal landed on their four feet was recorded. For the contact righting test, mice were gently restrained in a transparent 3.2 cm diameter round acrylic holder (Med Associates, Inc. model: ENV-263A). The tube was then quickly rotated 180 degrees to place the mouse upside down and the time taken by the mouse to right itself was recorded.
Tail-suspension
The tail suspension test was performed using a Square Test Cubicle equipped with a strain gauge (Med Associates, Inc. Tail suspension amplifier, ENV-505TS, serial number: 425). Mice were suspended by the tail with tape to the strain gauge and were positioned such that the base of their tail is parallel with the bar. Each mouse was given one trial of 6 minutes. The device constantly recorded movement and immobility was defined as movements below the preset threshold of 3. The total duration of immobility during the 6 min trial was calculated. None of the mice attempted to escape by climbing onto their tails.
Forced swim
The force swim test was conducted according to the method of Porsolt [36]. Mice were plunged into vertical Plexiglas cylinders (height: 30 cm, diameter: 18 cm) containing 15 cm of 28°C water. They were unable to reach the bottom with their feet or tail. The water surface was lower than the top of the cylinder to prevent animals from jumping out. Mice were forced to swim for a period of 6 minutes, and the test was recorded by video tape. After the swim period, the mice were moved out immediately and transferred into a warm enclosure padded with dry paper towel. The mice were kept in the paper towel for at least 15 min to allow them to dry before being returned to their home cages. Mice were deemed immobile when they floated passively in the water, their head just above the surface; and with only minimal movements to maintain themselves above water.
Statistical analysis
All data were analyzed using two-tailed unpaired T-test (GraphPad Prism 3.0). Data were noted to be significant at P < 0.05 and highly significant at P < 0.001.
Results
SPAK knockout mice were generated by homologous recombination in embryonic stem cells, as described in an earlier publication [16]. The mice did not display any overt adverse phenotype, with the exception of a reduced breeding efficiency. Indeed, only 57% of SPAK knockout matings (19 out of 33) resulted in progeny, versus 90.5% (19 out of 21) for wild-type mice. Furthermore, the number of mice per litter was significantly lower for knockouts versus wild-types: 4.5 ± 1.9 (n = 19 litters) versus 7.0 ± 1.7 (n = 17 litters), t35, P = 0.0002. Also on average, knockout mice delivered 18.7 ± 4.3 (n =19) days past the projected due date based on the mating date, compared to 5.2 ± 1.2 (n = 19) days for wild-type, a difference that reached statistical significance (t37, P = 0.0042). We have not yet determined the cause of this phenotype, nor did we assess whether the deficit was coming from males, females or both sexes. For the general neurological screen, a subset of tests from the Irwin Gross Neurological Screen [19] was performed. First, physical characteristics such as coat color and consistency, skin color, appearance of fur, presence of whiskers and wounds were examined. This examination revealed an unsmooth coat for the SPAK knockout animals, giving them a larger appearance. Interestingly, this was mostly observed when the animals were active on the rotarod cylinder. Growth curves, however, showed no difference between knockout and wild-type mice. At the age of 5 months, mice from the two genotypes presented at identical weights (25.94 ± 1.19 g (n = 6 wild-types) versus 26.03 ± 2.46 g (n = 6 knockouts)).The mice were placed in an empty cage for 3 min, and responses to the novel environment, such as wild running, excessive grooming or freezing as well as abnormal gait or postures, piloerection, palpebral closure, tail elevation, urination, and defecation were scored. No differences in these general behaviors were observed between the SPAK knockout mice and their wild-type counterparts.
We tested acute heat-evoked pain sensitivity in wild-type and SPAK knockout mice. The latency to respond to noxious heat was tested using the tail flick and hot plate assays to assess spinal and supraspinal nociception, respectively. The tail flick response was elicited by immersing ~2 cm of the tail in water at one control, non-noxious temperature (38°C) and two noxious temperatures (46°C and 52°C). Figure 1A shows increased latency of the knockout responses at 52°C (t20, P < 0.001). A similar increased latency to respond to 52°C heat was demonstrated in knockouts animals using the hot plate assay (see Figure 1B). The results from these tests demonstrate a difference in pain sensitivity in SPAK knockout mice.
Figure 1.
