Abstract
Background & Aims
Ethanol abuse can lead to hepatic steatosis and evolve into cirrhosis and hepatocellular carcinoma. Pigment epithelium-derived factor (PEDF) is a multifunctional secreted glycoprotein that is expressed by hepatocytes. Proteomic, experimental, and clinical studies implicate PEDF’s role in lipid regulation. Because matrix metalloproteinase (MMP)-2/9 activity regulates PEDF levels, we investigated whether PEDF degradation by MMPs has a permissive role in ethanol-induced hepatic steatosis.
Methods
PEDF levels were examined in liver biopsy specimens from patients with ethanol-induced steatosis. Hepatic PEDF levels and MMP activity were assessed in 2 animal models of ethanol feeding (rats on an alcohol-containing liquid diet and mice given intragastric infusion of ethanol). The consequences of PEDF depletion in the liver were examined in PEDF-null mice.
Results
Liver biopsy samples from patients with ethanol-induced steatosis had reduced PEDF levels, compared with normal liver samples. Ethanol-fed animals had histologic steatosis and increased liver triglyceride content (P < .05), as well as reduced levels of hepatic PEDF and increased MMP-2/9 activity. Ethanol-exposed hepatic lysates degraded PEDF in a MMP-2/9-dependent manner, and liver sections demonstrated abundant MMP-2/9 activity in situ. Addition of recombinant PEDF to PEDF-null hepatocytes, reduced their triglyceride content.
Conclusions
Ethanol exposure leads to marked loss of hepatic PEDF in human livers and in 2 animal models of ethanol feeding. Loss of PEDF contributes to the accumulation of lipids in ethanol-induced hepatic steatosis.
Hepatic steatosis from ethanol use has traditionally been considered a benign and reversible condition. Emerging information, however, indicates that patients with steatosis can develop more advanced alcohol-related liver disease including cirrhosis and hepatocellular carcinoma. 1 The pathogenesis of ethanol-induced steatosis is multifactorial and involves changes in cellular redox potential and the reciprocal regulation of transcription factors that control lipogenesis and fat metabolism.2 Identification of new regulators of cellular lipid metabolism that are altered by ethanol may be relevant to the pathogenesis of ethanol-induced steatosis and liver injury.
Recently, an endogenous inhibitor of angiogenesis, pigment epithelium-derived factor (PEDF), has been identified in proteomic studies as a potential regulator of lipid metabolism.3–5 PEDF is a conserved, 50-kilodalton, hypoxia-suppressed glycoprotein with multiple actions in several organ systems.6,7 Best known as a neurotrophic factor and a potent inhibitor of angiogenesis,6 its mechanism of action remains largely unknown. PEDF is highly expressed in the adult liver.8 In liver diseases characterized by pathologic neovascularization such as hepatocellular carcinoma, PEDF expression is decreased.9 Recently, adipose triglyceride lipase (ATGL) was identified as a putative receptor for PEDF.10 PEDF, moreover, was able to reduce hepatocyte TG content in vitro.11 These findings have clinical relevance because serum PEDF levels appear to correlate with conditions associated with hepatic steatosis including the metabolic syndrome.12–14
Tissue levels of PEDF can be regulated; PEDF levels are markedly suppressed by hypoxia.6,15 Two mechanisms could potentially regulate PEDF levels: changes in synthesis or degradation. In this context, PEDF can be proteolytically degraded by matrix metalloproteinases (MMP) such as MMP-2 and MMP-9,15 which are generated in response to hypoxic stimuli. Activated hepatic stellate cells (HSC) along with endothelial cells and inflammatory cells are a major source of MMPs in the injured liver,16 and ethanol-induced steatosis alone has been found to stimulate hypoxic responses and activate HSC.17–19 Because PEDF is highly expressed in the normal liver and implicated in lipid metabolism, we hypothesized that ethanol might contribute to hepatocyte fat accumulation through MMP-mediated depletion of hepatic PEDF. In this study, liver biopsy specimens from patients with ethanol-induced steatosis show markedly reduced PEDF expression. We further find that ethanol feeding in 2 rodent models results in hepatic steatosis, PEDF depletion, and induction of MMP-2/9 activity. Restoring PEDF protein reduces hepatocyte TG content in vitro. These findings suggest that loss of hepatic PEDF is permissive for the development of liver steatosis after ethanol exposure.
Materials and Methods
Human Tissue Samples
Archival histologic specimens were obtained from patients with a clinical and pathologic diagnosis consistent with ethanol-induced steatosis (n = 6). Liver biopsy specimens were excluded if there was a clinical history or laboratory values that were consistent with a diagnosis of alcoholic hepatitis or if histologic evidence of fibrosis/inflammation was observed. Patients who had biopsies for increased serum levels of liver enzymes, but who were found to have a normal liver biopsy, served as controls (n = 6). An experienced pathologist (Dr Susan Crawford, Northwestern University) performed all histologic evaluations in a blinded manner. Relative intensity of PEDF labeling was assessed using National Institutes of Health (Bethesda, MD) software ImageJ. Measurements were done by placing a fixed box over the cytoplasm. Average densitometry was calculated by measuring 5 random fields for each patient slide. Values were normalized to the average density of controls. The institutional review board of the VA CT Healthcare approved the study.
Animal Models of Ethanol Exposure and PEDF Deficiency
Male Wistar rats (Charles River Co, Wilmington, MA) with starting weight of ~120 g were housed in a climate-controlled room on a 12-hour light/dark cycle, fed standard laboratory chow, and allowed to acclimatize for 1 week. To determine the effects of alcohol on hepatic PEDF content, animals were fed ethanol using the Lieber-DeCarli protocol for 6 weeks.20 In brief, pairs of age-matched rats were fed, with one of each pair receiving an alcohol-containing liquid diet (36% of total calories) or control diet (Dyets, Bethlehem, PA). The paired control animal received equivalent calories of glucose replacing alcohol. Diets were adjusted so that they received equal caloric density by volume. Livers from mice given intragastric infusion of ethanol or control low-fat diet for 4 weeks (gift of Dr Hide Tsukamoto, USC Alcohol Research Center) were assayed for hepatic PEDF content. Livers from this mouse model demonstrate steatosis in the absence of necrosis or inflammatory infiltrates when given for 4 weeks.21 PEDF null and age-matched control hepatocytes were used to assess the functional effects of PEDF restoration.7 Procedures were approved by the Institutional Animal Care and Use Committee of VA Connecticut and the VA Greater Los Angeles Healthcare Systems.
Cell Cultures, Primary Isolations, and Recombinant PEDF Production
The rat hepatic stellate cell line HSC-T6 has been described.22 HSC-T6 cells exposed to ethanol 10–50 mmol/L overnight in serum-free media were wrapped in Parafilm. Conditioned medium (CM) was concentrated using a Centricon filter (Pall, Ann Arbor, MI) and normalized for protein content. Lysates were prepared using a commercial buffer (Pierce, Rockford, IL). For primary HSC isolations, Sprague–Dawley rats were anesthetized, and liver matrix was digested in situ by Pronase E (2.4 mg/mL) and collagenase B (0.3– 0.45 mg/mL) (Roche, Basel, Switzerland) perfusion. Single cell suspensions of HSC were subjected to density gradient centrifugation using 9%–11% Nycodenz (Sigma, St Louis, MO). HSC were used from days 2, 7, and 10 after isolation.
