Abstract
30S ribosomal subunits from Escherichia coli were reacted with three protein reagents. After reaction, the ribosomal proteins were extracted and examined; of the 20 proteins known to compose the particle, nine did not react with any of the three reagents. We have tentatively classified these proteins as „internal,” and the remaining eleven as „external.” A strong correlation was found between these results and the sequence of assembly. Those proteins which enter the assembly process early are classed as internal, whereas those proteins found to participate later in the assembly sequence are classed as external.
Full text
PDF

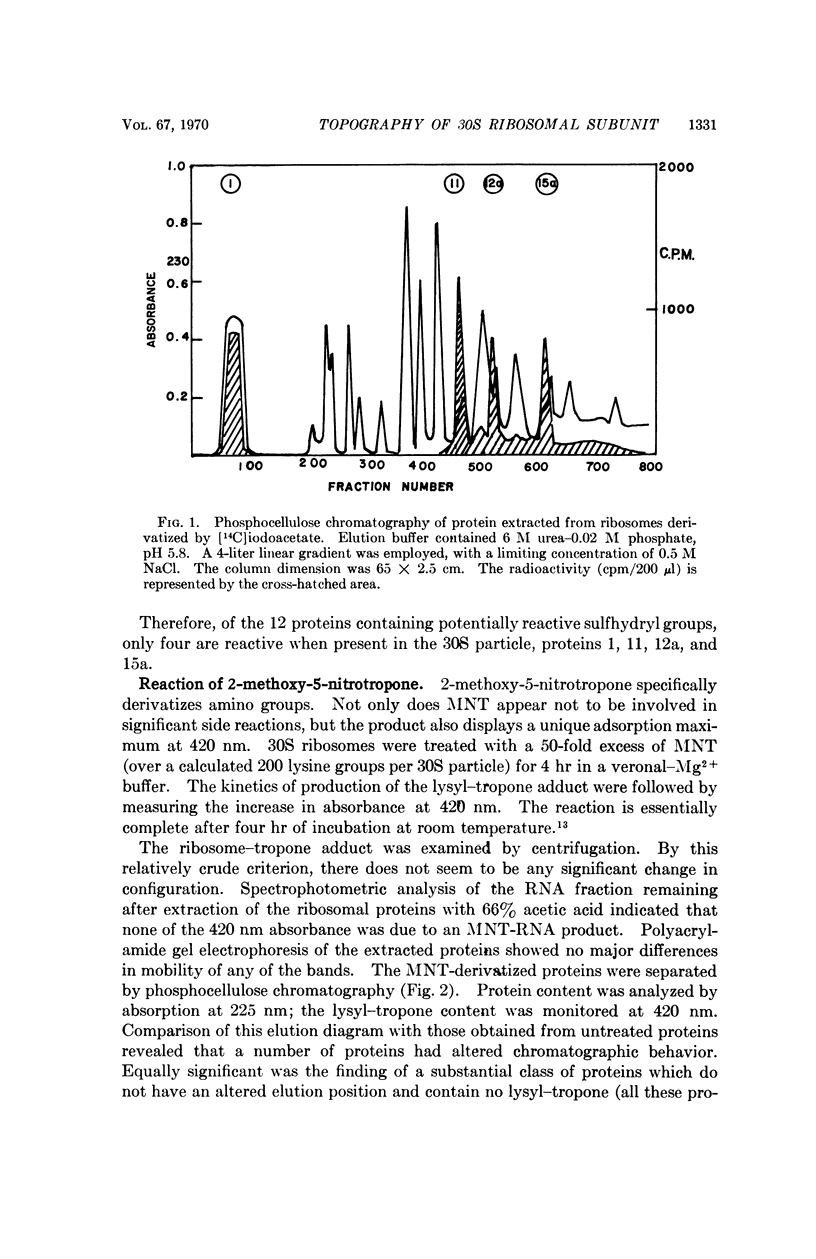

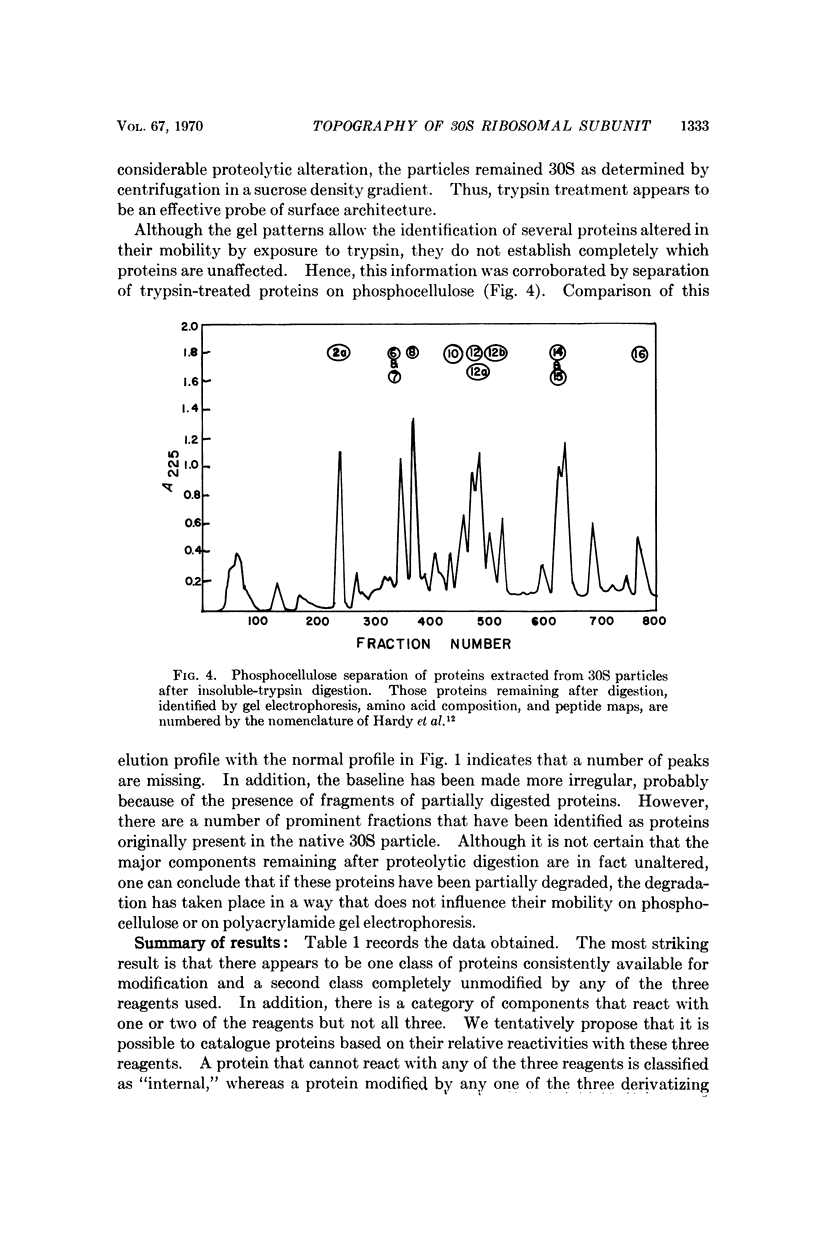

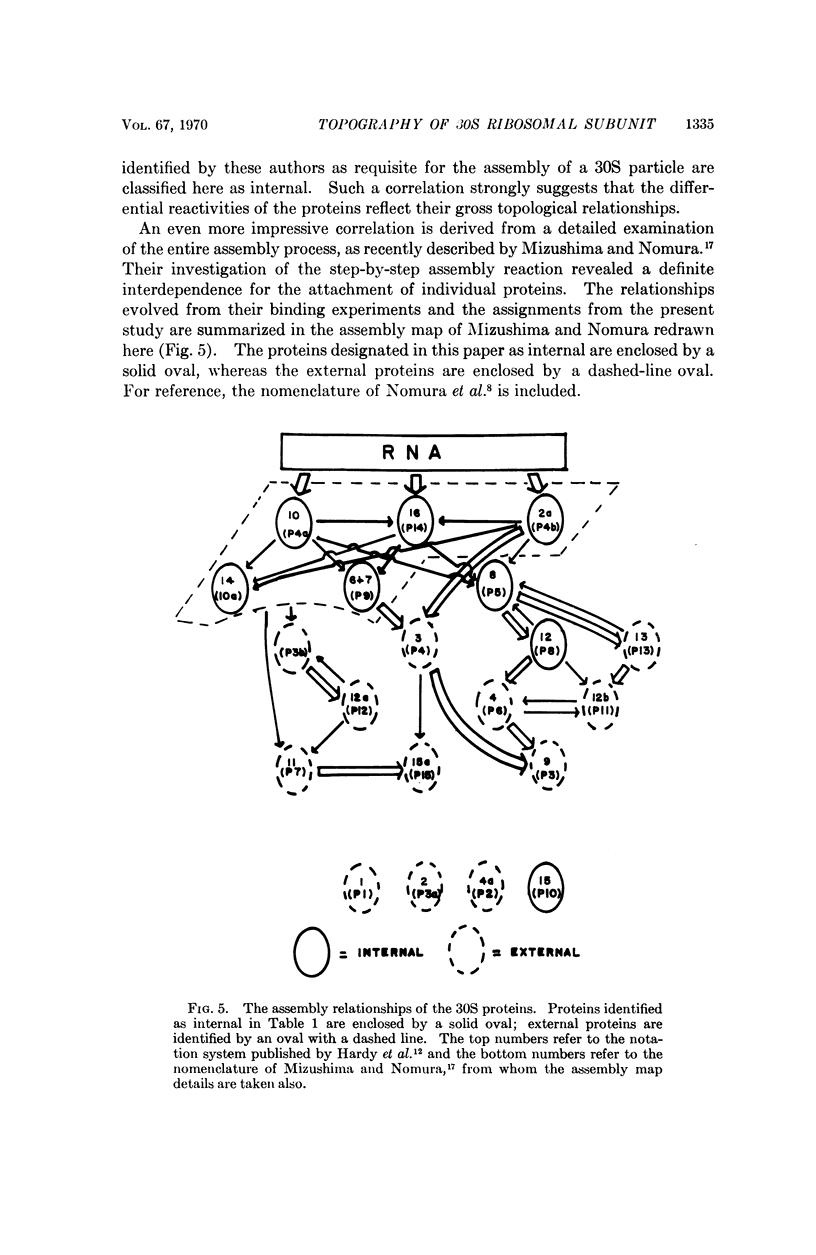

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang F. N., Flaks J. G. Topography of the Escherichia coli 30S ribosomal subunit and streptomycin binding. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1321–1328. doi: 10.1073/pnas.67.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven G. R., Voynow P., Hardy S. J., Kurland C. G. The ribosomal proteins of Escherichia coli. II. Chemical and physical characterization of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2906–2915. doi: 10.1021/bi00835a032. [DOI] [PubMed] [Google Scholar]