Nociception tests in SPAK wild-type and knockout mice. A, Tail flick response was measured at three temperatures. The cut-off times were 30 seconds for 38°C and 15 seconds at 46°C and 52°C to prevent injury. There were no statistical differences between the two genotypes at either 38°C (t20, P = 0.71) or 46°C (t20, P = 0.11). Note the significant difference in response to 52°C (t20, P = 0.001). B, Hot plate assay was performed with the same mice. The assay was first conducted at 38°C with all mice reaching the cut-off time of 15 seconds without any response (data not shown). Note the significant difference between control and SPAK knockout mice at 52°C (t20, P = 0.001). Bars represent means ± SEM (n = 11 wild-types, 10 knockouts).
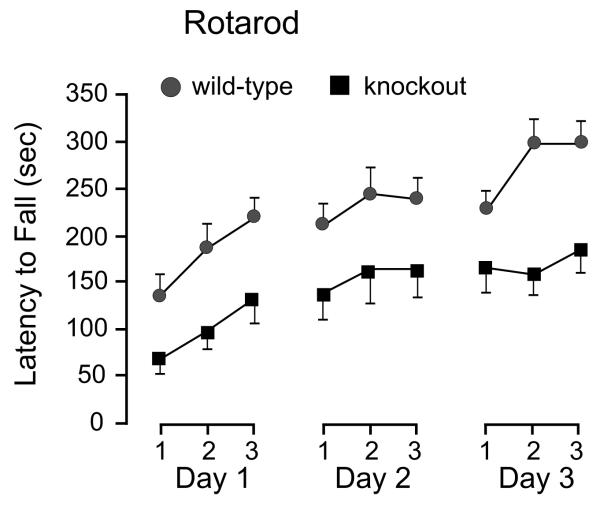
To evaluate motor coordination and balance, we tested wild-type and SPAK knockouts on an accelerating rotarod. To assess the learning component of this motor task, we subjected the mice for three trials a day for 3 consecutive days. There was a significant difference between the abilities of wild-type and knockout mice to maintain their balance on the rotating cylinder (Figure 2). Although impaired compared to wild-types (t20, P < 0.05 for each day), performance of the SPAK knockout mice improved significantly over the nine testing sessions, increasing from 69 ± 18 s on the first trial of day one to 185 ± 26 s on the third trial of the third day (t20, P = 0.001). This demonstrates that the SPAK knockout mice retain an ability to learn motor skills in spite of their reduced motor ability.
Figure 2.
Motor coordination and balance through the rotarod test. Control (n = 11) and SPAK knockouts (n = 10) were placed on a rotating rod of ~3 cm in diameter, and the latency to fall was measured. The test was performed three times a day for three consecutive days. Note the differences in latency between the two genotypes as well as the improvement in locomotor skills for both genotypes over repeated sessions.
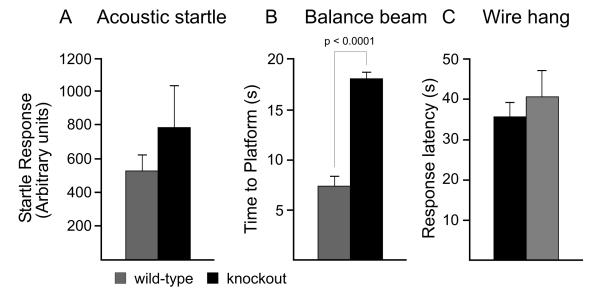
There are many possible reasons that mice can exhibit deficits in the rotarod test. For example, it could stem from an inner ear deficit, as balance relies on the proper function of the vestibular labyrinth. To assess whether the SPAK knockout mice exhibit an inner ear phenotype, we used an acoustic startle test (Figure 3A). Both SPAK wild-type and knockout mice responded similarly to a moderately loud (120 dB) acoustic stimulus, indicating their ability to hear properly.
Figure 3.
Acoustic startle response (audition), beam walk and wire hang tests. A, For determination of possible auditory changes, each mouse was restrained and startle responses to 120 dB sounds spaced every 15 sec were recorded. Data were not statistically different (P = 0.7). B, For the beam task, each mouse was placed on the lower end of an 80 cm long round plastic beam with a diameter of 2 cm. The mouse was allowed to walk to the upper platform. The time to reach the platform was measured and walking pattern was observed. Note that the SPAK-KO mice displayed a significantly longer latency to reach the platform (P < 0.0001). C, The wire hang test was performed by placing the mice on a wire suspended 50 cm above bedding. The latency for the mouse to fall was recorded with a 60 sec cut-off time. No difference was recorded between the two genotypes (t11, P = 0.31). Bars represent means ± SEM (n = 6 mice per genotype).