Hepatocytes were prepared as described.11 Briefly, livers were perfused with Hanks’ A and then Hanks’ B medium containing 0.05% collagenase (Boehringer Mannheim Biochemicals, Mannheim, Germany) and 0.8 U trypsin inhibitor (Sigma). Cells (1 × 106) were plated on 35-mm collagen I coated plates (BD Biosciences, Bedford, MA) in Williams’ medium E supplemented with 10% fetal calf serum (FCS), 10 mmol/L HEPES, 2 mmol/L L-glutamine, 1 µmol/L dexamethasone, 4 mg/L insulin, and antibiotics. Cells were used 24–48 hours after isolation. Recombinant PEDF (rPEDF) was made as described.6
Immunoblotting
Antibodies against PEDF6 and the catalytic domains of MMP-2 and -9 (Chemicon, Temecula, CA) were used for immunoblotting as described.7 β-actin (Sigma) and Coomassie staining served as the loading standard for lysates and CM, respectively. National Institutes of Health ImageJ was used to compare band intensities.
MMP-Dependent Degradation of PEDF
In vitro degradation of PEDF by MMP-2 was assessed using rPEDF 500 ng and commercial MMP-2 100 ng (human 66-kilodalton intermediate form; Calbiochem, Gibbstown, NJ) in a buffer containing CaCl2 5 mmol/L in the absence and presence of the gelatinase activator 4-aminophenylmercuric acid (APMA) (Sigma) 1 mmol/L. PEDF purity, the processing of precursor MMP-2 to active forms, and degradation of PEDF by MMP-2 were evaluated by electrophoretic separation on SDS (10%)-PAGE gels and silver stain (Pierce).
A specific cyclic peptide inhibitor, CTTHWGFTLC (CTT) (BIOMOL, Plymouth Meeting, PA), was used at 10–100 µmol/L to inhibit MMP-2/9 activities. At 50 µmol/L, CTT has been shown to inhibit 90% of MMP-2 activity without affecting the activity of other MMP family members or serine proteases, which can activate pro-MMPs.23 To examine PEDF degradation over time, 250 ng of rPEDF was incubated in a reaction buffer (50 mmol/L Tris, 5 mmol/L CaCl2, 200 mmol/L NaCl) at 37°C with 100–150 µg of lysates from control or ethanol-treated lysates from 0 to 24 hours in the presence and absence of CTT 100 µmol/L.
SDS-Gelatin and In Situ Zymography
To detect MMP activities in hepatic lysates, CM, and liver sections, SDS-gelatin and in situ zymography were performed as described.24,25 Details are described online (see supplementary methods and Supplementary Figure 1 online at www.gastrojournal.org).
TG Determination
Quantitative TG measurements were performed with an enzymatic method (Stanbio Laboratory, Boerne, TX) as described.11 Samples were normalized to protein content.
Immunohistochemistry
Immunohistochemistry was performed as described. 7 Tissue samples were stained with H&E and antibodies against PEDF and α-smooth muscle actin (α-SMA).
Statistical Analysis
P values were calculated using 2-tailed Student t tests on Prism, version 4.0 (La Jolla, CA), with P < .05 deemed statistically significant. Values were stated as mean ± SE.
Results
Ethanol-Induced Hepatic Steatosis in Patients Results in Hepatic PEDF Suppression
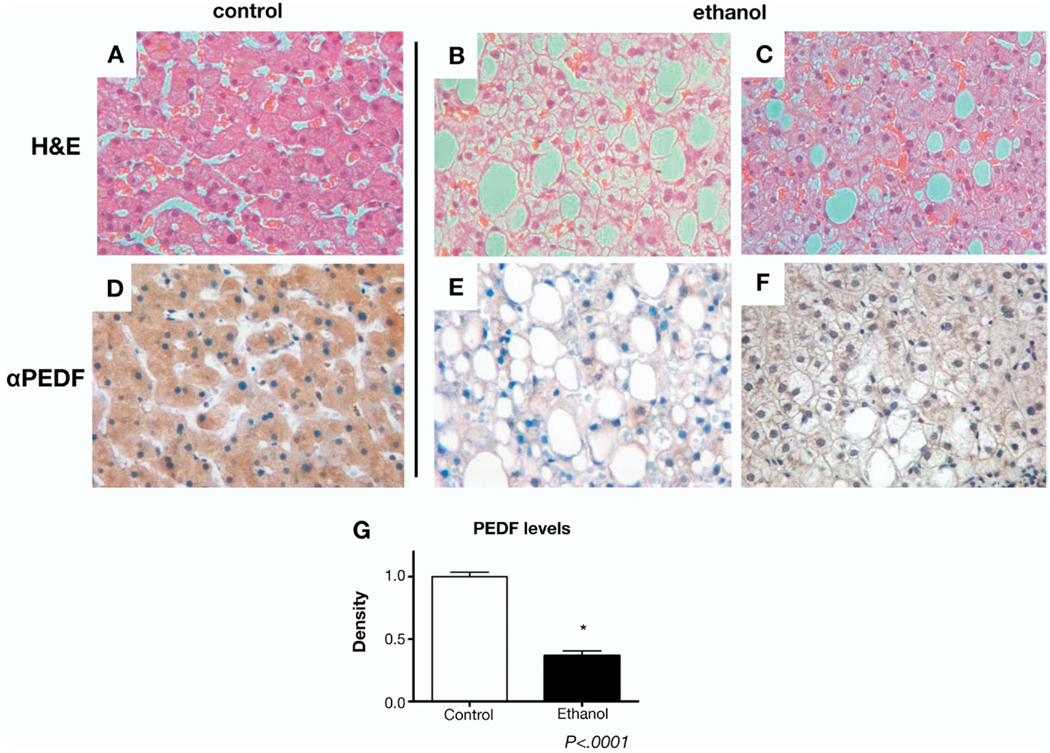
H&E stains of liver sections from patients with ethanol-induced hepatic steatosis demonstrated macro- and/or microvesicular steatosis in the absence of inflammatory infiltrates or hepatic fibrosis (Figure 1B and C) compared with controls (Figure 1A). Immunohistochemical labeling for PEDF in controls demonstrated abundant cytoplasmic PEDF immunoreactivity (Figure 1D) consistent with its high degree of hepatic expression.8 Hepatic PEDF labeling was markedly diminished in all patients with ethanol-induced steatosis compared with controls (Figure 1E and F). The average intensity of PEDF staining within ethanol-induced steatosis specimens was decreased 64% compared with controls (Figure 1G, P < .0001).
Figure 1.
Liver biopsy specimens from patients with ethanol-induced steatosis demonstrate loss of PEDF. (A–C) H&E-stained sections from normal human liver (A). PEDF labeling in tissues from patients with chronic alcohol histories demonstrate marked macrovesicular steatosis (B) and mixed macro-/microvesicular steatosis (C) in the absence of inflammatory or fibrotic changes. (D–F) Immunohistochemical staining for PEDF (brown label) shows abundant expression in the cytoplasm of normal hepatocytes (D) with loss of expression in hepatocytes with lipid accumulation in alcoholic patients (E and F). Results are representative of 6 controls and 6 patients with ethanol-induced steatosis. (G) PEDF labeling measured by densitometry shows markedly reduced PEDF in ethanol-exposed livers (*P < .0001).
