Abstract
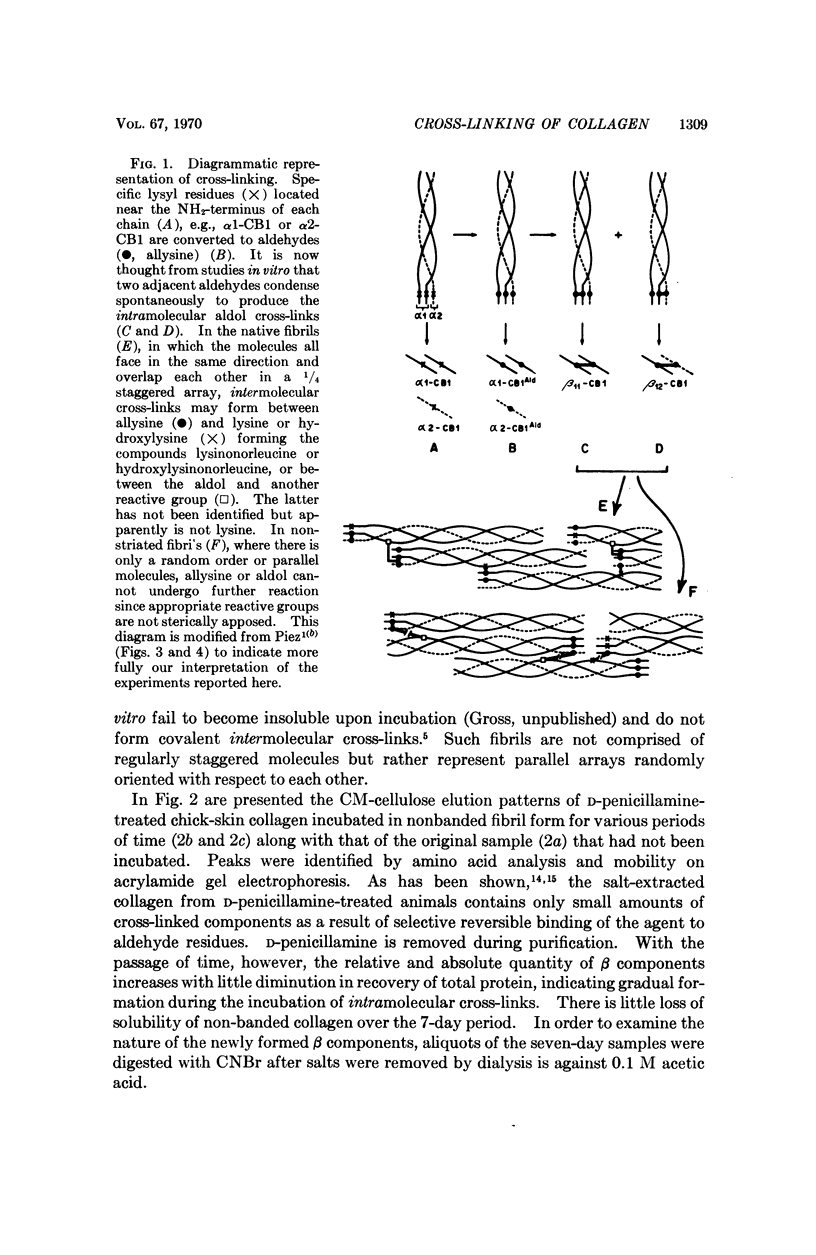
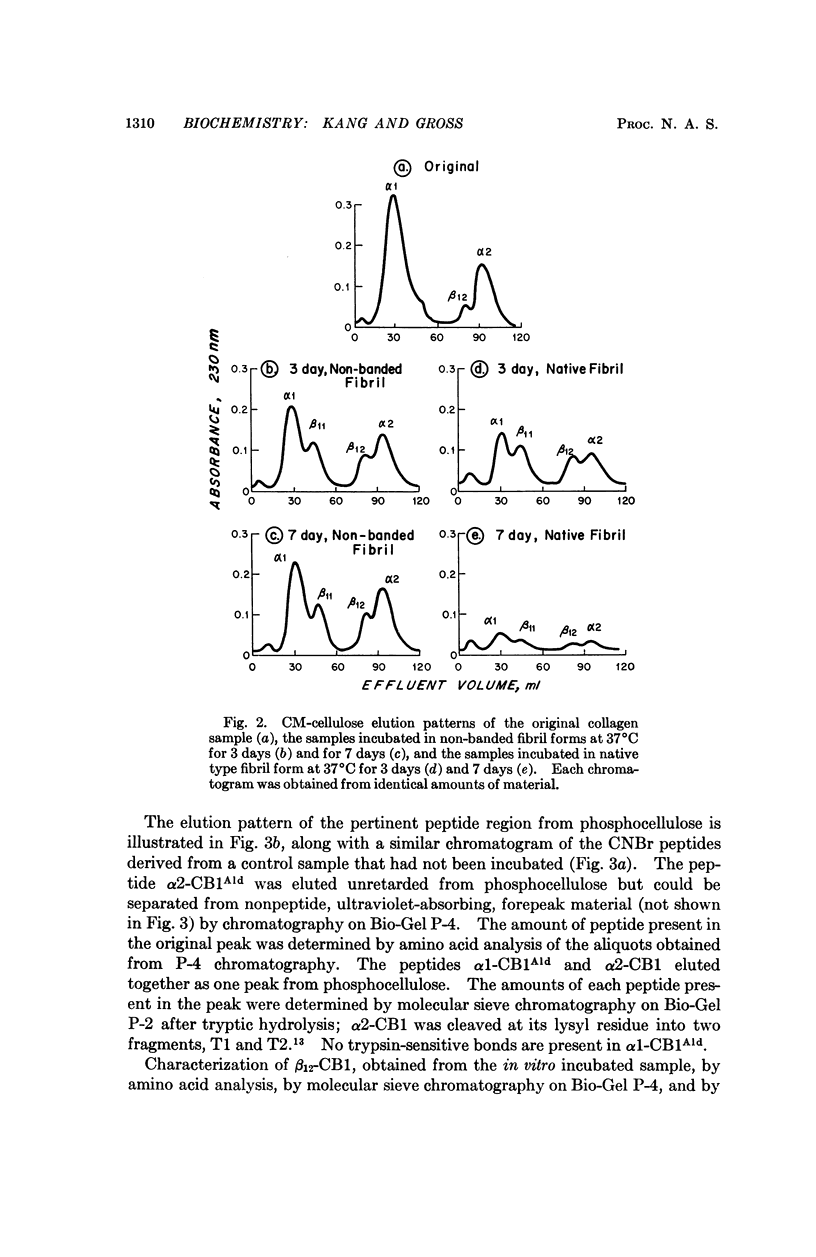
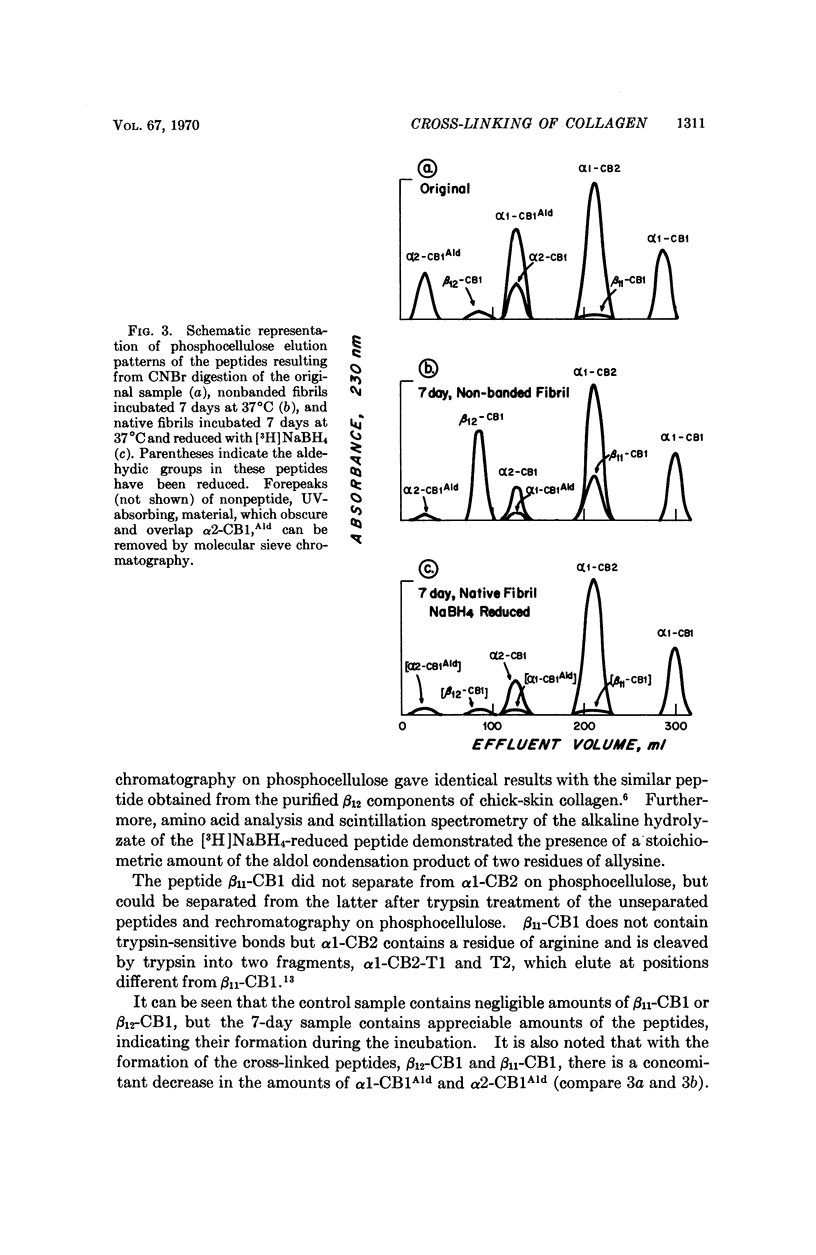
Spontaneous cross-linking in vitro, during incubation of purified collagen in nonstriated and native type fibrils, was studied to elucidate the relationship between intra and intermolecular cross-links. In nonbanded fibrils, the aldol condensation products of two allysyl residues, previously shown to constitute the intramolecular cross-link, formed spontaneously and could be isolated as such, whereas in native type fibrils, these compounds were found to be incorporated into an intermolecular cross-link; after chemical reduction they could not be isolated. Similar results were obtained from studies on a native collagenous tissue, rat tail tendon. It is suggested that the intramolecular cross-link is not a separate entity but only an intermediate of an intermolecular cross-link and that its existence in solubilized collagen is a result of the extraction procedure. The quantitative formation of intramolecular cross-links in vitro and identification of the cross-link compound are also reported.