Balance was also assessed using a suspended beam attached to a platform. The mouses’ability to keep its balance and walk properly to the platform was recorded. As seen in Figure 3B, SPAK knockout mice spent a significantly longer amount of time on the beam (t11, P < 0.0001). Since they were, however, able to maintain their balance and were able to reach the platform without falls, limb slippages, or overtly uncoordinated movements, we concluded that the knockout mice did not display a balance phenotype. Thus, a lack of balance is an unlikely explanation for their decreased ability on the rotarod. The extended amount of time that the SPAK knockout mice spent on the beam can be explained by the open field data (see below).
Another possible explanation for the impaired locomotor activity is muscle weakness. To determine whether the SPAK knockout mice display a neuromuscular phenotype, we performed a wire hang test. Mice were allowed to grasp on an elevated wire with their front paws and were observed for a period of 60 seconds maximum. The latency to release/fall, a measure of muscle strength, was recorded. As seen in Figure 3C, there was no difference in latency to release between the two genotypes.
Locomotor activity was recorded in an open field chamber equipped with infrared beams. Each wild-type and SPAK knockout mouse were separately monitored for distance traveled, number of jumps and rearings over a one hour period. As shown in Figure 4, SPAK knockout mice traveled significantly less than their wild-type counterparts (t20, P < 0.01). Vertical activity (jumping and rearing) were also comparatively diminished in the SPAK knockouts, relative to controls.
Figure 4.
Open-field test was performed to assess locomotor activity in wild-type and SPAK knockout mice. Distance traveled (cm/h) and number of jumps and rearings (counts/h) were quantified by the number of beam crossings. Note the significant difference in these 3 parameters. Bars represent means ± SEM (n = 11 wild-types, 10 knockouts).
As the locomotor phenotype could not be explained by balance or muscle weakness deficits, we tested proprioception by performing righting tests. Indeed, righting reflexes are a measure of proprioception (neuromuscular and sensory integration) and vestibular function [29; 18; 43]. We did not find any difference between the two genotypes in either contact or air righting. Indeed, in the air righting test, both knockout and wild-type animals landed on their four feet when dropped from a 45-cm height. In the contact righting, both genotypes were able to right themselves immediately after rotation of the tube.
To assess whether the absence of the kinase affected behaviors that have a CNS origin, we tested the mice for anxiety- and depression-like behaviors. Although exploratory activity is the primary parameter that is evaluated in the open field test, the data also indicated anxiety-like behavior when comparing time spent in close proximity to the walls versus the center of the test area. Indeed, the open space in the center of the open field box is more anxiogenic and thus more aversive than areas near the walls. We observed that the SPAK knockout animals spent significantly more time close to the surrounding walls rather than the center area (t20, P < 0.001), whereas the wild-type mice spent similar amounts of time in both areas (Figure 5A). In order to confirm that this behavior results from increased anxiety, we also performed the light-dark box test (Figure 5B). In this test, the ratio of time spent in the dark compartment versus the time spent in the light compartment is an index of anxiety [4]. Anxiety is considered to be high if this ratio is high. Statistical analysis revealed that the SPAK knockout mice exhibited more anxiety than their wild-type counterparts (t20, P < 0.01).
Figure 5.
Increased anxiety in SPAK knockout mice. A, Light/dark box. The ratio of time spent in the light (anxiogenic) and dark (anxiolytic) areas was decreased in the knockout (t20, P < 0.05). B, Open field measurements of time spent in the center versus periphery of the chamber. There was no difference observed for wild-type mice (t20, P > 0.05), but a significant difference observed with the knockout mice (t20, P < 0.001). Bars represent means ± SEM (n = 11 wild-types, 10 knockouts).