Ethanol-Fed Livers Demonstrate MMP-2/9-Dependent Proteolysis of PEDF
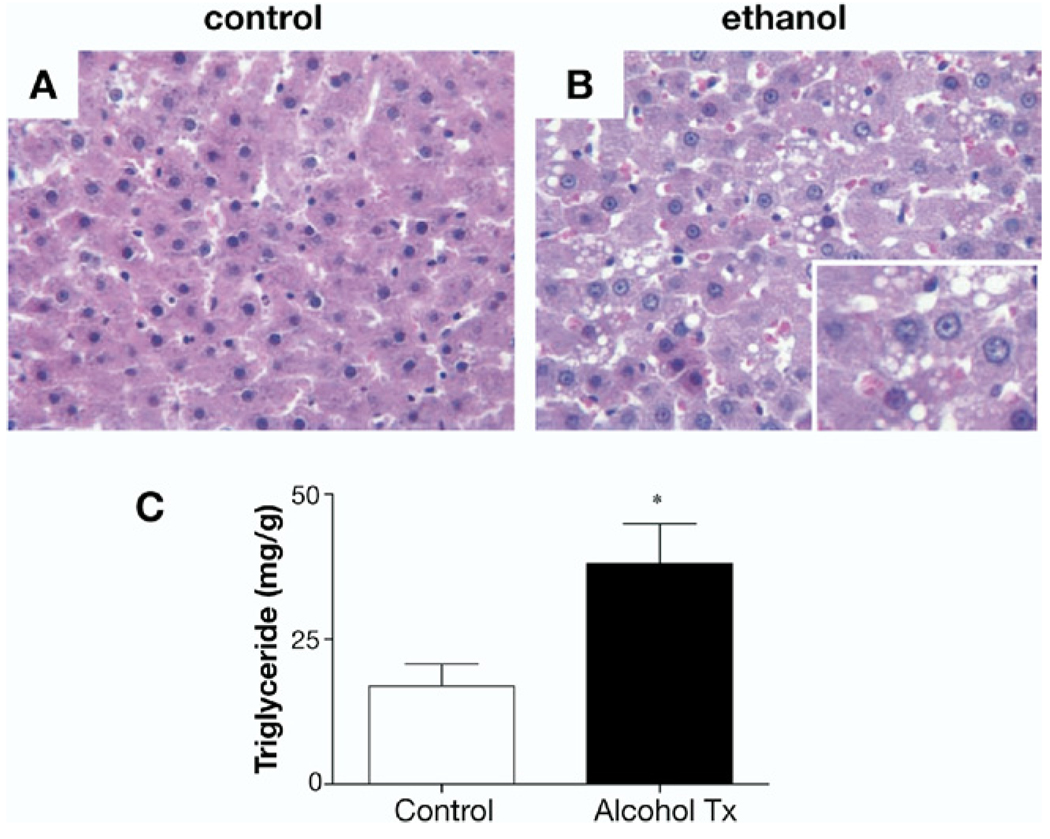
Control and ethanol-fed rats showed no significant differences in body weight (405 ± 6.3 vs 397 ± 8.7 g, respectivey, P = NS) at the end of the study period. H&E staining of control animals (Figure 2A) showed normal liver architecture, whereas ethanol-fed rats showed hepatocyte lipid accumulation (Figure 2B). Hepatic TG levels in animals fed the ethanol diet were more than 2-fold higher than the control diet group (Figure 2C; 38.1 ± 6.9 vs 16.9 ± 3.9, mg/g TG/g of tissue, respectively, P < .05). There was no liver fibrosis or inflammatory changes seen with the Lieber–DeCarli diet, consistent with this model.20 We next examined the effects of ethanol on hepatic PEDF expression.
Figure 2.
Livers from rats on a Lieber–DeCarli diet demonstrate steatosis but no inflammation or fibrosis. (A and B) H&E-stained sections from control rat livers (A) and rats fed ethanol for 6 weeks (B). (C) Triglyceride content increased more than 2-fold in the ethanol-fed group compared with controls: 38 ± 6.9 vs 17 ± 3.9 mg TG/g liver, respectively (*P < .05).
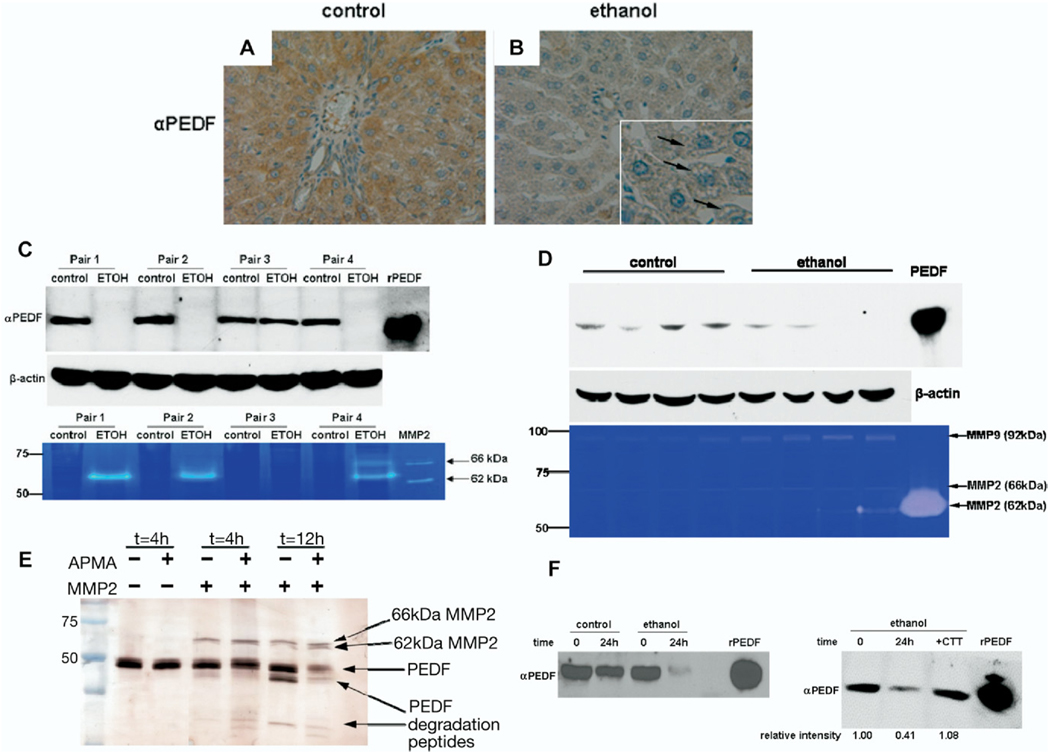
Ethanol feeding in both rats and mice had a marked suppressive effect on hepatic PEDF levels. In livers from control rats, PEDF immunoreactivity was most intense over hepatocytes (Figure 3A) and was also present by immunoblotting (Figure 3C). However, in animals fed ethanol, PEDF tissue labeling was markedly decreased (Figure 3B), and PEDF was undetectable by immunoblotting in 3 of the 4 ethanol pair-fed animals (Figure 3C). In other tissues, PEDF levels can be regulated by MMP-2 and -9.15 To detect such proteases in the liver, zymography was performed. In ethanol-fed rats, there was a prominent increase in MMP-2 activity compared with controls (Figure 3C). The intermediate (66 kilodaltons) and fully active (62 kilodaltons) forms of MMP-2 were present (Figure 3C). No MMP-9 activity was identified in rat hepatic lysates. In the 1 ethanol-fed animal, in which PEDF levels were minimally changed compared with its control (pair No. 3), there was undetectable MMP-2 activity by zymography. In this pair of animals, the ethanol-fed animal had the smallest increase in hepatic TG accumulation, suggesting that its ethanol ingestion might have been inadequate and that a threshold of lipid accumulation might be required to see the loss of PEDF.
Figure 3.
Ethanol feeding results in loss of PEDF, increased MMP-2 activity, and MMP-2/9-mediated degradation of PEDF in livers from 2 rodent species. (A and B) Immunohistochemistry for PEDF demonstrates abundant cytoplasmic staining in control rat livers (A, brown label), whereas ethanol feeding results in accumulation of intracellular lipids (inset, arrows) and loss of PEDF (B). (C) Immunoblotting of hepatic lysates from control and ethanol-fed pairs of rats reveal absence of hepatic PEDF levels in 3 of the 4 animals fed ethanol. β-actin loading controls shown below. (C) Zymography of hepatic lysates show expression of the fully active form of MMP-2 (62 kilodaltons) in animals in which PEDF expression is lost. The intermediate form of MMP-2 (66 kilodaltons) was included as a positive control and is converted to the fully active form in incubation buffer. In pair 3, in which there is no appreciable loss of PEDF with ethanol exposure, MMP-2 activity was not detected. (D) Loss of PEDF also occurs in a mouse model of ethanol feeding. In 2 animals in which PEDF levels could not be detected by immunoblotting, the presence of the fully active form of MMP-2 (62 kilodaltons) was detected on zymography. Ethanol feeding increased levels of the precursor form of MMP-9 (92 kilodaltons). (E) MMP-2 degrades rPEDF in vitro. Incubation of human MMP-2 (66 kilodaltons) with rPEDF results in autoactivation of MMP-2 to its fully active form (62 kilodaltons) and PEDF degradation over time. APMA, an MMP-2 activator, leads to additional PEDF degradation. (F, left) Incubation of rPEDF with control and ethanol-fed rat liver lysates reveals degradation of PEDF at 24 hours. (F, right) Degradation of rPEDF by ethanol-exposed hepatic lysates is MMP-2 dependent; addition of the specific MMP-2 inhibitor, CTT, preserves PEDF.