- Gesteland R. F. Unfolding of Escherichia coli ribosomes by removal of magnesium. J Mol Biol. 1966 Jul;18(2):356–371. doi: 10.1016/s0022-2836(66)80253-x. [DOI] [PubMed] [Google Scholar]
- Hardy S. J., Kurland C. G., Voynow P., Mora G. The ribosomal proteins of Escherichia coli. I. Purification of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2897–2905. doi: 10.1021/bi00835a031. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt E., Dzionara M., Donner D., Wittmann H. G. Ribosomal proteins. I. Isolation, amino acid composition, molecular weights and peptide mapping of proteins from E. coli ribosomes. Mol Gen Genet. 1967;100(4):364–373. doi: 10.1007/BF00334063. [DOI] [PubMed] [Google Scholar]
- Kurland C. G., Voynow P., Hardy S. J., Randall L., Lutter L. Physical and functional heterogeneity of E. coli ribosomes. Cold Spring Harb Symp Quant Biol. 1969;34:17–24. doi: 10.1101/sqb.1969.034.01.006. [DOI] [PubMed] [Google Scholar]
- LEBOY P. S., COX E. C., FLAKS J. G. THE CHROMOSOMAL SITE SPECIFYING A RIBOSOMAL PROTEIN IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Dec;52:1367–1374. doi: 10.1073/pnas.52.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima S., Nomura M. Assembly mapping of 30S ribosomal proteins from E. coli. Nature. 1970 Jun 27;226(5252):1214–1214. doi: 10.1038/2261214a0. [DOI] [PubMed] [Google Scholar]