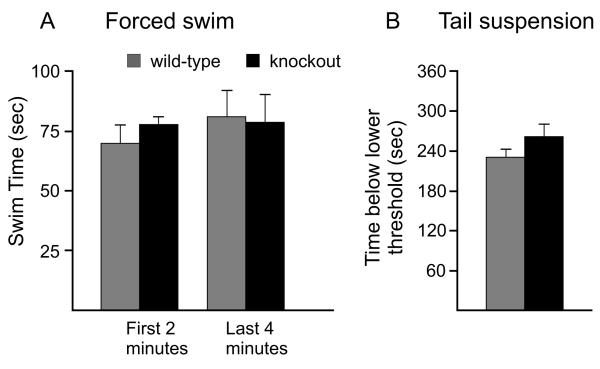
We first used the forced swim test [36; 30] as a screen for depression. In this test, time of immobility is an index of depression. Wild-type and SPAK knockout mice were placed in the water tank for a period of 6 min. The analysis was performed for the first 2 and the last 4 min. Data presented in Figure 6A show no significant difference in swimming time, and therefore immobility, between knockout and wild-type mice. To confirm these data, we also subjected mice to the tail suspension test, which also measures depression. In contrast to the forced swim test, the tail suspension test avoids any possible confounds induced by hypothermic exposure. Furthermore, the tail suspension test does not rely on proper motor coordination. As shown in Figure 6B, the tail suspension data were also not significantly different between the two genotypes. Our data therefore indicate that the SPAK knockout mice do not display behaviors that would indicate a depression-like phenotype.
Figure 6.
Two tests are used to measure depression-like behaviors in wild-type and SPAK knockout mice. A, Forced swim test. Mice were placed in a water tank for 6 min and their time spent swimming versus immobility was assessed. Data were analyzed for the first two minutes (no difference, t11 P = 0.13) and the last 4 minutes (no difference, t11, P = 0.94). B, Tail suspension test. Mice were hung by the tail for 6 min on a devise sensitive to movement. The movement thresholds were set at 3 (minimum) and 50 (maximum). There was no difference between genotypes for the amount of time spent below the minimal threshold (t11, P = 0.24). Bars represent means ± SEM (n = 6 mice per genotype).
Discussion
Work from this and other laboratories has shown that the kinases SPAK and OSR1 modulate the activity of electroneutral cation-chloride cotransporters such as NKCC1 [11; 2; 14], NKCC2 [38; 35], NCC [37], and KCC2 [14; 15]. They affect transport activity by binding to specific amino acid motifs located in cytosolic tails of the cotransporters [32; 13; 44] and phosphorylating them [12; 27; 45; 37]. SPAK and OSR1 are two closely related Ste20p-like kinases [5; 6], with SPAK originating from gene duplication during late vertebrate evolution [9]. It is interesting that the knockout of the older gene, OSR1, is embryonic lethal, whereas the knockout of the more recent gene, SPAK, is viable with no overt phenotype [16]. These observations indicate that these two functionally similar kinases are not identical.
The present study demonstrates that SPAK knockout mice exhibit several neurobehavioral phenotypes: a higher nociceptive threshold, decreased locomotor activity, and increased anxiety. The nociceptive phenotype is evident by the increased latency to respond to heat-evoked noxious stimuli in both the tail flick and hot plate assays (Figure 3). The tail flick response measures a spinal reflex involving cutaneous nociceptors, afferent conduction, central conduction, and efferent conduction to muscles. This reflex is however modulated by supraspinal structures, which have excitatory and inhibitory effects on the activity of dorsal horn neurons [21]. One location where the kinase could play a significant role is in dorsal root ganglion neurons. Indeed, an early SPAK paper identified high expression levels of the kinase in these sensory neurons [24]. Furthermore, these neurons highly express Na-K-2Cl cotransporters, which through its role in accumulating intracellular Cl−, promote GABA-mediated primary afferent depolarization and presynaptic inhibition. Interestingly, the SPAK knockout exhibits a phenotype similar to the NKCC1 knockout, i.e., delayed response to nociceptive stimuli. However, we demonstrated in a previous study that NKCC1 in DRG neurons is regulated equally by SPAK and OSR1 and that NKCC1 activity is about half that of wild-type DRG neurons in the SPAK knockout [16]. Moreover, reduced NKCC1 function in NKCC1 heterozygote mice did not result in a nociceptive phenotype [41; 22]. Thus, in light of the behavioral data of the NKCC1 heterozygote mouse, it is difficult to attribute the nociceptive phenotype on ~50% loss of NKCC1 function or a presynaptic deficit. Besides the dorsal root ganglion, SPAK is