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey A. J., Peach C. M. Isolation and structural identification of a labile intermolecular crosslink in collagen. Biochem Biophys Res Commun. 1968 Dec 9;33(5):812–819. doi: 10.1016/0006-291x(68)90233-7. [DOI] [PubMed] [Google Scholar]
- Bornstein P., Piez K. A. The nature of the intramolecular cross-links in collagen. The separation and characterization of peptides from the cross-link region of rat skin collagen. Biochemistry. 1966 Nov;5(11):3460–3473. doi: 10.1021/bi00875a012. [DOI] [PubMed] [Google Scholar]
- Deshmukh A. D., Nimni M. E. In vitro formation of intramolecular crosslinks in tropocollagen. Biochem Biophys Res Commun. 1969 Jun 27;35(6):845–853. doi: 10.1016/0006-291x(69)90701-3. [DOI] [PubMed] [Google Scholar]
- Franzblau C., Kang A. H., Faris B. In vitro formation of intermolecular crosslinks in chick skin collagen. II. Kinetics. Biochem Biophys Res Commun. 1970 Jul 27;40(2):437–444. doi: 10.1016/0006-291x(70)91028-4. [DOI] [PubMed] [Google Scholar]
- GROSS J., KIRK D. The heat precipitation of collagen from neutral salt solutions: some rate-regulating factors. J Biol Chem. 1958 Aug;233(2):355–360. [PubMed] [Google Scholar]
