Abstract
Dietary long chain fatty acids are absorbed in the intestine, esterified to triacylglycerol, and packaged in the unique lipoprotein of the intestine, the chylomicron. The rate-limiting step in the transit of chylomicrons through the enterocyte is the exit of chylomicrons from the endoplasmic reticulum in prechylomicron transport vesicles (PCTV) that transport chylomicrons to the cis-Golgi. Because chylomicrons are 250 nm in average diameter and lipid absorption is intermittent, we postulated that a unique SNARE pairing would be utilized to fuse PCTV with their target membrane, cis-Golgi. PCTV loaded with [3H]triacylglycerol were incubated with cis-Golgi and were separated from the Golgi by a sucrose step gradient. PCTV-chylomicrons acquire apolipoprotein-AI (apoAI) only after fusion with the Golgi. PCTV became isodense with Golgi upon incubation and were considered fused when their cargo chylomicrons acquired apoAI but docked when they did not. PCTV, docked with cis-Golgi, were solubilized in 2% Triton X-100, and proteins were immunoprecipitated using VAMP7 or rBet1 antibodies. In both cases, a 112-kDa complex was identified in non-boiled samples that dissociated upon boiling. The constituents of the complex were VAMP7, syntaxin 5, vti1a, and rBet1. Antibodies to each SNARE component significantly inhibited fusion of PCTV with cis-Golgi. Membrin, Sec22b, and Ykt6 were not found in the 112-kDa complex. We conclude that the PCTV-cis-Golgi SNARE complex is composed of VAMP7, syntaxin 5, Bet1, and vti1a.
The villus structure and especially the brush border of intestinal absorptive cells provide an expanded surface area for the absorption of lipids and other nutrients. Since control over uptake rates of diet-derived fatty acids is limited, large amounts may flood enterocytes upon ingestion of a fatty meal requiring rapid disposal or risk cellular injury (1). This is accomplished either by binding the fatty acids to L-FABP (liver fatty acid-binding protein) (2) or by their rapid incorporation into triacyl-glycerols (TAG)2 (3) at the endoplasmic reticulum (ER). The TAG is incorporated into the unique lipoprotein of the intestine, the chylomicron, whose diameter averages 250 nm. The exit of chylomicrons from the ER in a specialized vesicle, the prechylomicron transport vesicle (PCTV) (4), is the rate-limiting step in lipid absorption (3). After exiting the ER, PCTV travel to the cis-Golgi by an anterograde mechanism (4, 5).
There is considerable evidence to suggest that chylomicrons pass through the Golgi and are modified while in this compartment. Apolipoprotein B48 (apoB48) becomes associated with chylomicrons in the ER and undergoes glycosylation changes in the Golgi (6, 7). The lipid composition of chylomicrons changes in the Golgi (7), and they acquire apolipoprotein A-I (apoAI) in this compartment (4). Finally, microsomal triglyceride transport protein is found in the Golgi and may participate in chylomicron biogenesis (8). After being modified in the Golgi, chylomicrons subsequently undergo exocytosis at the basolateral membrane of the enterocyte.
Because of their large size to accommodate the chylomicron cargo, and the intermittent nature of their production associated with eating, we hypothesized that PCTV generation and its targeting to the cis-Golgi was associated with unique trafficking and targeting protein(s) as compared with nascent protein-transporting vesicles, which are 80 nm in diameter (9). In support of this thesis, we found that PCTV budding from ER membranes was independent of COPII proteins but that COPII proteins were required for delivery of the chylomicrons to the cis-Golgi (4). The present study focuses on the proteins that mediate the interactions of PCTV with the Golgi.
Vesicles arriving at their target compartment, such as the Golgi, are first tethered and then dock and finally fuse with the membrane, resulting in the mixing of the interior contents of the vesicle with the luminal contents of the target (10, 11). Fusion requires soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins that form 4-α helix coiled-coil structures (12) necessary for membrane fusion (11). Since we have observed that PCTV fuse only with intestinal cis-Golgi and not liver or kidney cis-Golgi (13), the possibility arises that in the intestine, there is a unique SNARE pairing for PCTV with cis-Golgi. Further, PCTV generated in the absence of COPII proteins do not become associated with the cis-Golgi (4), suggesting that COPII proteins or cargo selected by the COPII complex may be required for Golgi targeting or fusion.
In the current study, we have sought to define the SNARE complex for PCTV as the vesicle and cis-Golgi as the target membrane. We provide evidence that VAMP7 (vesicle-associated membrane protein 7) (TI-VAMP), a vesicle-associated SNARE, or v-SNARE (R-SNARE), thought to be primarily used in the endosomal system (14) but recently identified in rat intestinal ER (9), is the v-SNARE for PCTV and forms part of the PCTV-cis-Golgi SNARE complex. Further, we document that PCTV fuses with the Golgi by demonstrating delivery of PCTV content to the cis-Golgi.
EXPERIMENTAL PROCEDURES
Materials
[3H]oleic acid (9.2 Ci/mm) was obtained from PerkinElmer Life Sciences. Immunoblot reagents were purchased from Bio-Rad. ECL reagents were procured from Amersham Biosciences. Protease inhibitor mixture tablets were obtained from Roche Applied Science. Other biochemicals used were of analytical grade and were purchased from local companies. Rats, 150–200 g, were purchased from Harlan (Indianapolis, IN).
Antibodies
Polyclonal antibodies against rat VAMP7 were raised in rabbits against amino acids 105–123 of rat VAMP7 (9). The antibody recognized VAMP7 but did not cross-react with VAMP1,2 by immunoblot (9). Mouse monoclonal antibodies to rBet1, GOS-28, and membrin were procured from Stressgen (Vancouver, Canada). Mouse anti-vti1a monoclonal antibodies were purchased from BD Biosciences. Rabbit polyclonal anti-apolipoprotein AI (apoAI) antibodies were a gift of Dr. Patrick Tso (University of Cincinnati, Cincinnati, OH). Rabbit polyclonal antibody to syntaxin 5 was a gift of Dr. William Balch (Scripps Research Institute, La Jolla, CA). Polyclonal anti-Sec22b antibodies were a generous gift of Dr. Jesse Hay (University of Montana). Goat polyclonal antibodies to calnexin, Rab11, and Ykt6 were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Goat anti-rabbit IgG conjugated with agarose beads was purchased from Sigma. Goat anti-rabbit IgG, goat anti-mouse IgG, and goat anti-rabbit IgG conjugated with horseradish peroxidase were purchased from Santa Cruz Biotechnology.
Isolation of ER, Cytosol, and Golgi and Metabolic Labeling of Enterocytes
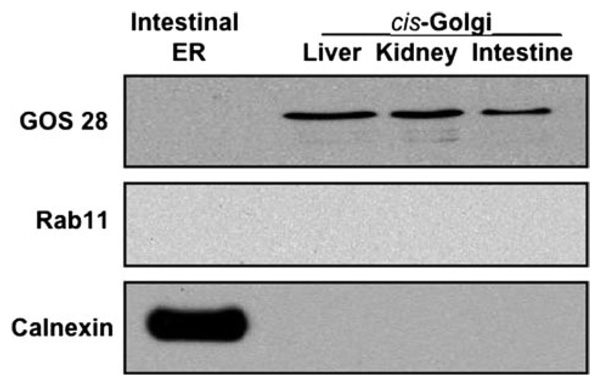
The surface mucosal cells, enterocytes, from the proximal half of rat small intestine were isolated and radiolabeled with [3H]TAG as described (13). In brief, enterocytes were stripped from intestinal villi, collected, incubated with albumin-bound [3H]oleate, and washed with albumin to remove excess [3H]oleate. The cells were homogenized, and the ER were isolated using a sucrose step gradient, which was repeated to purify the ER. The [3H]TAG-loaded ER preparation contained no Golgi, endosomes, or lysosomes (9) (Fig. 1). cis-Golgi was isolated from nonradiolabeled rat enterocytes (13) as was cytosol (4). The cis-Golgi preparation contained GOS28 but not calnexin (4) (Fig. 1).