also expressed in spinal cord and brain. In spinal cord, SPAK likely regulates the K-Cl cotransporter KCC2. In this case, the absence of SPAK expression and function would increase cotransporter function [14]. This hypothesis is supported by the observation that decreased KCC2 expression is associated with increased pain perception [3; 23; 28; 20; 25; 47]. Further work involving crosses between the SPAK knockout and KCC2 hetereozygote mice, or intrathecal injection of novel KCC2 specific inhibitors [7], could resolve this issue. We indeed expect that decreasing KCC2 expression by inactivation one allele or decreasing KCC2 function through the use of inhibitors would reverse the phenotype associated with the absence of SPAK
SPAK knockout mice also exhibit a significant locomotor phenotype. Indeed, both deficits on the accelerating rotarod and decreased open field locomotor activity were observed in the SPAK knockout mice. Moreover, the beam test also indicated a significant decrease in the activity of the knockout mice. Because inner ear balance, muscle strength, and proprioception were all eliminated as possible causes of the locomotor deficits, it is likely that the deficit involves the central nervous system. As mentioned above, a likely target of SPAK in the CNS is KCC2. As this cotransporter facilitates GABAA-mediated hyperpolarization [39; 48], increased KCC2 function may lead to sedative-like effects, and the decreased locomotor activity might be similar to the one induced by non-selective benzodiazepines [46]. Decreased exploratory activity of SPAK knockouts in the open field chamber may indicate a possible deficiency in motivated or goal-directed behaviors [40]. Although the decreased latencies on the accelerating rotarod may point to coordination or balance problems that could involve sensory deficits due to SPAK deletion, there remains the possibility that motor coordination may not be affected per se, but poor rotarod performance could be the result of general hypoactivity and/or motivation. Decreased exploratory behavior could also stem from anxiety.
Some of the phenotypes associated with the absence of the kinase in the knockout mouse might not be related to effects on cation-chloride cotransporters, but to effects on other substrates of the kinase. In a systematic search of the mouse proteome, we identified 137 mouse proteins that contain SPAK binding sequences [8]. Therefore, these proteins are candidate targets for SPAK phosphorylation and regulation. Furthermore, studies in C. elegans have demonstrated the regulation of the CLH3 chloride channel by GCK-3, the worm orthologue of OSR1 [10]. Because this Cl− channel is the worm equivalent of the mammalian ClC2, it is possible that CLC2 is regulated by SPAK and this regulation is affected in the SPAK knockout mouse.
Another phenotype observed in the SPAK knockout is increased anxiety. Indeed, as compared to wild-type mice, the knockout exhibited increased anxiety-like behaviors by spending more time in the dark versus light section of a box and increasing thigmotaxis in the open field chamber. Anxiety-like behaviors are complex and the relationship between SPAK and this phenotype is unknown. Benzodiazepines constitute a group of drugs that is particularly effective in treating generalized anxiety disorders. It is therefore intriguing if the absence of the kinase results in increased KCC2 function and GABAA-mediated hyperpolarization, which leads to anxiety, especially since anxiety-like behavior has been demonstrated in KCC2 hypomorphic mice [42]. The SPAK knockout did not show any signs of behavioral despair (or depression) in either the forced swim or tail suspension tests. This mood disorder is attributed to serotonin and norepinephrine pathways that originate from brain stem nuclei (raphe and locus coeruleus, respectively) and project to mid brain and forebrain structures.
In summary, neurobehavioral analyses of the SPAK knockout mouse demonstrate nociceptive, locomotor, and anxiety-like phenotypes that could stem from increased function of the neuronal-specific K-Cl cotransporters, KCC2. These intriguing data indicate the need for further studies of the role of SPAK in modulating KCC2 function in both brain and spinal cord.