- Moore P. B., Traut R. R., Noller H., Pearson P., Delius H. Ribosomal proteins of Escherichia coli. II. Proteins from the 30 s subunit. J Mol Biol. 1968 Feb 14;31(3):441–461. doi: 10.1016/0022-2836(68)90420-8. [DOI] [PubMed] [Google Scholar]
- Nomura M., Mizushima S., Ozaki M., Traub P., Lowry C. V. Structure and function of ribosomes and their molecular components. Cold Spring Harb Symp Quant Biol. 1969;34:49–61. doi: 10.1101/sqb.1969.034.01.009. [DOI] [PubMed] [Google Scholar]
- Sypherd P. S. Amino acid differences in a 30S ribosomal protein from two strains of Escherichia coli. J Bacteriol. 1969 Aug;99(2):379–382. doi: 10.1128/jb.99.2.379-382.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sypherd P. S., O'Neil D. M., Taylor M. M. The chemical and genetic structure of bacterial ribosomes. Cold Spring Harb Symp Quant Biol. 1969;34:77–84. doi: 10.1101/sqb.1969.034.01.012. [DOI] [PubMed] [Google Scholar]
- Tamaoki H., Murase Y., Minato S., Nakanishi K. Reversible chemical modification of protein by 2-methoxy-5-nitrotropone. Renaturation of nitrotroponyl taka-amylase A. J Biochem. 1967 Jul;62(1):7–14. doi: 10.1093/oxfordjournals.jbchem.a128638. [DOI] [PubMed] [Google Scholar]
- WADE H. E., ROBINSON H. K. ABSENCE OF RIBONUCLEASE FROM THE RIBOSOMES OF PSEUDOMONAS FLUORESCENS. Nature. 1963 Nov 16;200:661–663. doi: 10.1038/200661a0. [DOI] [PubMed] [Google Scholar]