Similar to rats on the Lieber–DeCarli diet, immunoblots of hepatic lysates from mice given intragastric ethanol infusion for 4 weeks displayed markedly decreased or absent PEDF compared with controls (Figure 3D). The fully active isoform of MMP-2 (62 kilodaltons) was detected in the hepatic lysates in which PEDF was absent (Figure 3D), illustrating the reciprocal regulation of PEDF and MMP-2. Unlike the rat livers, we found induction of the precursor (92 kilodaltons) but not the active form of MMP-9 compared with controls. These findings are consistent with those reported in other organs in which hypoxic stress up-regulates MMP-2/9-mediated PEDF degradation.15
Time-dependent proteolysis of PEDF was examined in vitro; it demonstrated that the intermediately active form of MMP-2 (66 kilodaltons) could activate itself and degrade PEDF in the absence and presence of the MMP activator APMA (Figure 3E). Finally, we observed that ethanol-exposed rat liver lysates proteolytically digested most of a fixed quantity of rPEDF substrate compared with control lysates at 24 hours (Figure 3F, left). Blocking the MMP-2/9 activity in the liver lysate with CTT 100 µmol/L protected PEDF from degradation (Figure 3F, right). These findings suggest that PEDF degradation in the ethanol-fed rat liver is MMP-2/9 dependent.
Studies of PEDF, MMP-2/9, MT1-MMP, and TIMP1/2 gene expression revealed that PEDF messenger RNA (mRNA) levels are not significantly altered, whereas MMP-2/9 expression increases with ethanol-induced hepatic steatosis (see supplementary methods, Supplementary Figure 1, and Supplementary Table). This preserved PEDF expression is consistent with previous reports that observed that hypoxia significantly reduced PEDF protein but did not alter PEDF gene expression.6,15 Thus, degradation of hepatic PEDF protein by MMP-2/9 plays the major role in regulating its levels after ethanol exposure.
Because HSC are thought to be an important source of MMPs, we next examined the potential for HSC to secrete MMP-2/9 and degrade PEDF.
HSC-Derived MMP-2/9 Degrades PEDF
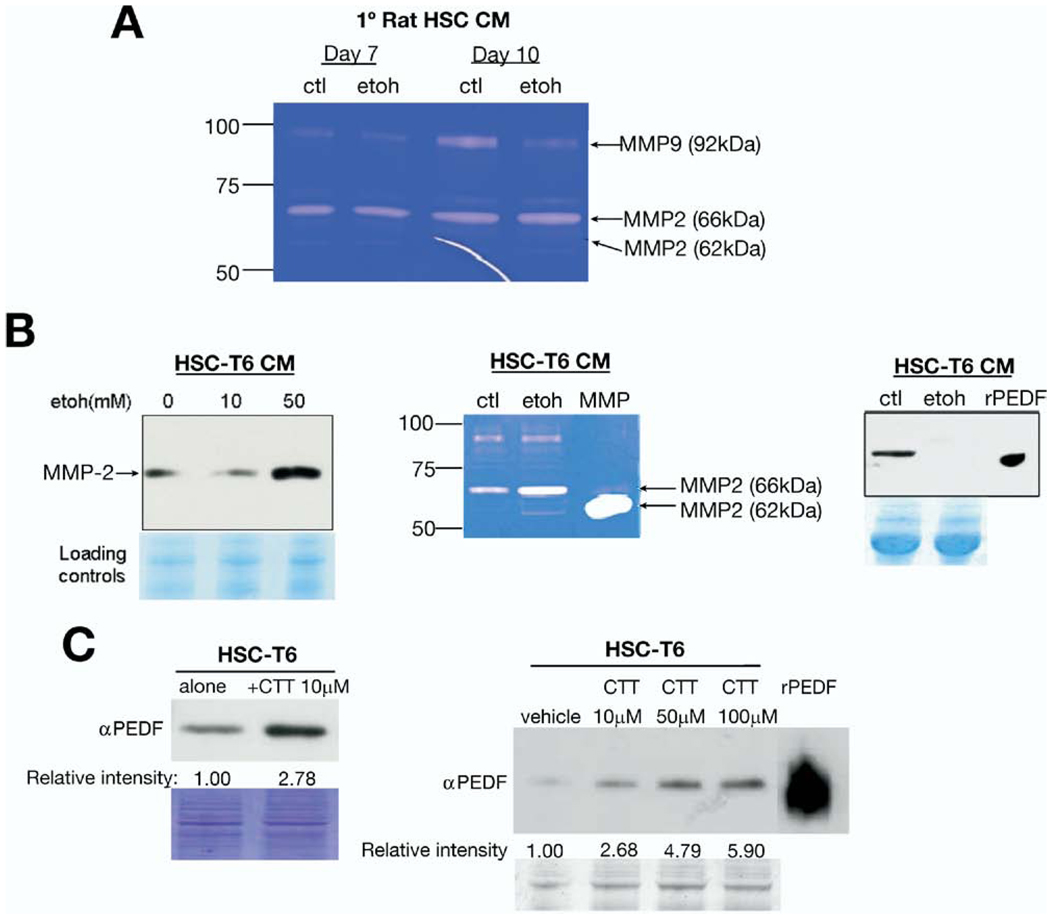
In various liver diseases, activated HSC degrade the normal matrix by secreting MMPs such as MMP-2/9 in response to hypoxia, a condition caused by ethanol feeding.18 To determine whether acute ethanol exposure had the same effect as hypoxia, we exposed primary isolated rat HSC (days 2, 7, and 10) to 50 mmol/L ethanol overnight and assayed gelatinase activity. Primary rat HSC at day 2 demonstrated minimal activity on zymography in the absence or presence of acute ethanol treatment (data not shown). Primary HSC from days 7 and 10 demonstrated gelatinase activity corresponding to all 3 forms of MMP-2 and the precursor form of MMP-9 (Figure 4A); however, there was no increase in activities with acute ethanol exposure.
Figure 4.
Hepatic stellate cell (HSC)- derived MMP-2 degrades PEDF. (A) Primary rat HSC from days 7 and 10 after isolation exposed to ethanol 50 mmol/L did not show increased MMP-2/-9 activity as assessed by zymography. (B) Conditioned medium (CM) from HSC-T6 demonstrate increased MMP-2 levels with immunoblotting (left), enhanced MMP-2 activity (center), and a reciprocal decrease in PEDF expression (right) with acute ethanol exposure. (C) Incubation with the specific MMP-2/9 inhibitor, CTT 50 µmol/L, results in a nearly 3-fold increase in PEDF signal from HSC-T6 (left), indicating that PEDF is secreted from these cells and that its levels are MMP-2 dependent. CTT (10–100 µmol/L) causes a concentration-dependent increase in PEDF levels in cultured rat hepatic stellate cells (right). Coomassie stain demonstrated equivalent protein loading.