- Kang A. H., Faris B., Franzblau C. Intramolecular cross-link of chick skin collagen. Biochem Biophys Res Commun. 1969 Aug 7;36(3):345–349. doi: 10.1016/0006-291x(69)90570-1. [DOI] [PubMed] [Google Scholar]
- Kang A. H., Faris B., Franzblau C. The in vitro formation of intermolecular cross-links in chick skin collagen. Biochem Biophys Res Commun. 1970 Apr 8;39(1):175–182. doi: 10.1016/0006-291x(70)90774-6. [DOI] [PubMed] [Google Scholar]
- Kang A. H., Gross J. Amino acid sequence of cyanogen bromide peptides from the amino-terminal region of chick skicollagen. Biochemistry. 1970 Feb 17;9(4):796–804. doi: 10.1021/bi00806a012. [DOI] [PubMed] [Google Scholar]
- Kang A. H., Piez K. A., Gross J. Characterization of the alpha-chains of chick skin collagen and the nature of the NH2-terminal cross-link region. Biochemistry. 1969 Sep;8(9):3648–3655. doi: 10.1021/bi00837a023. [DOI] [PubMed] [Google Scholar]
- Kang A. H., Piez K. A., Gross J. Characterization of the cyanogen bromide peptides from the alpha 1 chain of chick skin collagen. Biochemistry. 1969 Apr;8(4):1506–1514. doi: 10.1021/bi00832a029. [DOI] [PubMed] [Google Scholar]
- Piez K. A. Cross-linking of collagen and elastin. Annu Rev Biochem. 1968;37:547–570. doi: 10.1146/annurev.bi.37.070168.002555. [DOI] [PubMed] [Google Scholar]