FIGURE 1. Immunoblot of cis-Golgi from rat intestine, liver, and kidney and from intestinal ER for organelle-specific proteins.
Samples containing ER or cis-Golgi (30 µg of protein each) isolated from the indicated organ were separated on SDS-PAGE, transblotted onto a nitrocellulose membrane, and probed with specific antibodies to GOS28, Rab11, and calnexin as indicated (see “Experimental Procedures”). Detection was by ECL.
SDS-PAGE, Calculation of Protein Mr, and Immunoblots
Proteins were separated by SDS-PAGE (4). Protein molecular weights were calculated from a plot of the log Mr of standard molecular weight proteins versus their Rf (relative mobility) on SDS-PAGE. A linear regression line and y intercept were calculated from the data, and the formula was used to determine the log Mr of unknown proteins or protein complexes using their Rf values.
For immunoblotting, proteins, separated by SDS-PAGE, were transblotted onto nitrocellulose membranes (Bio-Rad) (4). After incubations with specific primary and then secondary antibodies, labeled proteins were detected using ECL reagents and exposing the developed blots to Biomax film (Eastman Kodak Co., Rochester, NY).
In Vitro PCTV Formation
PCTV, containing chylomicron-[3H]TAG, were formed from [3H]TAG-loaded ER (4). In brief, ER was incubated at 37 °C for 30 min with cytosol and an ATP-regenerating system in the absence of Golgi acceptor. The incubation mixture was resolved on a continuous sucrose gradient, and PCTV were isolated from the light portions of the gradient. PCTV thus formed were concentrated to 5.0 mg/ml protein using a Centricon-10 filter (Millipore Corp., Bedford, MA).
In Vitro PCTV Docking and Fusion with the Golgi
In experiments where PCTV docking with Golgi was required, PCTV (150 µg of protein) containing [3H]TAG were incubated with cis-Golgi membranes (300 µg of protein) for 30 min at 4 °C in the presence of 500 µg of cytosolic protein. Reactions contained 0.5 mm ATPγS, 1 mm EDTA, and buffer A (0.25 m sucrose, 30 mm HEPES, pH 7.2, 30 mm KCl, 5 mm CaCl2, 2 mm dithiothreitol; total volume, 500 µl). In experiments where PCTV fusion with the Golgi was required, similar amounts of PCTV, cis-Golgi membrane, and cytosol protein were used, but the incubation (35 °C) contained an ATP-regenerating system, 5 mm MgCl2, and buffer A but no ATPγS or EDTA. Throughout, “ATP” refers to an ATP-regenerating system (1mm ATP, 5mm phosphocreatine, and 5 units of creatine phosphokinase). Reactions were stopped by adding cold buffer A and placing the tubes on ice. The density of the reaction mix was adjusted to 1.22m sucrose (total volume 3 ml). This was overlaid with 2.6 ml each of 1.15, 0.86, and 0.25 m sucrose, and the gradient was centrifuged (5). Under these conditions, PCTV not associated with the Golgi float to the top of the gradient (4, 5). To release Golgi luminal contents, the cis-Golgi fraction (0.86/1.15 m sucrose interface) was incubated with 100 mm carbonate, pH 11, for 1 h at 4 °C. The incubation mix was diluted with 10 ml of 0.15 m NaCl, the suspension was centrifuged (15), and the fraction containing the chylomicrons, which were visible as a white band at the top of the gradient, was isolated (15). Chylomicron proteins were delipidated, separated on SDS-PAGE (4), and probed for apoAI by immunoblot.
Transport of Newly Synthesized TAG to the Golgi
The delivery of PCTV-TAG to the Golgi was determined using PCTV loaded with newly synthesized [3H]TAG (4). The PCTV were incubated with Golgi from unlabeled cells in the presence of cytosol, an ATP-regenerating system, Mg2+, and buffer A at 35 °C for 30 min (4).
Isolation of the SNARE Complex
PCTV were docked (see above, and see “Results”) with cis-Golgi, and the cis-Golgi were isolated on a sucrose step gradient (13). Under these conditions, undocked PCTV are separated from the Golgi and float to the top of the gradient. Putative PCTV-Golgi membranes were solubilized using 2% Triton X-100 in phosphate-buffered saline (PBS) and incubated overnight at 4 °C with either rabbit anti-VAMP7 or anti-rBet1 antibodies bound to agarose beads. The beads were washed thoroughly to remove unbound proteins (as judged by the absence of VAMP7 on immunoblot after six of a total of 10 washes when the anti-VAMP7 antibody was used; data not shown), and the beads were either boiled or not boiled in Laemmli buffer as indicated. These procedures were slightly modified from those described by others in that no N-ethylmaleimide (NEM) was used (16, 17). The immunoprecipitated proteins were separated by SDS-PAGE and transblotted onto a nitrocellulose membrane. The same membrane was probed with antibodies to syntaxin 5, VAMP7, rBet1, and vti1a. For reprobing membranes with different antibodies, membranes were washed with PBS containing 0.05% Tween 20 (PBS-T) three times, incubated in stripping buffer (62.5 mm Tris-HCl, pH 6.7), 100 mm 2-mercaptoethanol, and 2% SDS) for 45 min at 56 °C, and washed three times with PBS-T, which was followed by blocking in 5% blotto (Bio-Rad) in PBS-T overnight.
Measurement of TAG Radioactivity
TAG radioactivity was quantitated as described (13).
Statistical Analysis
Comparisons between means were carried out using a statistical package supplied by GraphPad Software (Instat, GraphPad Software, Inc., San Diego, CA) using a two-tailed t test.
RESULTS
PCTV Associate with the cis-Golgi
To define the components of the fusion complex, it was first necessary to develop conditions whereby PCTV associate with the cis-Golgi but do not fuse (i.e. the PCTV dock with the cis-Golgi) (18–20). We postulated that the proteins important for docking would be components of the fusion complex and identifiable as a multimeric, SDS-stable complex (21), which dissociates on boiling. We further postulated that docking would stabilize the components of the fusion complex, which otherwise would quickly dissociate (22).
As a first step toward this goal, the purity of our subcellular organelle preparations was established. The intestinal ER preparations from which PCTV are derived have been shown to be free of Golgi, lysosomal, or endosomal contamination (4, 9) but do contain the ER resident protein, calnexin (Fig. 1). The cis-Golgi preparations used have the Golgi protein, GOS28, but do not contain either calnexin or Rab11 (Fig. 1), ruling out significant ER or endosomal/lysosomal contamination, respectively. These results suggest that our PCTV and cis-Golgi preparations were of adequate purity for studies of compartment interactions.