To verify whether ethanol had effects on MMP levels and activity in an activated HSC cell line, immunoblotting for PEDF and MMP-2/9 was assessed on CM from rat-derived HSC-T6.22 These data showed an increase in MMP-2 levels and activity with ethanol exposure and a corresponding decrease in PEDF (Figure 4B). We could not detect MMP-9 levels using a commercial antibody (data not shown). To confirm the role of MMPs in regulating PEDF levels, we assessed MMP-2/9 inhibition in HSC-T6 cells. Using a specific inhibitor, CTT, resulted in an almost 3-fold increase in PEDF levels (Figure 4C, left) in HSC-T6 CM. The effects of MMP-2/9 inhibition with CTT on PEDF levels were concentration dependent (Figure 4C, right). These observations suggest that, in the course of HSC activation, MMP-2/9 activity increases and degrades PEDF.
Ethanol Exposure Results in Gelatinase Activity In Situ
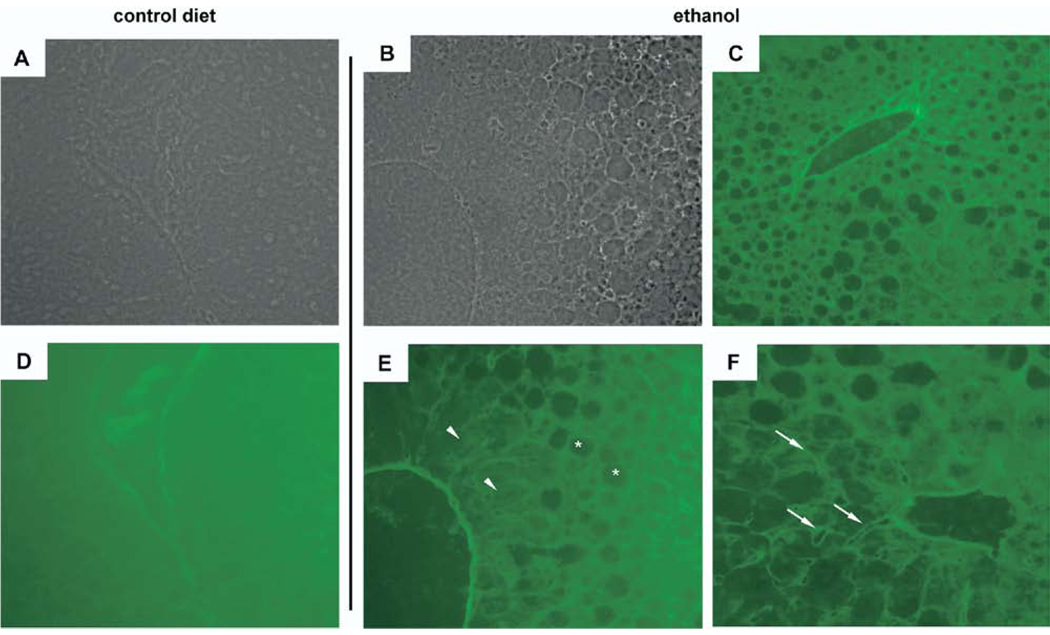
Because several cell types in the liver can secrete MMP-2/9, in situ zymography was done to localize activity. 25 In control livers (Figure 5A [phase] and D), uniform staining indicated absence of gelatinase activity. Sections from ethanol-fed mouse livers demonstrated discrete areas of obvious clearing corresponding to gelatin digestion (Figure 5C, E, and F). The most evident areas corresponded to regions of prominent hepatocyte steatosis (Figure 5E). However, even in regions in which hepatocyte steatosis was absent, areas of clearing adjacent to hepatocytes consistent with the perisinusoidal space (Figure 5F) were apparent. These findings indicate that ethanol- induced hepatic steatosis results in the induction of gelatinase activity within the liver. However, because the fully active form of MMP-2 and -9 are secreted and several cell types are implicated in their release, precise identification of which cell types are responsible in the ethanol-exposed liver remains unclear.
Figure 5.
In situ zymography (ISZ) reveals gelatinase activity in livers from ethanol-fed mice. (A and D) Representative phase (A) and corresponding ISZ (D) image of control livers show uniform green stain consistent with lack of gelatinase activity. (B and E) Phase image (B) shows abundant lipid accumulation around a central vein. ISZ image (E) shows gelatin digestion in areas overlying hepatocyte steatosis (asterisks) and diminished but preserved gelatin staining in a cuff of hepatocytes without steatosis (arrowheads). (C) Low-power ISZ image illustrates discrete regions of gelatin degradation that correlate to areas with the most lipid accumulation. (F) Restricted areas of gelatin digestion corresponding to the perisinusoidal space (arrows) occur in areas in which hepatocyte steatosis is less evident.
HSC Activation Is Found in Ethanol-Exposed and PEDF Null Livers
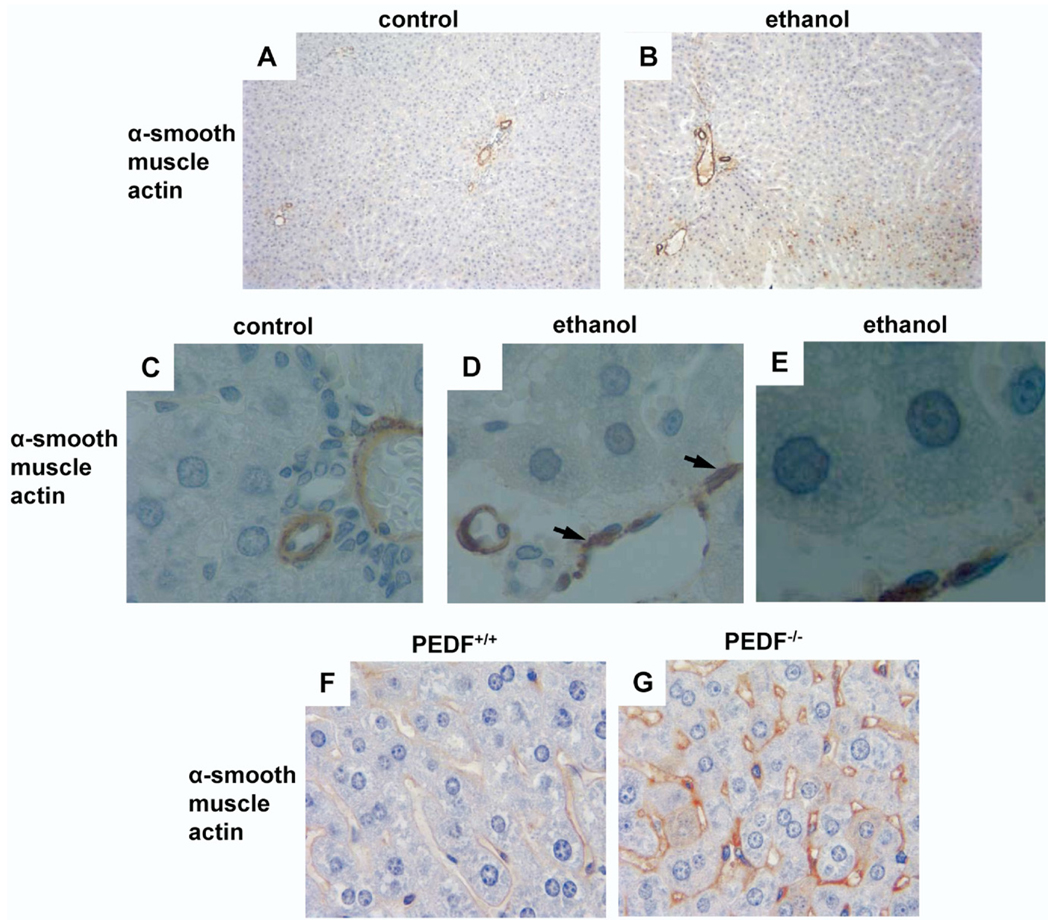
Because a previous clinical study indicated that ethanol-induced steatosis alone can be associated with HSC activation,17 we examined rat livers for HSC activation after alcohol feeding using an antibody to α-SMA. In the periportal regions of control animals, α-SMA labeling was absent except for labeling of vascular structures (Figure 6A). With ethanol feeding, α-SMA labeling occurred in a patchy distribution (Figure 6B, brown label). Under high power, α-SMA labeling could be seen in the perisinusoidal space (Figure 6D), but it was absent in controls (Figure 6C). A magnified image (Figure 6E) of ethanol-fed hepatocytes shows intracellular lipid accumulation. Because HSC activation was observed with ethanol-induced hepatic steatosis, a condition associated with PEDF depletion, similar changes were evaluated for in animals with genetic PEDF deletion. Age-matched controls displayed no α-SMA labeling (Figure 6F), whereas PEDF-null livers demonstrated abundant perisinusoidal α-SMA labeling (Figure 6G). Together, these data indicate that ethanol feeding is associated with enhanced hepatic MMP-2/9 activity and proteolytic degradation of PEDF. Moreover, the presence of α-SMA positivity in PEDF-null livers suggests that PEDF could have a role in suppressing HSC activation that is similar to the reported cell-cycle effects of PEDF on mesenchymal cells.26,27
Figure 6.