- Pinnell S. R., Martin G. R. The cross-linking of collagen and elastin: enzymatic conversion of lysine in peptide linkage to alpha-aminoadipic-delta-semialdehyde (allysine) by an extract from bone. Proc Natl Acad Sci U S A. 1968 Oct;61(2):708–716. doi: 10.1073/pnas.61.2.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojkind M., Gutierrez A. M., Zeichner M., Lent R. W. The nature of the intramolecular cross-link in collagen. Biochem Biophys Res Commun. 1969 Aug 7;36(3):350–356. doi: 10.1016/0006-291x(69)90571-3. [DOI] [PubMed] [Google Scholar]
- Schiffmann E., Martin G. R. Spontaneous generation of cross-links in aldehyde-containing collagen. Arch Biochem Biophys. 1970 May;138(1):226–232. doi: 10.1016/0003-9861(70)90302-4. [DOI] [PubMed] [Google Scholar]
- Tanzer M. L. Collagen reduction by sodium borohydride: effects of reconstitution, maturation and lathyrism. Biochem Biophys Res Commun. 1968 Sep 6;32(5):885–892. doi: 10.1016/0006-291x(68)90324-0. [DOI] [PubMed] [Google Scholar]
- Tanzer M. L. Intermolecular cross-links in reconstituted collagen fibrils. Evidence for the nature of the covalent bonds. J Biol Chem. 1968 Aug 10;243(15):4045–4054. [PubMed] [Google Scholar]