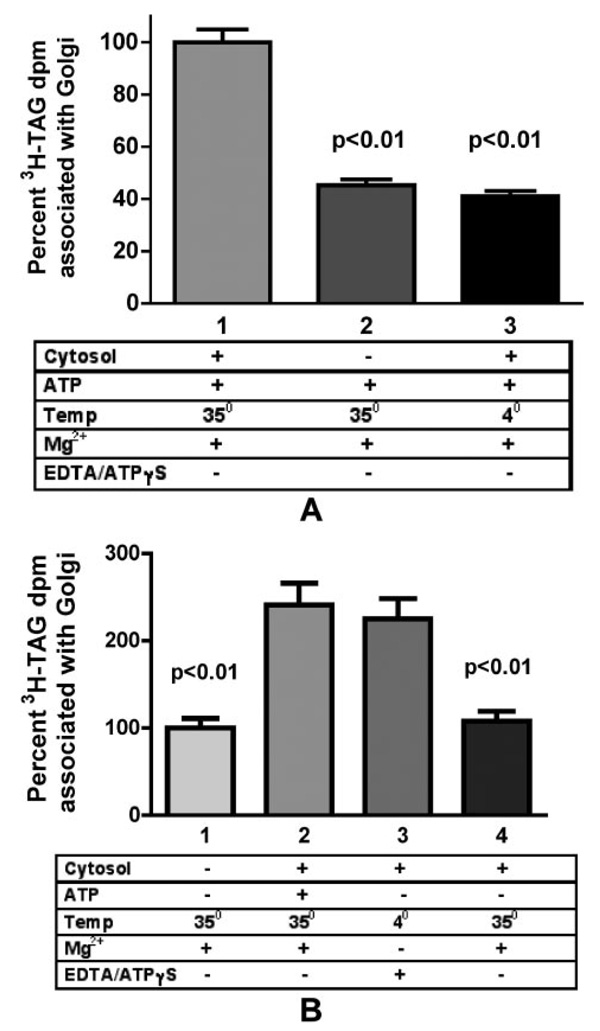
The first step in examining PCTV docking and fusion was to determine whether PCTV could associate with the cis-Golgi. For these studies, PCTV were incubated with cis-Golgi and various cofactors and then separated on a sucrose density gradient. An association of PCTV with Golgi was defined as a shift of PCTV from light fractions to heavier fractions containing Golgi. Incubations were performed at 35 °C in the expectation of fusion and 4 °C in the expectation of a potentially stabilized docking complex. The redistribution of PCTV was normalized to the maximal condition with PCTV, Golgi, cytosol, and ATP at 35 °C (Fig. 2A, bar 1). As shown in Fig. 2A, PCTV association with the cis-Golgi at 35 °C was enhanced by the addition of cytosol (bar 1 versus bar 2). However, the enhancing effects of cytosol were eliminated when incubations were performed at 4 °C (bar 1 versus bar 3). These findings demonstrate that the association of PCTV with Golgi is both temperature-and cytosol-dependent. Next, the effects of ATP on this interaction were examined. As shown in Fig. 2B, removal of ATP decreased the cytosol-dependent association of PCTV with Golgi at 35 °C (bar 2 versus bar 4). PCTV also did not interact with cis-Golgi upon incubation at 4 °C with ATP in the absence of cytosol (data not shown) or at 35 °C when both cytosol and ATP were excluded (Fig. 2B, bar 1). However, in the absence of ATP, the association of PCTV with Golgi was enhanced when incubation occurred with cytosol at 4 °C (Fig. 2B, bar 4 versus bar 3).
FIGURE 2. The association of PCTV with intestinal cis-Golgi is dependent on temperature, cytosol, and ATP.
A, dependence on cytosol and temperature. PCTV (150 µg of protein) loaded with [3H]TAG were incubated with intestinal cis-Golgi (300 µg of protein), ATP, and intestinal cytosol (500 µg of protein) as indicated. Incubations were with (bar 1) or without (bar 2) cytosol at 35 °C or with cytosol at 4 °C (bar 3). After incubation for 30 min, the cis-Golgi fraction was isolated on a sucrose step gradient and collected by aspiration of the 0.86/1.15 m interface, and the [3H]TAG dpm were determined. The cis-Golgi fraction [3H]TAG dpm obtained postincubation with PCTV, cytosol, and ATP at 35 °C (bar 1) were considered to be 100%. The data are the mean of four experiments ± S.E. p values of significant differences with bar 1 are placed above the bars as indicated. B, dependence on ATP. [3H]TAG-loaded PCTV (150 µg of protein) were incubated (35 °C) with intestinal cis-Golgi (300 µg of protein) and no cytosol or ATP (bar 1) or with ATP and intestinal cytosol (500 µg of protein) at 35 °C (bar 2) or with cytosol but without ATP and incubated at 4 °C (bar 3) or with cytosol but without ATP and incubated at 35 °C (bar 4). After incubation for 30 min at the indicated temperatures, the cis-Golgi was isolated on a sucrose step gradient, and the [3H]TAG dpm were determined. The cis-Golgi fraction [3H]TAG dpm postincubation at 35 °C with PCTV and no cytosol or ATP (bar 1) were considered to be 100%. The data are the mean of four experiments ± S.E. The p values indicating significant differences between bars 1 and 4 and bars 2 and 3 are shown above bars 1 and 4. The p values between bars 1 and 4 were >0.05, and values between bars 2 and 3 were also >0.05.
Interestingly, when examined at 4 °C, ATP caused the opposite effect from that observed at 35 °C. At 4 °C, ATP decreases the association of PCTV with Golgi (compare Fig. 2A, bar 3 with Fig. 2B, bar 3), whereas at 35 °C, ATP increases the association of PCTV with Golgi (compare Fig. 2B, bar 2, with Fig. 2B, bar 4). There are several potential explanations for these observations. First, the mechanism of the PCTV shift to the cis-Golgi fraction may not be the same at 4 and 35 °C. For example, whereas fusion events can occur at 35 °C, they cannot at 4 °C. Thus, it is possible that the PCTV association with Golgi at 35 °C is in part due to fusion, whereas at 4 °C the association is due to docking. Whereas the mechanism for the enhanced PCTV-Golgi association with ATP elimination is unclear, one explanation is that ATP destabilizes a docking complex that forms at 4 °C. This conclusion is supported by the work of Söllner et al. (23), who suggested that in the presence of ATP, the SNARE complex quickly disassociates even at 4 °C. In summary, these data demonstrate that PCTV can associate with the Golgi but do not discriminate between docking and fusion.
Docking and Fusion of PCTV with cis-Golgi
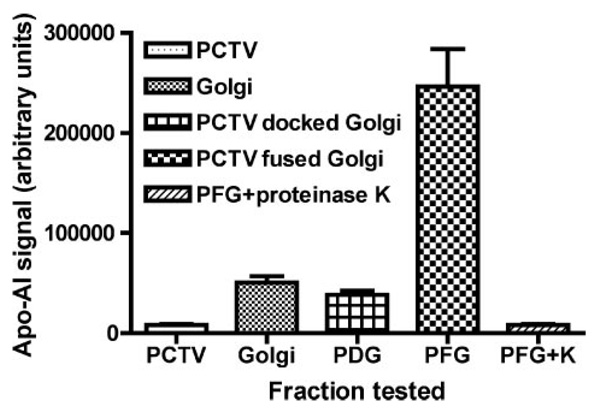
To differentiate between PCTV being docked or fused with the Golgi, we took advantage of the fact that PCTV-chylomicrons do not contain apoAI (4), a lipoprotein constituent of mature chylomicrons that is added in the Golgi complex (4). Thus, chylomicron acquisition of apoAI can be equated to the delivery of PCTV-chylomicrons to the Golgi lumen, suggesting a fusion event. In contrast, PCTV docked at the Golgi would undergo the density shift to the position of the Golgi, but its cargo chylomicrons would not acquire apoAI, whereas PCTV fused with the Golgi would also undergo the same density shift, but its PCTV-chylomicrons would acquire apoAI. To test the acquisition of apoAI by chylomicrons, the chylomicrons were released from the Golgi membranes by carbonate treatment, and the apoAI content assayed by immunoblot (see “Experimental Procedures”). Apoproteins remain associated with chylomicrons in the presence of carbonate.