HSC activation occurs in ethanol-induced hepatic steatosis and in PEDF null livers. (A–D) Labeling for α-smooth muscle actin (α-SMA) in control rat livers was confined to vascular structures (A, low power and C, high power). α-SMA labeling (B, brown stain and D, arrows) in livers from ethanol-fed rats was consistent with HSC activation. (E) Magnified image of ethanol-fed hepatocytes reveals intracellular lipid accumulation. (F and G) α-SMA labeling was prominent in PEDF null livers (G, arrow) compared with age-matched controls (F).
Restoration of PEDF Reduces TG Content in Null Hepatocytes
To determine whether restoring PEDF could reduce hepatocyte TG content in vitro, we treated isolated wild-type and PEDF null hepatocytes with rPEDF 1000 ng/mL for 2 hours in the absence and presence of ethanol 50 mmol/L. Wild-type hepatocytes treated with PEDF (n = 6) (Figure 7A) demonstrated no change in TG content with PEDF treatment or ethanol exposure. PEDF-null hepatocytes treated with PEDF (n = 6) (Figure 7B) showed a significant reduction in TG content, 35.8 ± 1.4 vs 26.7 ± 1.4 µg TG/mg protein, respectively (P = .001). Overnight ethanol exposure tended to increase TG content (P = .09); this effect was significantly reduced in the presence of rPEDF: 41.7 ± 2.7 vs 29.5 ± 1.4 µg TG/mg protein, respectively (P = .004).
Figure 7.
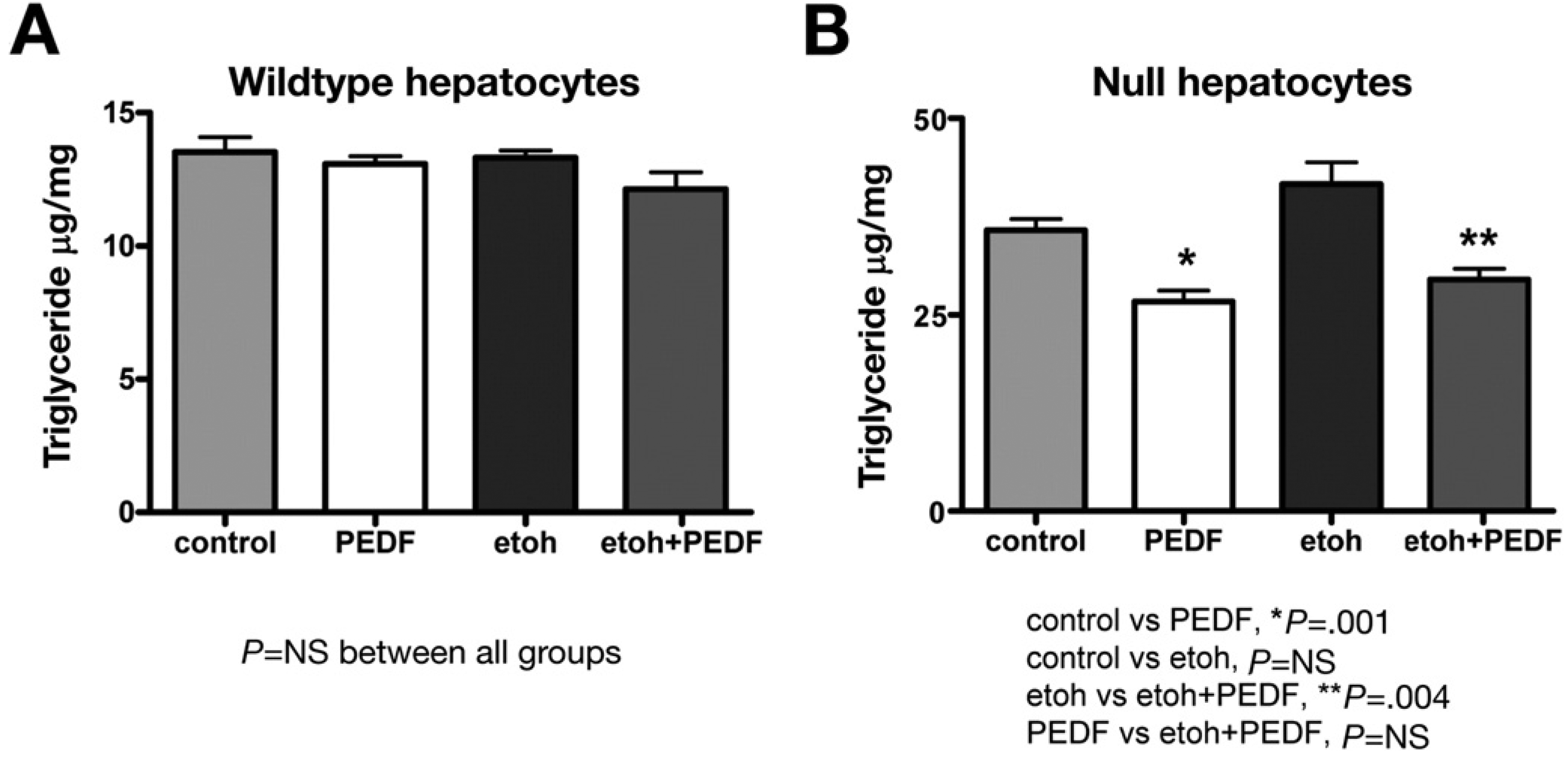
Addition of rPEDF to PEDF-null hepatocytes in vitro reduces TG content. (A) Adding rPEDF to isolated wild-type hepatocytes had no effect on TG content either in the absence or presence of ethanol. (B) Restoring PEDF to null hepatocytes significantly (*P = .001) reduced TG content compared with controls. There was a trend (P = .09) toward increased TG content with ethanol exposure in null hepatocytes. Adding rPEDF to ethanol-exposed hepatocytes significantly (**P = .004) decreased TG content.
Discussion
The current study investigated the expression and regulation of PEDF, a putative regulator of cellular lipid content, in ethanol-induced hepatic steatosis. In liver biopsy specimens from patients with ethanol-induced steatosis, PEDF levels were dramatically reduced. This finding is consistent with recent proteomic, experimental, and clinical data that support a role for PEDF in lipid homeostasis.3–5,10–14,28 To evaluate PEDF regulation, 2 experimental ethanol feeding models demonstrated marked suppression of hepatic PEDF. Accompanying the loss of PEDF was a reciprocal increase in hepatic MMP-2 activity in a rat model and an increase in MMP-2 and -9 in mice. Furthermore, the expression of hepatic MMP-2/9 activity that could degrade PEDF was induced by chronic ethanol feeding.