Acknowledgements
Experiments and data analysis were performed in part through the use of the Murine Neurobehavior Core lab at the Vanderbilt University Medical Center. We would like to thank Dr. John Allison for his technical advice. This work was supported by NIH grant GM74771 to ED.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Alvarez-Leefmans FJ, Gamiño SM, Giraldez F, Nogueron I. Intracellular chloride regulation in amphibian dorsal root ganglion neurons studied with ion-selective microelectrodes. J Physiol (Lond) 1988;406:225–246. doi: 10.1113/jphysiol.1988.sp017378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Anselmo AN, Earnest S, Chen W, Juang YC, Kim SC, Zhao Y, et al. WNK1 and OSR1 regulate the Na+, K+, 2Cl− cotransporter in HeLa cells. Proc Natl Acad Sci USA. 2006;103:10883–10888. doi: 10.1073/pnas.0604607103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, et al. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- [4].Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- [5].Dan I, Watanabe NM, Kusumi A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 2001;11:220–230. doi: 10.1016/s0962-8924(01)01980-8. [DOI] [PubMed] [Google Scholar]
- [6].Delpire E. The Mammalian family of Sterile20p-like protein kinases Pflügers. Arch Eur J Physiol. 2009;458:953–967. doi: 10.1007/s00424-009-0674-y. [DOI] [PubMed] [Google Scholar]
- [7].Delpire E, Days E, Mi D, Lewis M, Kim K, Lidnsley C, et al. Small molecule screen identifies inhibitors of the neuronal K-Cl cotransporter KCC2. Proc Natl Acad Sci U S A. 2009;106:5383–5388. doi: 10.1073/pnas.0812756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Delpire E, Gagnon KB. Genome-wide analysis of SPAK/OSR1 binding motifs. Physiol Genomics. 2007;28:223–231. doi: 10.1152/physiolgenomics.00173.2006. [DOI] [PubMed] [Google Scholar]
- [9].Delpire E, Gagnon KB. SPAK and OSR1: STE20 kinases involved in the regulation of ion homoeostasis and volume control in mammalian cells. Biochem J. 2008;409:321–331. doi: 10.1042/BJ20071324. [DOI] [PubMed] [Google Scholar]
- [10].Denton J, Nehrke K, Yin X, Morrison R, Strange K. GCK-3, a newly identified Ste20 kinase, binds to and regulates the activity of a cell cycle-dependent ClC anion channel. J Gen Physiol. 2005;125:113–125. doi: 10.1085/jgp.200409215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dowd BF, Forbush B. PASK (Proline-Alanine-rich STE20-related Kinase), a Regulatory Kinase of the Na-K-Cl Cotransporter (NKCC1) J Biol Chem. 2003;278:27347–27353. doi: 10.1074/jbc.M301899200. [DOI] [PubMed] [Google Scholar]
- [12].Gagnon KB, England R, Delpire E. Characterization of SPAK and OSR1, regulatory kinases of the Na-K-2Cl cotransporter. Mol Cell Biol. 2006;26:689–698. doi: 10.1128/MCB.26.2.689-698.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gagnon KB, England R, Delpire E. A single binding motif is required for SPAK activation of the Na-K-2Cl cotransporter. Cell Physiol Biochem. 2007;20:131–142. doi: 10.1159/000104161. [DOI] [PubMed] [Google Scholar]
- [14].Gagnon KB, England R, Delpire E. Volume sensitivity of cation-chloride cotransporters is modulated by the interaction of two kinases: SPAK and WNK4. Am J Physiol Cell Physiol. 2006;290:C134–C142. doi: 10.1152/ajpcell.00037.2005. [DOI] [PubMed] [Google Scholar]
- [15].Garzón-Muvdi T, Pacheco-Alvarez D, Gagnon KB, Vázquez N, Ponce-Coria J, Moreno E, et al. WNK4 kinase is a negative regulator of K+-Cl− cotransporters. Am J Physiol Renal Physiol. 2007;292:F1197–F1207. doi: 10.1152/ajprenal.00335.2006. [DOI] [PubMed] [Google Scholar]
- [16].Geng Y, Hoke A, Delpire E. The Ste20 kinases SPAK and OSR1 regulate NKCC1 function in sensory neurons. J Biol Chem. 2009;284:14020–14028. doi: 10.1074/jbc.M900142200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Granados-Soto V, Arguelles CF, Alvarez-Leefmans FJ. Peripheral and central antinociceptive action of Na—K—2Clcotransporter blockers on formalin-induced nociception in rats. Pain. 2005;114:231–238. doi: 10.1016/j.pain.2004.12.023. [DOI] [PubMed] [Google Scholar]