The ability of PCTV to dock with intestinal cis-Golgi at 4 °C and fuse at 35 °C was next examined. Fig. 3 shows the apoAI content, a marker of PCTV fusion with the Golgi, of released chylomicrons under varying conditions. Chylomicrons released from PCTV contained minimal amounts of apoAI (Fig. 3, PCTV), consistent with our prior data (4). Chylomicrons released from native cis-Golgi had a modest increase in apoAI content over that found in PCTV (Fig. 3, PCTV versus Golgi). Importantly, chylomicrons released from cis-Golgi incubated at 4 °C with PCTV and cytosol but without ATP (Fig. 3, PDG) contained amounts of apoAI similar to that found in native Golgi. As judged by the [3H]TAG signal, however, under these incubation conditions (PDG), the PCTV become isodense with the cis-Golgi (Fig. 2B, bar 3). Since the chylomicrons in PCTV incubated with Golgi at 4 °C as in Fig. 3 (PDG) or Fig. 2B (bar 3) became associated with the Golgi but did not acquire apoAI (Fig. 3, PDG), we concluded that the PCTV were docked with but not fused with the Golgi. These data suggest that at 4 °C, PCTV docking with the cis-Golgi requires cytosolic factors and is ATP-independent; the requirements are consistent with the conditions for docking described by Wilson et al. (24). These data suggest that at 4 °C, PCTV dock but do not fuse with the Golgi.
FIGURE 3. Acquisition of apoAI by PCTV-chylomicrons isolated from intestinal cis-Golgi depends on fusion of PCTV with the Golgi.
PCTV (150 µg of protein) were incubated with intestinal cis-Golgi (300 µg of protein) and intestinal cytosol (500 µg of protein) under various conditions. Postreaction, the cis-Golgi was isolated using a sucrose density step gradient and incubated with 100 mm carbonate buffer (pH 11). The released chylomicrons were floated by centrifugation and collected; the delipidated proteins were separated by SDS-PAGE and transblotted to a nitrocellulose membrane; and the amount of apoAI associated with the chylomicrons was measured using ECF. The intensity of the ECF signal, indicating the amount of apoAI associated with the chylomicrons under each incubation condition, is displayed on the ordinate in arbitrary units. PCTV, apoAI content of chylomicrons released from PCTV without prior incubation; Golgi, apoAI content of chylomicrons released from native cis-Golgi; PDG, apoAI content of chylomicrons released from cis-Golgi incubated with cytosol and PCTV at 4 °C in the absence of ATP; PFG, apoAI content of chylomicrons released from cis-Golgi incubated with cytosol, PCTV, and ATP at 35 °C. PFG+K, apoAI content of chylomicrons released from cis-Golgi incubated with cytosol, PCTV, and ATP at 35 °C. The released chylomicrons were treated with proteinase K. The data are the mean ± S.E. (n = 3).
Our previous work provided preliminary evidence that PCTV might fuse with Golgi when combined with warm cytosol (4, 5). To directly demonstrate this phenomenon, PCTV were incubated with intestinal cis-Golgi, cytosol, Mg2+, and ATP at 35 °C, and the amount of apoAI associated with postincubation chylomicrons was assayed (Fig. 3, PFG). After incubation in the warm conditions, there was a 6.4-fold increase in the amount of apoAI associated with the released chylomicrons (Fig. 3, PFG) as compared with when the PCTV were incubated with Golgi under conditions that favored docking (Fig. 3, PDG). These data support the conclusion that the PCTV fused with the cis-Golgi under these conditions (Fig. 3, PFG), and their chylomicron cargo acquired apoAI as a consequence. When chylomicrons were released from the postincubation Golgi (as in Fig. 3, PFG) and incubated with proteinase K, the apoAI signal was completely attenuated (Fig. 3, PFG+K). These data support the conclusion that the chylomicrons that acquired apoAI in Fig. 3 (PFG) were within the Golgi lumen and that when released they were free of membrane and could be successfully attacked by proteinase K. The findings demonstrate that at 35 °C, PCTV fuse with the Golgi in an ATP- and cytosol-dependent manner.
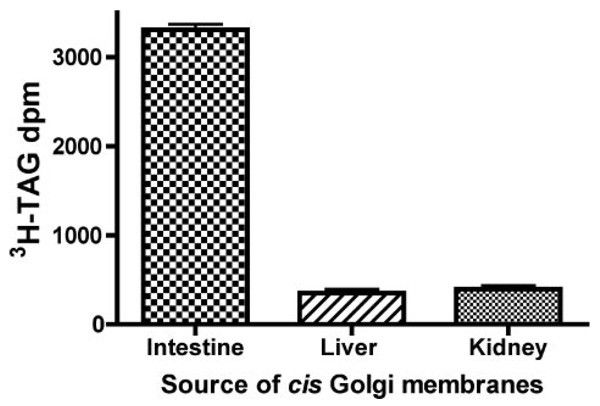
To determine the specificity of PCTV docking for intestinal Golgi, PCTV were incubated with cis-Golgi from liver and kidney. We tested both liver and kidney Golgi because liver exports a TAG-rich lipoprotein, the very low density lipoprotein, whereas kidney represents a non-TAG-rich lipoprotein-exporting organ. There was minimal association of PCTV-[3H]TAG with the Golgi derived from either organ compared with the intestinal Golgi control (Fig. 4). These data support the thesis that the interaction between PCTV and intestinal Golgi is unique. The combined data are consistent with the interpretation that the luminal contents of PCTV and intestinal Golgi mix upon incubation under warm conditions in the presence of ATP and cytosol, suggesting that the membranes fused and that such a PCTV-Golgi fusion event is specific for intestinal cis-Golgi. Although intestinal cytosol was used for these experiments (Fig. 4), cytosol from either liver or kidney has been previously shown to support ER to Golgi transport of chylomicrons (13), indicating that the cytosolic components of the reaction do not provide specificity.
FIGURE 4. PCTV docks with intestinal cis-Golgi but not with liver or kidney cis-Golgi.
cis-Golgi were isolated from rat intestine, liver, and kidney on a sucrose step gradient as for intestine. PCTV (150 µg of protein) preloaded with [3H]TAG, were incubated at 4 °C with intestinal cytosol (500 µg of protein) and cis-Golgi (300 µg of protein) from the organs as indicated in the absence of ATP. Postincubation, the cis-Golgi were separated on a sucrose step gradient and isolated, and their [3H]TAG content was determined. The data are the mean ± S.E., n = 4.