Previous studies of PEDF expression in hypoxia have suggested that PEDF regulation is primarily post-translational. 6,15 Because the loss of hepatic PEDF occurred in 2 animal models of ethanol feeding and in archival human tissue, a common pathway of PEDF degradation in ethanol-induced steatosis was explored. We first examined PEDF protein regulation by MMP-2 since we detected its activity, but not MMP-9, in rats. PEDF protein could be proteolytically degraded by recombinant MMP-2 within hours in vitro. Rat hepatic lysates from the ethanol-fed animals could also digest PEDF, and this activity was blocked by an MMP-2/9 inhibitor. These findings were confirmed in mice fed ethanol, but the precursor form of MMP-9 was also increased in the murine model. Moreover, PEDF protein, but not mRNA expression, was decreased in mouse livers in which fully activated MMP-2 was not detected by zymography, suggesting that other proteases such as MMP-9 might degrade PEDF.
Activated HSCs are an important source of MMPs in the liver.16 Because α-SMA staining was detected in the ethanol-fed livers, HSC may represent one source of MMP-2/9 after chronic alcohol exposure. We observed that cultured and isolated HSC secrete both PEDF and MMP-2/9; inhibition of the proteases led to increased PEDF levels in cultured cells. Although the contribution of other cell types cannot be excluded, these findings implicate HSC-derived MMP-2/9-mediated proteolysis as a regulator of PEDF in the liver. Similar regulatory mechanisms occur in other organs. For example, endogenous PEDF has a critical role in maintaining vascular quiescence in the eye; with hypoxia, PEDF levels are reduced by MMP-2/9-dependent degradation.15 Although the current study did not evaluate for MMP levels or activity in human tissue, a study of archival human tissue has reported that HSC activation correlates with the degree of steatosis caused by ethanol.17
The reciprocal regulation of MMP-2/9 and PEDF in this study supports the idea that increased expression of proteases responsible for matrix turnover are coupled to the loss of endogenous proteins like PEDF that can maintain vascular and stromal quiescence.7,27 MMPs, a family of zinc-dependent proteases, degrade multiple components of the extracellular matrix.29 Distinct MMPs have been implicated in both perpetuating and limiting experimental liver injury.30,31 In nutritional models of hepatic steatosis, nonspecific blockade of MMP activity can ameliorate steatosis, suggesting that lipid accumulation and alterations in the matrix are coupled processes.32 MMP-2, in particular, has been implicated as critical for HSC invasion in response to chemotactic stimuli.33 Thus, induction of MMP-2 activity and PEDF depletion after chronic ethanol exposure may represent a critical step in the development of progressive alcoholic liver disease.
This study highlighted a role for HSC-derived MMPs in PEDF degradation; however, we cannot exclude the potential contribution of other cell types that are known to secrete gelatinases. MMP-2 expression in human liver specimens has been identified in stellate cells, endothelial cells, and inflammatory cells.16 Inflammatory infiltrates were not present in our patient biopsy specimens and are not a component of the Lieber–DeCarli diet or the Tsukamoto-French murine model for 4 weeks.20,21 However, endothelial cells are known to express MMP-2 and MT1-MMP in liver diseases16 and could also contribute to the MMP activities and reduced PEDF levels observed in this study.
A related point is the absence of any increase in MMP-2 or -9 activities with acute ethanol exposure using primary rat stellate cells. We used primary HSC at multiple times after isolation and detected no increase in MMP-2/9 activity with acute ethanol exposure. Thus, MMP-2/9 activity seen with tissue lysates as opposed to the primary HSC may reflect the contribution of other cells including the endothelium, activation of precursor zymogens by the lysis/renaturation process itself,25 the short exposure time to ethanol using primary HSC, or a combination of these factors. Finally, we selected human archival tissue with ethanol-induced steatosis in the absence of confounding clinical and histologic features such as alcoholic or viral hepatitis or the presence of inflammation/fibrosis. This limited the number of human specimens available. However, by restricting biopsies to those with steatosis only, we highlighted PEDF’s role as a novel lipid regulator that is suppressed with lipid accumulation. Future studies evaluating PEDF in other conditions associated with hepatocyte lipid accumulation such as chronic hepatitis C virus infection and nonalcoholic fatty liver disease are needed to determine the relevance of our findings to other diseases.
Our results are consistent with recent studies that have identified angiogenic factors as regulators of lipid content. 34 Proteomic screens had raised the possibility of PEDF’s involvement in lipid regulation,3–5 but the lack of an identifiable receptor had made investigation of this activity difficult. Notari et al reported that ATGL binds avidly to PEDF and induces lipase activity in a cell-free system.10 Subsequently, we demonstrated that ATGL is present in murine liver and human hepatocellular carcinoma cell lines, immunoprecipitates with rPEDF, and has functional activity in vitro.11 Consistent with an endogenous role in lipid metabolism, restoration of PEDF to null, but not wild-type, hepatocytes significantly reduced TG content. Whether our findings with ethanol feeding will be relevant to other models of hepatic steatosis is unknown. However, in studies using the methionine-choline deficient diet, steatosis was associated with a marked increase in MMP-2 expression,35 suggesting that loss of PEDF may be contributory in nutritional models of hepatic steatosis.
In summary, ethanol-induced hepatic steatosis in humans and in 2 animal models of ethanol feeding leads to marked suppression of PEDF. Regulation of PEDF occurs through its proteolysis by enhanced MMP-2/9 activity. Absence of PEDF is associated with TG accumulation in hepatocytes, which can be reversed with exogenous PEDF. Endogenous PEDF appears to play a role in regulating lipid homeostasis within the normal liver. Its loss may contribute to some of the key phenotypic features associated with chronic ethanol exposure and potentially other diseases characterized by hepatic steatosis.
Supplementary Material
Acknowledgments
The authors thank Dr James L. Boyer (Yale University) for insightful comments, Dr Marie Robert (Yale University) for additional archival liver tissue, Nina Sheung and Dr Jon Dranoff (Yale University) for HSC isolations, and the USC Research Center for Alcoholic Liver and Pancreatic Disease (P50-AA11999) for livers from the Lieber–DeCarli and mouse intragastric ethanol models.
The authors disclose the following: Supported by an American Liver Foundation grant; NIH Liver Center Core DK34989 (to C.C.), P60 AA11999 (to S.J.P.), DK54021; and VA Merit Award (to F.S.G.).
Abbreviations used in this paper
- HSC
hepatic stellate cell
- MMP-2/9
matrix metalloproteinase-2/9
- PEDF
pigment epithelium-derived factor
- TG
triglyceride
- TIMP
tissue inhibitor of metalloproteinase
Footnotes
Supplementary Data
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi: 10.1053/j.gastro.2008.09.065.