- [18].Heyser CJ. Assessment of developmental milestones in rodents. Curr Protoc Neurosci. 2004 doi: 10.1002/0471142301.ns0818s25. Chaper 8: Unit 8.18. [DOI] [PubMed] [Google Scholar]
- [19].Irwin S. Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia. 1968;13:222–257. doi: 10.1007/BF00401402. [DOI] [PubMed] [Google Scholar]
- [20].Jolivalt CG, Lee CA, Ramos KM, Calcutt NA. Allodynia and hyperalgesia in diabetic rats are mediated by GABA and depletion of spinal potassium-chloride co-transporters. Pain. 2008;140:48–57. doi: 10.1016/j.pain.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Karl T, Pabst R, von Horsten S. Behavioral phenotyping of mice in pharmacological and toxicological research. Exp Toxicol Pathol. 2003;55:69–83. doi: 10.1078/0940-2993-00301. [DOI] [PubMed] [Google Scholar]
- [22].Laird JM, Garcia-Nicas E, Delpire EJ, Cervero F. Presynaptic inhibition and spinal pain processing in mice: a possible role of the NKCC1 cation-chloride co-transporter in hyperalgesia. Neurosci Lett. 2004;361:200–203. doi: 10.1016/j.neulet.2003.12.015. [DOI] [PubMed] [Google Scholar]
- [23].Mantyh PW, Hunt SP. Setting the tone: superficial dorsal horn projection neurons regulate pain sensitivity. Trends Neurosci. 2004;27:582–584. doi: 10.1016/j.tins.2004.07.007. [DOI] [PubMed] [Google Scholar]
- [24].Miao N, Fung B, Sanchez R, Lydon J, Barker D, Pang K. Isolation and expression of PASK, a serine/threonine kinase during rat embryonic development, with special emphasis on the pancreas. J Histochem Cytochem. 2000;48:1391–1400. doi: 10.1177/002215540004801009. [DOI] [PubMed] [Google Scholar]
- [25].Miletic G, Miletic V. Loose ligation of the sciatic nerve is associated with TrkB receptor-dependent decreases in KCC2 protein levels in the ipsilateral spinal dorsal horn. Pain. 2008;137:532–539. doi: 10.1016/j.pain.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Morales-Aza BM, Chillingworth NL, Payne JA, Donaldson LF. Inflammation alters cation chloride cotransporter expression in sensory neurons. Neurobiol Dis. 2004;17:62–9. doi: 10.1016/j.nbd.2004.05.010. [DOI] [PubMed] [Google Scholar]
- [27].Moriguchi T, Urushiyama S, Hisamoto N, Iemura SI, Uchida S, Natsume T, et al. WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem. 2006;280:42685–42693. doi: 10.1074/jbc.M510042200. [DOI] [PubMed] [Google Scholar]
- [28].Nomura H, Sakai A, Nagano M, Umino M, Suzuki H. Expression changes of cation chloride cotransporters in the rat spinal cord following intraplantar formalin. Neurosci Res. 2006;56:435–440. doi: 10.1016/j.neures.2006.08.012. [DOI] [PubMed] [Google Scholar]
- [29].Pellis SM, Pellis VC, Chen YC, Barzci S, Teitelbaum P. Recovery from axial apraxia in the lateral hypothalamic labyrinthectomized rat reveals three elements of contact-righting: cephalocaudal dominance, axial rotation, and distal limb action. Behav Brain Res. 1989;35:241–251. doi: 10.1016/s0166-4328(89)80144-5. [DOI] [PubMed] [Google Scholar]
- [30].Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl) 2005;177:245–55. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- [31].Piechotta K, Garbarini NJ, England R, Delpire E. Characterization of the interaction of the stress kinase SPAK with the Na+-K+-2Cl- cotransporter in the nervous system: Evidence for a scaffolding role of the kinase. J Biol Chem. 2003;278:52848–52856. doi: 10.1074/jbc.M309436200. [DOI] [PubMed] [Google Scholar]
- [32].Piechotta K, Lu J, Delpire E. Cation-chloride cotransporters interact with the stress-related kinases SPAK and OSR1. J Biol Chem. 2002;277:50812–50819. doi: 10.1074/jbc.M208108200. [DOI] [PubMed] [Google Scholar]