Identification of the SNARE Complex Involved in PCTV Docking Fusion with cis-Golgi
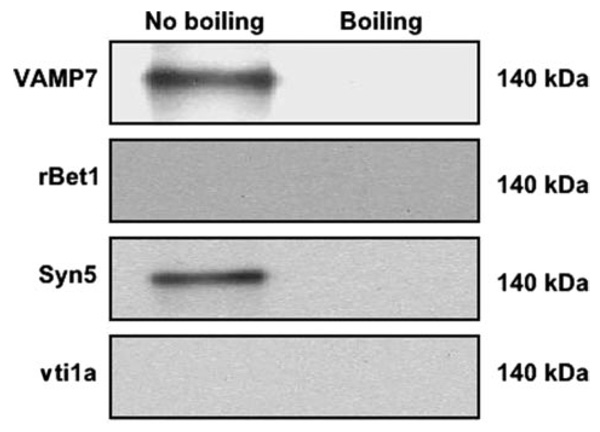
To isolate the SNARE complex involved in the fusion of PCTV with the Golgi, PCTV were docked with intestinal cis-Golgi, and the PCTV-Golgi complexes were isolated on a sucrose step gradient. PCTV-Golgi membranes were solubilized in 2% Triton X-100 and incubated with anti-VAMP7 antibodies conjugated to agarose beads. VAMP7 is a putative v-SNARE that we have found is associated with PCTV (9). The beads were isolated and washed, and the proteins attached to the VAMP7 antibodies were probed for potential SNAREs using immunoblot analysis. Fig. 5 shows, respectively, the presence of VAMP7, syntaxin 5, rBet1, and vti1a in a band corresponding to 112 kDa when the samples were not boiled, suggesting that they formed a heteromeric complex. Importantly, only a single membrane was used for the immunoblots that were sequentially probed with the indicated antibodies after stripping. These four SNARE proteins migrating at 112 kDa are proposed to contain the SNAREs that form the PCTV-cis-Golgi SNARE complex. Further, Fig. 5 shows that the complex resisted disassociation upon exposure to SDS, a criterion of SNARE formation (25). Upon boiling, each of the component SNARE proteins disassociated from the 112-kDa complex and was found to migrate at its expected monomeric Mr (data not shown). The Mr of these four SNAREs as monomers (25, 17, 42, and 29 kDa, respectively) totals 113,000 as compared with the 112,000 calculated for the Mr of the complete SNARE complex.
FIGURE 5. Distinct proteins co-immunoprecipitate with VAMP7 in a 112-kDacomplex after PCTV docking with intestinal cis-Golgi.
PCTV (150 µg of protein) preloaded with [3H]TAG, were incubated at 4 °C with intestinal cis-Golgi (300 µg of protein), intestinal cytosol (500 µg of protein), and no ATP. The PCTV-Golgi complex was isolated on a sucrose step gradient and obtained by aspiration. The PCTV-Golgi complex was solubilized in 2% Triton X-100 and incubated with anti-VAMP7 antibodies bound to agarose beads at 4 °C overnight. The beads were washed and placed in Laemmli buffer and either boiled or not as indicated prior to electrophoresis. The proteins were separated on SDS-PAGE and probed with antibodies against potential SNARE components as indicated. A single membrane was used, which was sequentially probed with the indicated antibodies after washing. Protein bands at 112 kDa are shown. Detection was by ECL. The data are representative of three trials.
To confirm the composition of the SNARE complex described in the legend to Fig. 5, beads coupled to rBet1 antibodies were used to bind rBet1-associated proteins in solubilized membranes from PCTV docked with intestinal cis-Golgi as outlined in Fig. 5. The results (Fig. 6) revealed that the same components of the SNARE complex that were associated with VAMP7 were found with rBet1, increasing the likelihood of the specificity of our findings.
FIGURE 6. Distinct proteins co-immunoprecipitate with rBet1 in a 112-kDa complex after PCTV docking with intestinal cis-Golgi.
PCTV were docked with intestinal cis-Golgi, and the proteins were solubilized with 2% Triton X-100. Bead-bound anti-rBet1 antibody was used to probe the solubilized proteins. A single lane on the same membrane was probed sequentially with anti-VAMP7, rBet1, and syntaxin 5 antibodies. A separate lane on the same membrane was probed with anti-vti1a antibodies. In each case, the sample was either boiled or not prior to electrophoresis as indicated. The proteins to which the specific antibodies were directed are indicated beside each blot. Proteins migrating at 112 kDa are shown. The data are representative of three trials.
Because other SDS-resistant SNARE complexes could have similar apparent Mr on SDS-PAGE, we tested the complex for the presence of other potential SNARE proteins present on PCTV or intestinal ER membranes. However, the potential PCTV ER-SNARE proteins, membrin, Sec22b, and Ykt6, were not identified in the 112-kDa complex associated with VAMP7 (Fig. 5). Further, the Golgi SNARE protein GOS28 also was not present in the 112-kDa complex (Fig. 5).
SDS can nonspecifically induce intermolecular SH bonding and multiprotein complex formation (26). To exclude the possibility that SDS had nonspecifically generated a complex with an Mr of 112,000, two studies were performed. First, intestinal cis-Golgi alone were incubated under the same conditions we used to immunoprecipitate the 112-kDa complex with the VAMP7 antibody. These conditions resulted in a 140-kDa complex that contained VAMP7 and syntaxin 5 but neither vti1a nor rBet1 (Fig. 7), clearly differentiating it from the 112-kDa complex shown in Fig. 5 and Fig. 6. Second, it is also possible that SDS or Triton solubilization of the PCTV-Golgi membranes induced a nonspecific complex migrating at 112 kDa by cross-linking SNAREs or other proteins. To address this question, we immunoprecipitated Triton-solubilized PCTV-Golgi membranes with anti-membrin antibodies bound to beads, separated the proteins by SDS-PAGE under nonboiling conditions, and immunoblotted for SNARE proteins found in our 112-kDa complex. We identified only monomer species of membrin, syntaxin 5, and rBet1 migrating at their expected Mr on immunoblot (data not shown). Membrin was chosen for these studies because it is a t-SNARE and is present in the ER, PCTV, and Golgi (Fig. 8). These data support the conclusion that nonspecific cross-linking mediated by SDS or Triton did not result in a 112-kDa complex, which included membrin. Together, these studies demonstrate that the generation of the 112-kDa complex after Triton solubilization and SDS treatment of PCTV-Golgi was not due to nonspecific cross-linking effects of the detergents. In summary, the data support the conclusion that the 112-kDa complex formed as a result of the interaction of PCTV with cis-Golgi prior to detergent solubilization and that docking requires both PCTV and cis-Golgi.
FIGURE 7. Incubation of intestinal cis-Golgi without PCTV produces a 140-kDa complex upon immunoprecipitation using anti-VAMP7 antibodies.
Intestinal cis-Golgi (300 µg of protein), intestinal cytosol (500 µg of protein), and no ATP were incubated for 30 min at 4 °C (conditions for docking). PCTV were excluded from the incubation mix. Postincubation, the Golgi membranes were solubilized and probed with bead-bound anti-VAMP7 antibodies, and the immunoprecipitated proteins were separated by SDS-PAGE. Samples were boiled or not as indicated prior to electrophoresis. The proteins were transblotted to a nitrocellulose membrane and probed for the indicated proteins. The only high molecular mass band identified was at 140 kDa as shown. The data are representative of two trials.
FIGURE 8. The presence of membrin in PCTV, intestinal ER, and cis-and trans-Golgi as identified by immunoblot.
Proteins from the intestinal subcellular organelles or PCTV (30 µg of protein each) were separated by SDSPAGE and transblotted to a nitrocellulose membrane, and the membrane was probed with anti-membrin antibody. Identification of the protein bands was by ECL.
To determine the organ specificity for fusion of PCTV with Golgi membranes, we sought to determine if [3H]TAG-loaded PCTV became isodense with cis-Golgi isolated from liver or kidney under conditions as in Fig. 3 (PFG), in which fusion would be expected. However, no [3H]TAG was found to be associated with the cis-Golgi of either liver or kidney (data not shown) indicating a lack of fusion, consistent with our prior observations (13).
