Abstract
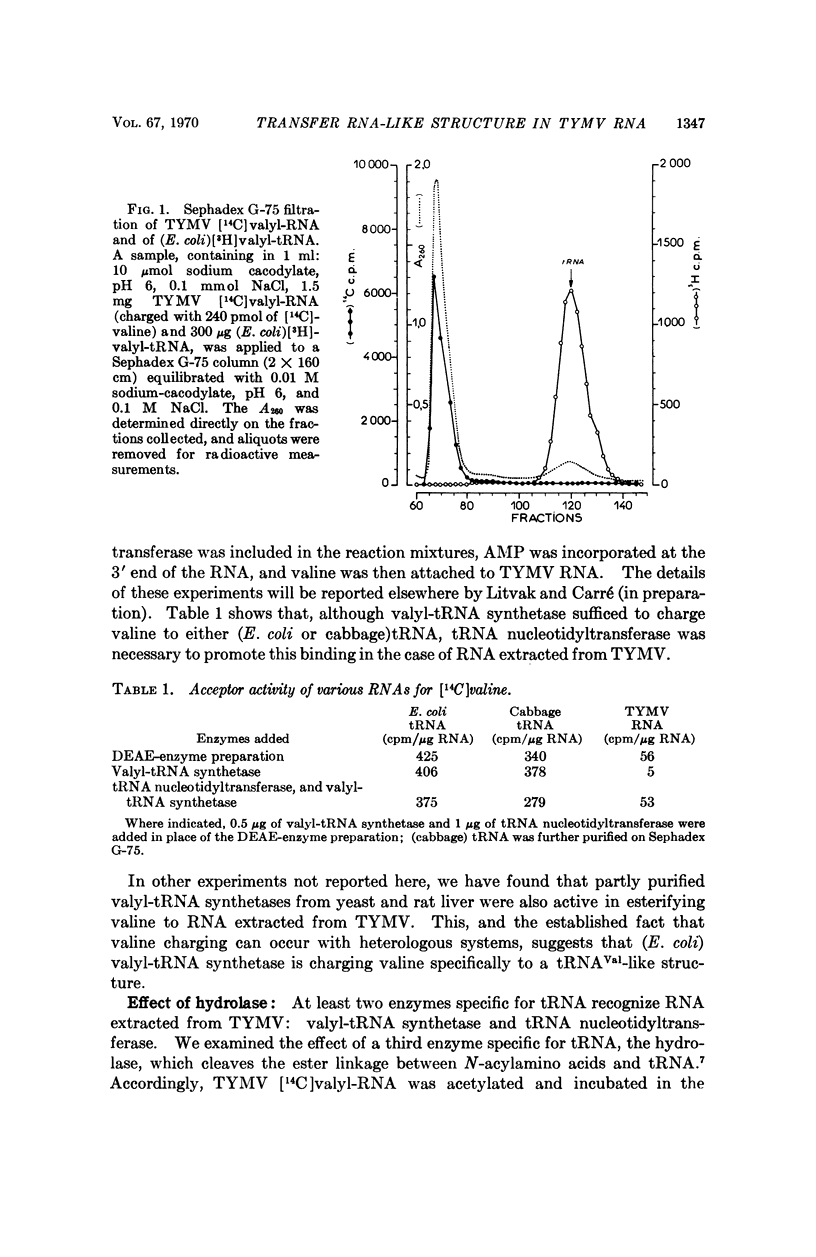
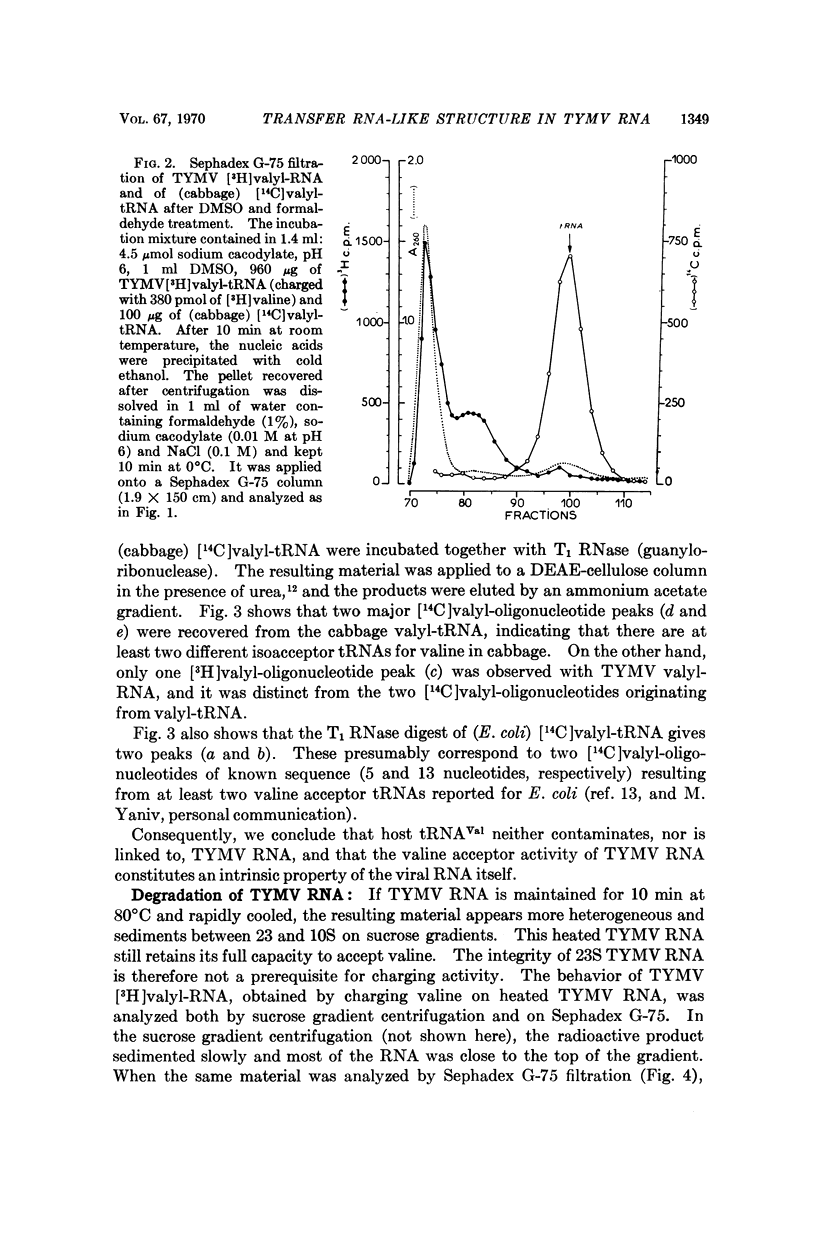
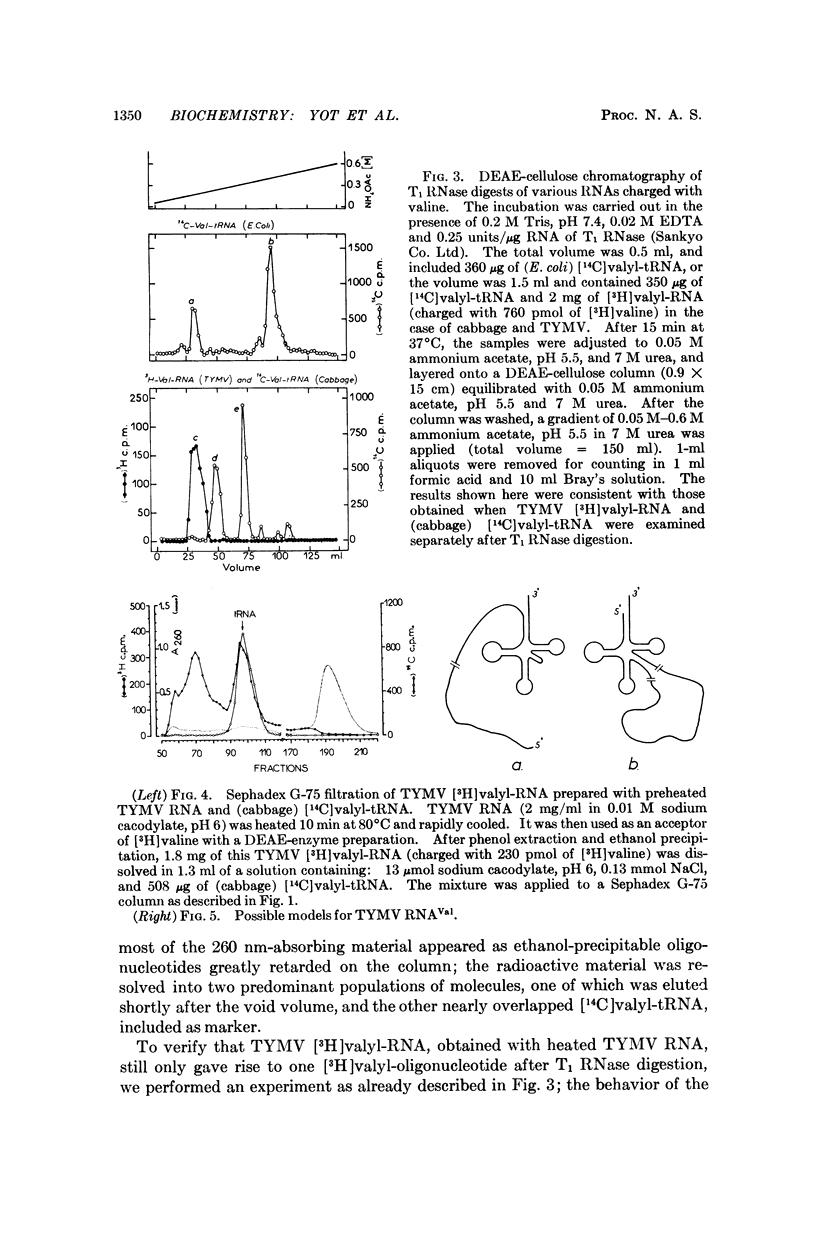
The 3′ terminal nucleotide of turnip yellow mosaic virus (TYMV) RNA (23-25 S) may be esterified with valine in the presence of ATP and an enzyme preparation from Escherichia coli. The nucleotide composition near the valine-binding site is different for TYMV RNA and tRNAVal from cabbage, as shown by comparison of the valine adducts of nucleotides labeled with radioactive valine in T1 RNase digests. Consequently, host tRNAVal is not involved in the observed charging of TYMV RNA with valine. The TYMV RNA