References
- 1.Teli MR, Day CP, Burt AD, et al. Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet. 1995;346:987–990. doi: 10.1016/s0140-6736(95)91685-7. [DOI] [PubMed] [Google Scholar]
- 2.You M, Crabb DW. Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1–G6. doi: 10.1152/ajpgi.00056.2004. [DOI] [PubMed] [Google Scholar]
- 3.Kratchmarova I, Kalume DE, Blagoev B, et al. A proteomic approach for identification of secreted proteins during the differentiation of 3T3-L1 preadipocytes to adipocytes. Mol Cell Proteomics. 2002;1:213–222. doi: 10.1074/mcp.m200006-mcp200. [DOI] [PubMed] [Google Scholar]
- 4.Wang P, Mariman E, Keijer J, et al. Profiling of the secreted proteins during 3T3-L1 adipocyte differentiation leads to the identification of novel adipokines. Cell Mol Life Sci. 2004;61:2405–2417. doi: 10.1007/s00018-004-4256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zvonic S, Lefevre M, Kilroy G, et al. Secretome of primary cultures of human adipose-derived stem cells: modulation of serpins by adipogenesis. Mol Cell Proteomics. 2007;6:18–28. doi: 10.1074/mcp.M600217-MCP200. [DOI] [PubMed] [Google Scholar]
- 6.Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 7.Doll JA, Stellmach VM, Bouck NP, et al. Pigment epithelium-derived factor regulates the vasculature and mass of the prostate and pancreas. Nat Med. 2003;9:774–780. doi: 10.1038/nm870. [DOI] [PubMed] [Google Scholar]
- 8.Tombran-Tink J, Mazuruk K, Rodriguez IR, et al. Organization, evolutionary conservation, expression and unusual Alu density of the human gene for pigment epithelium-derived factor, a unique neurotrophic serpin. Mol Vis. 1996;2:11. [PubMed] [Google Scholar]
- 9.Matsumoto K, Ishikawa H, Nishimura D, et al. Antiangiogenic property of pigment epithelium-derived factor in hepatocellular carcinoma. Hepatology. 2004;40:252–259. doi: 10.1002/hep.20259. [DOI] [PubMed] [Google Scholar]
- 10.Notari L, Baladron V, Aroca-Aguilar JD, et al. Identification of a lipase-linked cell membrane receptor for pigment epithelium-derived factor. J Biol Chem. 2006;281:38022–38037. doi: 10.1074/jbc.M600353200. [DOI] [PubMed] [Google Scholar]
- 11.Chung C, Doll JA, Gattu AK, et al. Anti-angiogenic pigment epithelium-derived factor regulates hepatocyte triglyceride content through adipose triglyceride lipase (ATGL) J Hepatol. 2008;48:471–478. doi: 10.1016/j.jhep.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 12.Ogata N, Matsuoka M, Matsuyama K, et al. Plasma concentration of pigment epithelium-derived factor in patients with diabetic retinopathy. J Clin Endocrinol Metab. 2007;92:1176–1179. doi: 10.1210/jc.2006-2249. [DOI] [PubMed] [Google Scholar]
- 13.Yamagishi S, Adachi H, Abe A, et al. Elevated serum levels of pigment epithelium-derived factor in the metabolic syndrome. J Clin Endocrinol Metab. 2006;91:2447–2450. doi: 10.1210/jc.2005-2654. [DOI] [PubMed] [Google Scholar]
- 14.Jenkins AJ, Zhang SX, Rowley KG, et al. Increased serum pigment epithelium-derived factor is associated with microvascular complications, vascular stiffness and inflammation in type 1 diabetes. Diabet Med. 2007;24:1345–1351. doi: 10.1111/j.1464-5491.2007.02281.x. [DOI] [PubMed] [Google Scholar]
- 15.Notari L, Miller A, Martinez A, et al. Pigment epithelium-derived factor is a substrate for matrix metalloproteinase type 2 and type 9: implications for down-regulation in hypoxia. Invest Ophthalmol Vis Sci. 2005;46:2736–2747. doi: 10.1167/iovs.04-1489. [DOI] [PubMed] [Google Scholar]
- 16.Takahara T, Furui K, Yata Y, et al. Dual expression of matrix metalloproteinase-2 and membrane-type 1-matrix metalloproteinase in fibrotic human livers. Hepatology. 1997;26:1521–1529. doi: 10.1002/hep.510260620. [DOI] [PubMed] [Google Scholar]
- 17.Reeves HL, Burt AD, Wood S, et al. Hepatic stellate cell activation occurs in the absence of hepatitis in alcoholic liver disease and correlates with the severity of steatosis. J Hepatol. 1996;25:677–683. doi: 10.1016/s0168-8278(96)80238-8. [DOI] [PubMed] [Google Scholar]
- 18.Friedman SL. Stellate cell activation in alcoholic fibrosis—an overview. Alcohol Clin Exp Res. 1999;23:904–910. [PubMed] [Google Scholar]
- 19.French SW. The role of hypoxia in the pathogenesis of alcoholic liver disease. Hepatol Res. 2004;29:69–74. doi: 10.1016/j.hepres.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Lieber CS, DeCarli LM, Sorrell MF. Experimental methods of ethanol administration. Hepatology. 1989;10:501–510. doi: 10.1002/hep.1840100417. [DOI] [PubMed] [Google Scholar]
- 21.Tsukamoto H, French SW, Benson N, et al. Severe and progressive steatosis and focal necrosis in rat liver induced by continuous intragastric infusion of ethanol and low fat diet. Hepatology. 1985;5:224–232. doi: 10.1002/hep.1840050212. [DOI] [PubMed] [Google Scholar]
- 22.Vogel S, Piantedosi R, Frank J, et al. An immortalized rat liver stellate cell line (HSC-T6): a new cell model for the study of retinoid metabolism in vitro. J Lipid Res. 2000;41:882–893. [PubMed] [Google Scholar]
- 23.Koivunen E, Arap W, Valtanen H, et al. Tumor targeting with a selective gelatinase inhibitor. Nat Biotechnol. 1999;17:768–774. doi: 10.1038/11703. [DOI] [PubMed] [Google Scholar]
- 24.Theret N, Musso O, L’Helgoualc’h A, et al. Activation of matrix metalloproteinase-2 from hepatic stellate cells requires interactions with hepatocytes. Am J Pathol. 1997;150:51–58. [PMC free article] [PubMed] [Google Scholar]
- 25.Frederiks WM, Mook OR. Metabolic mapping of proteinase activity with emphasis on in situ zymography of gelatinases: review and protocols. J Histochem Cytochem. 2004;52:711–722. doi: 10.1369/jhc.4R6251.2004. [DOI] [PubMed] [Google Scholar]
- 26.Pignolo RJ, Francis MK, Rotenberg MO, et al. Putative role for EPC-1/PEDF in the G0 growth arrest of human diploid fibroblasts. J Cell Physiol. 2003;195:12–20. doi: 10.1002/jcp.10212. [DOI] [PubMed] [Google Scholar]
- 27.Pollina EA, Legesse-Miller A, Haley EM, et al. Regulating the angiogenic balance in tissues: a potential role for the proliferative state of fibroblasts. Cell Cycle. 2008;7:2056–2070. doi: 10.4161/cc.7.13.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakamura K, Yamagishi SI, Adachi H, et al. Serum levels of pigment epithelium-derived factor (PEDF) are positively associated with visceral adiposity in Japanese patients with type 2 diabetes. Diabetes Metab Res Rev. 2008 doi: 10.1002/dmrr.820. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Nagase H, Woessner JF., Jr Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 30.Bansal MB, Kovalovich K, Gupta R, et al. Interleukin-6 protects hepatocytes from CCl4-mediated necrosis and apoptosis in mice by reducing MMP-2 expression. J Hepatol. 2005;42:548–556. doi: 10.1016/j.jhep.2004.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Popov Y, Patsenker E, Bauer M, et al. Halofuginone induces matrix metalloproteinases in rat hepatic stellate cells via activation of p38 and NFKB. J Biol Chem. 2006;281:15090–15098. doi: 10.1074/jbc.M600030200. [DOI] [PubMed] [Google Scholar]
- 32.Alwayn IP, Andersson C, Lee S, et al. Inhibition of matrix metalloproteinases increases PPAR-α and IL-6 and prevents dietary-induced hepatic steatosis and injury in a murine model. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1011–G1019. doi: 10.1152/ajpgi.00047.2006. [DOI] [PubMed] [Google Scholar]
- 33.Olaso E, Ikeda K, Eng FJ, et al. DDR2 receptor promotes MMP-2-mediated proliferation and invasion by hepatic stellate cells. J Clin Invest. 2001;108:1369–1378. doi: 10.1172/JCI12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117:2362–2368. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ip E, Farrell G, Hall P, et al. Administration of the potent PPARα agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology. 2004;39:1286–1296. doi: 10.1002/hep.20170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.