Antibodies to SNARE Complex Proteins Inhibit TAG Delivery to the Golgi
To confirm that the identified proteins of the putative PCTV-cis-Golgi SNARE complex were physiologically important in the fusion of PCTV with the cis-Golgi, we loaded PCTV with [3H]TAG and incubated the PCTV with VAMP7 antibodies or intestinal cis-Golgi with antibodies directed against rBet1, syntaxin 5, or vti1a for 1 h at 4 °C. Unbound antibodies were removed by washing, and the treated PCTV were incubated with native intestinal cis-Golgi or the treated cis-Golgi with native [3H]TAG-loaded PCTV at 35 °C. Antibodies to VAMP7, syntaxin 5, and rBet1 greatly reduced [3H]TAG delivery to the Golgi (Fig. 9). vti1a antibodies significantly, but more modestly, blocked the association of PCTV with Golgi. Under the same conditions, anti-Sec22b antibody treatment of PCTV did not affect delivery of PCTV-[3H]TAG to cis-Golgi (data not shown). These data are consistent with the physiological importance of each component of the proposed PCTV-cis-Golgi SNARE complex for the delivery of PCTV cargo to the cis-Golgi.
FIGURE 9. Effect of antibodies to PCTV-cis-Golgi SNARE proteins upon fusion of PCTV with intestinal cis-Golgi.
A, PCTV (150 µg of protein) containing [3H]TAG were incubated with anti-VAMP7 antibodies (PCTV + VAMP7 Ab) (10 µl) or preimmune IgG (PCTV + IgG) (10 µl) for 1 h at 4 °C, and the excess antibodies were removed by washing. Intestinal cis-Golgi (300 µg of protein) were incubated with either anti-rBet1 antibodies (Golgi + rBet1 Ab) (10 µl) or preimmune IgG (Golgi + IgG) (10 µl) (B), with anti-syntaxin 5 antibodies (Golgi + Syn5 Ab) (10 µl) or preimmune IgG (Golgi+IgG) (10 µl) (C), or with anti-vti1a antibodies (Golgi + vti1a Ab) (10 µl) or preimmune IgG (Golgi + IgG) (10 µl) (D) for 1 h at 4 °C. The cis-Golgi (B–D) were then washed to remove unbound antibody. After antibody incubation and washing, native intestinal cis-Golgi was added to tubes containing antibody-treated PCTV (A), and native [3H]TAG-loaded PCTV were added to tubes containing antibody-treated cis-Golgi (B–D). The PCTV and cis-Golgi were allowed to fuse by incubating them for 30 min at 35 °C with intestinal cytosol (500 µg of protein) and ATP. After incubation under warm conditions, the cis-Golgi were isolated on a sucrose step gradient. The number of Golgi-associated [3H]TAG dpm for each study is shown on the ordinate. Nonreacting PCTV floated to the top of the gradient and were excluded from analysis. The data are the mean ± S.E., n = 4. Significant p values are shown above the bars.
DISCUSSION
Targeted intracellular transport of proteins between different organelles requires SNARE proteins. SNAREs play a key role in docking and fusion between two intracellular compartments in the secretory pathway. Studies have shown that SNAREs form a stable four-helix complex with one helix localized on the vesicle membrane (v-SNARE) and the other three helices found on the opposing target membrane. These three may be composed of either three individual SNAREs (t-SNARES) or a synaptosome-associated protein (soluble N-ethylmaleimide-sensitive factor attachment protein), which contributes two SNARE regions, and an additional SNARE protein (27). Although these SNAREs are bound together through hydrophobic interactions, the center of the four-helix bundle contains a “0-layer,” at the center of which are one arginine and three glutamine residues (12). SNAREs contributing the arginine residue are classified as R-SNAREs, whereas those containing the glutamine residues are Q-SNAREs. Thus, SNARE complexes usually have the ratio of 3 Q-SNAREs:1 R-SNARE. Q-SNAREs are further divided into three subfamilies: QA-heavy chain or syntaxin-related, QB-light chain similar to the NH2 terminus of SNAP25, and QC-light chain similar to the carboxyl terminus of SNAP25. For fusion to occur, one member of each subfamily must be present; a Qc-SNARE cannot replace a QB-SNARE (28). The SNARE complex not only brings two membranes into close proximity, but the complex alone is able to initiate fusion between the two membranes in a liposome system (11).
Thus far in mammalian systems, only two SNARE complexes have been described for ER-derived vesicles that fuse with the cis-Golgi. In one, Sec22b was the v-SNARE, and syntaxin 5, membrin, and rBet1 were the t-SNAREs in NRK cells (29). However, in intestine, we found that Sec22b antibodies did not affect PCTV fusion with intestinal cis-Golgi. In the other ERGolgi SNARE complex that has been described, also in NRK cells, Ykt6 was the v-SNARE, with syntaxin 5, GOS28, and rBet1 as the t-SNAREs (30). An extensive tissue survey in rats has shown that Ykt6 is predominantly found in brain in a lysosome-related compartment (31). rBet1, another potential SNARE protein, is predominantly an ER-intermediate compartment resident protein recycling from the Golgi (32) and is present in intestinal ER and PCTV (4). However, rBet1, classified as a QC-SNARE (33), has not yet been shown to function as a v-SNARE in ER to Golgi transport. In support of this, antibodies directed against intestinal ER-localized rBet1 did not impede the fusion of PCTV with the cis-Golgi (data not shown).
Our SNARE complex of VAMP7 as the v-SNARE and syntaxin 5, vti1a, and rBet1 as the t-SNAREs (QA-, QB-, and QC-SNAREs, respectively) represents the third ER to Golgi SNARE pairing to be described and the first to be described in a primary tissue. The observations that the same components of the 112–113-kDa multimeric complex were found using either bead-bound anti-VAMP7 or anti-rBet1 antibodies, that the sum of the molecular weights of the individual components of the SNARE complex equals that of the multimeric complex, that one SNARE of each of the four SNARE families is present, and that alternative explanations for our observations were not found support our proposed composition of the PCTV-Golgi SNARE complex.
Because we propose that PCTV contains VAMP7 as its v-SNARE and PCTV are derived from intestinal ER (4), we questioned this unusual location for VAMP7, normally a post-Golgi-localized SNARE (34). However, in intestinal ER, VAMP7 was shown to co-localize with protein-diester isomerase by immunocytochemistry using deconvoluting microscopy, and VAMP7 was co-localized with Sar1 and rBet1 on PCTV using immunoelectron microscopy (9). Additionally, on iodixanol gradients, VAMP7 was shown to be isodense both with the ER markers calnexin and Sec22b and the endosomal markers Rab11 and syntaxin 8, suggesting that two separate pools exist in the intestine VAMP7 (9). Finally, it was shown that VAMP7 antibodies inhibited PCTV budding from intestinal ER but did not impede the generation of COPII-dependent protein vesicle buds (9).We conclude that VAMP7 is a bona fide, functional ER constituent in rat intestine.