- [33].Pieraut S, Matha V, Sar C, Hubert T, Méchaly I, Hilaire C, et al. NKCC1 phosphorylation stimulates neurite growth of injured adult sensory neurons. J Neurosci. 2007;27:6751–6759. doi: 10.1523/JNEUROSCI.1337-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pitcher MH, Price TJ, Entrena JM, Cervero F. Spinal NKCC1 blockade inhibits TRPV1-dependent referred allodynia. Mol Pain. 2007;3:17. doi: 10.1186/1744-8069-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ponce-Coria J, San-Cristobal P, Kahle KT, Vazquez N, Pacheco-Alvarez D, de Los Heros P, et al. Regulation of NKCC2 by a chloride-sensing mechanism involving the WNK3 and SPAK kinases. Proc Natl Acad Sci USA. 2008;105:8458–8463. doi: 10.1073/pnas.0802966105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- [37].Richardson C, Rafiqi FH, Karlsson HK, Moleleki N, Vandewalle A, Campbell DG, et al. Activation of the thiazide-sensitive Na+-Cl− cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci. 2008;121:675–684. doi: 10.1242/jcs.025312. [DOI] [PubMed] [Google Scholar]
- [38].Rinehart J, Kahle KT, de Los Heros P, Vazquez N, Meade P, Wilson FH, et al. WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl− cotransporters required for normal blood pressure homeostasis. Proc Natl Acad Sci USA. 2005;102:16777–16782. doi: 10.1073/pnas.0508303102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, et al. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- [40].Steiner H, Bonner TI, Zimmer AM, Kitai ST, Zimmer A. Altered gene expression in striatal projection neurons in CB1 cannabinoid receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96:5786–5790. doi: 10.1073/pnas.96.10.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sung K-W, Kirby M, McDonald MP, Lovinger DM, Delpire E. Abnormal GABAA-receptor mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J Neurosci. 2000;20:7531–7538. doi: 10.1523/JNEUROSCI.20-20-07531.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tornberg J, Voikar V, Savilahti H, Rauvala H, Airaksinen MS. Behavioural phenotypes of hypomorphic KCC2-deficient mice. Eur J Neurosci. 2005;21:1327–1337. doi: 10.1111/j.1460-9568.2005.03959.x. [DOI] [PubMed] [Google Scholar]
- [43].Van Laer L, Pfister M, Thys S, Vrijens K, Mueller M, Umans L, et al. Mice lacking Dfna5 show a diverging number of cochlear fourth row outer hair cells. Neurobiol Dis. 2005;19:386–399. doi: 10.1016/j.nbd.2005.01.019. [DOI] [PubMed] [Google Scholar]
- [44].Villa F, Goebel J, Rafiqi FH, Deak M, Thastrup J, Alessi DR, et al. Structural insights into the recognition of substrates and activators by the OSR1 kinase. EMBO Rep. 2007;8:839–845. doi: 10.1038/sj.embor.7401048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Vitari AC, Thastrup J, Rafiqi FH, Deak M, Morrice NA, Karlsson HK, et al. Functional interactions of the SPAK/OSR1 kinases with their upstream activator WNK1 and downstream substrate NKCC1. Biochem J. 2006;397:223–231. doi: 10.1042/BJ20060220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Vlainic J, Pericic D. Effects of acute and repeated zolpidem treatment on pentylenetetrazole-induced seizure threshold and on locomotor activity: comparison with diazepam. Neuropharmacology. 2009;56:1124–30. doi: 10.1016/j.neuropharm.2009.03.010. [DOI] [PubMed] [Google Scholar]
- [47].Zhang W, Liu L-Y, Xu T-L. Reduced potassium-chloride co-transporter expression in spinal cord dorsal horn neurons contributes to inflammatory pain hypersensitivity in rats. Neuroscience. 2008;152:502–510. doi: 10.1016/j.neuroscience.2007.12.037. [DOI] [PubMed] [Google Scholar]
- [48].Zhu L, Lovinger D, Delpire E. Cortical neurons lacking KCC2 expression show impaired regulation of intracellular chloride. J Neurophysiol. 2005;93:1557–1568. doi: 10.1152/jn.00616.2004. [DOI] [PubMed] [Google Scholar]