The cytosolic extensions of the SNAREs may contain sites that bind other proteins important to vesicle docking/fusion specificity. Recently, it has been shown in yeast that SNAREs bind to Sec24, a COPII component. Sec24 has three SNARE binding sites, one of which binds Sed5, another that binds both Sed5 and Bet1, and a third site that binds Sec22b (35, 36). SNAREs may also participate in cargo selection. In transport vesicles with glycosylphosphatidylinositol-anchored protein as cargo, v-SNAREs are required for sorting of appropriate cargo to the budding vesicle (37). It is well established that COPII proteins, including Sec24, are also involved in cargo selection (35, 36, 38). In this context, we have shown that the absence of COPII proteins on PCTV generated by Sar1 depletion resulted in a vesicle that did not fuse with the Golgi (4). This suggests the possibility that Sec24 is involved in selecting proteins on the surface of PCTV that are required for their fusion with target membranes. An alternative explanation for these data is that p58, which is required for COPI protein binding to the vesicle and subsequent fusion with the Golgi, was not present on Sar1-depleted PCTV (4, 39). In summary, current data would suggest that there is a cooperative interaction between COPII proteins and SNAREs both with respect to cargo selection and generation of a fusion and target-competent vesicle.
As defined by their cargo, there are five different vesicles generated from mammalian ER membranes. The bulk of secretory and other proteins are transported from the ER to the Golgi in COPII vesicles that fuse with the Golgi utilizing a well described SNARE complex (29). A second set of vesicles, which transport GPI-anchored proteins to the Golgi, has been described (40), but the components of the SNARE complex responsible for fusion with Golgi are unknown. We have described a third set of ER-derived vesicles that transport prechylomicrons to intestinal Golgi, PCTV. These vesicles do not require COPII proteins to bud from the ER but do require them to fuse with the Golgi (4). Very recently, the ability of vesicles like chylomicrons to exit the ER in the absence of associated COPII proteins has received support from studies of vaccinia primary viral membrane proteins. Viral proteins have been shown to be transported out of the ER to the ER-Golgi intermediate compartment in the presence of the dominant negative SAR1, SAR1H79G, an inhibitor of COPII coat formation (41). A fourth, less well defined group of vesicles, transports procollagen from the ER to the Golgi (42). The procollagen transport vesicle may represent the third example of an ER to Golgi transport vesicle that does not require COPII proteins for ER budding (43).A fifth vesicle with a distinctive cargo, apolipoprotein-B100-associated very low density lipoprotein, appears to be COPII-dependent for budding from liver ER (44). These findings suggest that diverse mechanisms regulate the egress of vesicles from the ER and raise the possibility that distinctive cellular machinery might mediate their fusion with the Golgi complex.
The SNAREs involved in the fusion of PCTV with the Golgi have not been previously investigated in the context of ER to Golgi transport and are the subject of this report. Intestinally derived PCTV selectively fuse with intestinal Golgi and not with either kidney or liver Golgi (13) and utilize VAMP7 as a v-SNARE. The localization of VAMP7 to intestinal ER is not replicated in the ER of the other TAG-exporting organ, the liver, nor is it present in kidney ER (9), supporting the thesis that VAMP7 may play a unique role in the intestine in the transport of PCTV to the Golgi. A possible mechanism linking the concentration of VAMP7 in PCTV to its cargo chylomicrons is that the N-terminal structural homologue of VAMP7, Sec22b, binds to apoB from liver ER (45). Despite its importance in chylomicron transport to the Golgi, VAMP7 plays no role in the movement of newly synthesized proteins to the Golgi in COPII-dependent vesicles (9), consistent with data from many other cell types. The presence of VAMP7 as the v-SNARE on PCTV suggests that the t-SNARE components of the PCTV-Golgi SNARE complex are unique. This thesis is consistent with the targeting function of SNAREs to specific intracellular membranes (46).
There is evidence that VAMP7 is required in intestinal ER for successful export of chylomicrons from intestinal cells. Intestinelike CaCo2 cells synthesize TAG rapidly (47) and synthesize the requisite lipoproteins associated with chylomicrons (48) but are unable to deliver TAG in an efficient manner to the basal medium. VAMP7 is expressed in CaCo2 cells, but it is not found in their ER (49), suggesting its absence from any PCTV formed. Any PCTV generated in CaCo2 cells would lack VAMP7 and may not have the targeting information required for their docking and fusion with the intestinal Golgi, resulting in impaired export of chylomicrons from the basolateral portion of the enterocyte.
In summary, this study confirms that PCTV generated from isolated enterocyte ER can bind to and fuse with the Golgi complex from the same intestinal cells. That PCTV do not fuse with Golgi from other cell types suggests that distinct proteins may regulate this interaction. In this context, we have identified a SNARE complex, the third ER to Golgi SNARE complex to be elucidated and the first in a primary tissue, that regulates PCTV fusion with the Golgi complex. This complex uses VAMP7 as the PCTV v-SNARE and syntaxin 5, rBet1, and vti1a as the intestinal cis-Golgi t-SNAREs.
Footnotes
This study was supported by NIDDK, National Institutes of Health (NIH), Grants DK38760 (to C. M. M.) and DK54201 (to F. S. G.) and NIH Medical Student Research Program Grant T35DK07405. The Stout Neuroscience Laboratory was supported by NIH Grant RR1052 and National Science Foundation Grant DB1960633, a Veterans Administration Senior Career Development Award (to F. S. G.), and Office of Research and Development (R & D) Medical Research Service, Department of Veteran Affairs, research funds (to C. M. M. and F. S. G.).
The abbreviations used are: TAG, triacylglycerol(s); ER, endoplasmic reticulum; PCTV, prechylomicron transport vesicle(s); SNARE, N-ethylmaleimide-sensitive factor attachment protein receptor; ATPγS, adenosine 5′-O-(thiotriphosphate); PBS, phosphate-buffered saline.
REFERENCES
- 1.Stralfors P. FEBS Lett. 1990;263:153–154. doi: 10.1016/0014-5793(90)80726-y. [DOI] [PubMed] [Google Scholar]
- 2.Hsu K-T, Storch J. J. Biol. Chem. 1996;271:13317–13323. doi: 10.1074/jbc.271.23.13317. [DOI] [PubMed] [Google Scholar]
- 3.Mansbach CM, II, Dowell R. J. Lipid Res. 2000;41:605–612. [PubMed] [Google Scholar]
- 4.Siddiqi SA, Gorelick FS, Mahan JT, Mansbach CM., II J. Cell Sci. 2003;116:415–427. doi: 10.1242/jcs.00215. [DOI] [PubMed] [Google Scholar]
- 5.Kumar NS, Mansbach CM., II Am. J. Physiol. 1999;276:G378–G386. doi: 10.1152/ajpgi.1999.276.2.G378. [DOI] [PubMed] [Google Scholar]
- 6.Berriot-Varoqueaux N, Dannoura AH, Moreau A, Verthier N, Sassoias A, Cadiot G, Lachaux A, Munck A, Schmitz J, Aggerbeck L, Samson-Bouma M-E. Gastroenterology. 2000;121:1101–1108. doi: 10.1053/gast.2001.29331. [DOI] [PubMed] [Google Scholar]
- 7.Olofsson SO, Bjursell G, Bostrom K, Carlsson P, Elovson J, Protter AA, Reuben MA, Bondjers G. Atherosclerosis. 1987;68:1–17. doi: 10.1016/0021-9150(87)90088-8. [DOI] [PubMed] [Google Scholar]
- 8.Levy E, Stan S, Delvin E, Menardi D, Shoulders C, Garofalo C, Slight I, Seidman E, Mayer G, Bendavan M. J. Biol. Chem. 2002;277:16470–16477. doi: 10.1074/jbc.M102385200. [DOI] [PubMed] [Google Scholar]
- 9.Siddiqi SA, Mahan JT, Siddiqi S, Gorelick FS, Mansbach CM., II J. Cell Sci. 2006;119:943–950. doi: 10.1242/jcs.02803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nickel W, Weber T, McNew JA, Parlati F, Sollner TH, Rothman JE. Proc. Natl. Acad. Sci. U. S. A. 1999;96:12571–12576. doi: 10.1073/pnas.96.22.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber T, Zemelman BV, McNew JA, Wetermann B, Gmachi M, Parlati F, Sollner TH, Rothman JE. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 12.Sutton R, Fasshauser D, Jahn R, Brunger A. Nature. 1998;395:347–357. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 13.Kumar NS, Mansbach CM., II Am. J. Physiol. 1997;273:G18–G30. doi: 10.1152/ajpgi.1997.273.1.G18. [DOI] [PubMed] [Google Scholar]
- 14.Advani R, Prekeris R, Lee K, Klumperman J, Scheller R. J. Cell Biol. 1999;146:765–775. doi: 10.1083/jcb.146.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mansbach CM, II, Arnold A. Am. J. Physiol. 1986;251:G263–G269. doi: 10.1152/ajpgi.1986.251.2.G263. [DOI] [PubMed] [Google Scholar]
- 16.Castillo-Flores A, Weinberger A, Robinson M, Gerst JE. J. Biol. Chem. 2005;280:34033–34041. doi: 10.1074/jbc.M507142200. [DOI] [PubMed] [Google Scholar]
- 17.Couve A, Gerst JE. J. Biol. Chem. 1994;269:23391–23394. [PubMed] [Google Scholar]
- 18.Rothman JE. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 19.Sollner T, Whitehart SW, Brunner M, Erdjumont-Bromage GS, Tempst P, Rothman JB. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 20.Sollner TH, Rothman JE. Cell Struct. Funct. 1996;21:407–412. doi: 10.1247/csf.21.407. [DOI] [PubMed] [Google Scholar]
- 21.Hag JC, Salem N, Peng XR, Kelly RB, Bennett MK. J. Neurosci. 1997;17:1596–1603. doi: 10.1523/JNEUROSCI.17-05-01596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng R, Gallwitz D. J. Cell Biol. 2002;157:645–655. doi: 10.1083/jcb.200202006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Söllner T, Bennett MK, Whiteheart S, Scheller RH, Rothman JE. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- 24.Wilson DW, Whiteheart SW, Wiedmann M, Bunner M, Rothman J. J. Cell Biol. 1992;117:531–538. doi: 10.1083/jcb.117.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang B, Gonzales L, Jr., Prekeris R, Steegmaier M, Advani RJ, Scheller RH. J. Biol. Chem. 1999;274:5649–5653. doi: 10.1074/jbc.274.9.5649. [DOI] [PubMed] [Google Scholar]
- 26.Johnston PA, Sudhof TC. J. Biol. Chem. 1990;265:8869–8873. [PubMed] [Google Scholar]
- 27.Katz L, Hanson PI, Heuser JE, Brenwald P. EMBO J. 1998;17:6200–6209. doi: 10.1093/emboj/17.21.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joglekar AP, Xu D, Rigotti DJ, Fairman R, Hay JC. J. Biol. Chem. 2003;278:14121–14133. doi: 10.1074/jbc.M300659200. [DOI] [PubMed] [Google Scholar]
- 29.Xu D, Joglekar A, Williams A, Hay J. J. Biol. Chem. 2000;275:39631–39639. doi: 10.1074/jbc.M007684200. [DOI] [PubMed] [Google Scholar]
- 30.Zhang T, Hong W. J. Biol. Chem. 2001;276:27480–27487. doi: 10.1074/jbc.M102786200. [DOI] [PubMed] [Google Scholar]
- 31.Hasegawa H, Zinsser S, Rhee Y, Vik-Mo EO, Davanger S, Hay JC. Mol. Biol. Cell. 2003;14:698–720. doi: 10.1091/mbc.E02-09-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hay J, Klumperman J, Oorschot V, Steegmaier M, Kup C, Scheller R. J. Cell Biol. 1998;141:1489–1502. doi: 10.1083/jcb.141.7.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hay JC. Exp. Cell Res. 2001;271:10–21. doi: 10.1006/excr.2001.5368. [DOI] [PubMed] [Google Scholar]
- 34.Advani RJ, Bae H-R, Bock JB, Chao DS, Doung Y-C, Prekeris R, Yoo J-S, Scheller RH. J. Biol. Chem. 1998;273:10317–10324. doi: 10.1074/jbc.273.17.10317. [DOI] [PubMed] [Google Scholar]
- 35.Miller EA, Beilharz TH, Malkus PN, Lee MCS, Hamamoto S, Orci L, Schekman R. Cell. 2003;114:497–509. doi: 10.1016/s0092-8674(03)00609-3. [DOI] [PubMed] [Google Scholar]
- 36.Mossessova E, Bickford LC, Goldberg J. Cell. 2003;114:483–495. doi: 10.1016/s0092-8674(03)00608-1. [DOI] [PubMed] [Google Scholar]
- 37.Morsomme P, Prescianotto-Baschong C, Riezman H. J. Cell Biol. 2003;162:403–412. doi: 10.1083/jcb.200212101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aridor M, Fish KN, Bannykh S, Weisman J, Roberts TH, Lippincott-Schwartz J, Balch WE. J. Cell Biol. 2001;152:213–229. doi: 10.1083/jcb.152.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tisdale EJ, Plutner H, Matteson J, Balch WE. J. Cell Biol. 1997;137:581–593. doi: 10.1083/jcb.137.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muñiz M, Nuoffer C, Hauri H-P, Riezman J. Cell Biol. 2000;148:925–930. doi: 10.1083/jcb.148.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Husain M, Moss B. J. Virol. 2003;77:11754–11766. doi: 10.1128/JVI.77.21.11754-11766.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stephens DJ, Pepperkok R. J. Cell Sci. 2002;115:1149–1160. doi: 10.1242/jcs.115.6.1149. [DOI] [PubMed] [Google Scholar]
- 43.Mironov AA, Mironov AA, Jr., Beznoussenko GV, Trucco A, Lupetti P, Smith JD, Geerts WJC, Koster AJ, Burger KNJ, Martone ME, Deerinck TJ, Ellisman MH, Luini A. Dev. Cell. 2003;5:583–594. doi: 10.1016/s1534-5807(03)00294-6. [DOI] [PubMed] [Google Scholar]
- 44.Gusarova V, Brodsky JL, Fisher EA. J. Biol. Chem. 2003;278:48051–48058. doi: 10.1074/jbc.M306898200. [DOI] [PubMed] [Google Scholar]
- 45.Rashid KA, Hevi S, Chen Y, LeCaherec F, Chuck SL. J. Biol. Chem. 2002;277:22010–22017. doi: 10.1074/jbc.M112448200. [DOI] [PubMed] [Google Scholar]
- 46.McNew JA, Parlati F, Fukuda R, Johnston RJ, Paz K, Paumet F, Sollne TH, Rothman JE. Nature. 2000;407:153–159. doi: 10.1038/35025000. [DOI] [PubMed] [Google Scholar]
- 47.Trotter PJ, Storch J. J. Lipid Res. 1991;32:293–304. [PubMed] [Google Scholar]
- 48.Traber MG, Kayden HJ, Rindler MJ. J. Lipid Res. 1987;28:1350–1363. [PubMed] [Google Scholar]
- 49.Galli T. Mol. Biol. Cell. 1998;9:1437–1438. doi: 10.1091/mbc.9.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]