appears to have a tRNA-like structure, at or near its 3′ end, that is recognized by three different enzymes which specifically catalyze reactions involving tRNA.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carnegie J. W., Deeney A. O., Olson K. C., Beaudreau G. S. An RNA fraction from myeloblastosis virus having properties similar to transfer RNA. Biochim Biophys Acta. 1969 Oct 22;190(2):274–284. doi: 10.1016/0005-2787(69)90079-3. [DOI] [PubMed] [Google Scholar]
- Cuzin F., Kretchmer N., Greenberg R. E., Hurwitz R., Chapeville F. Enzymatic hydrolysis of N-substituted aminoacyl-tRNA. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2079–2086. doi: 10.1073/pnas.58.5.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., SINGER B., TSUGITA A. Purification of viral RNA by means of bentonite. Virology. 1961 May;14:54–58. doi: 10.1016/0042-6822(61)90131-3. [DOI] [PubMed] [Google Scholar]
- GIERER A., SCHRAMM G. Infectivity of ribonucleic acid from tobacco mosaic virus. Nature. 1956 Apr 14;177(4511):702–703. doi: 10.1038/177702a0. [DOI] [PubMed] [Google Scholar]
- Haenni A. L., Chapeville F. The behaviour of acetylphenylalanyl soluble ribonucleic acid in polyphenylalanine synthesis. Biochim Biophys Acta. 1966 Jan 18;114(1):135–148. doi: 10.1016/0005-2787(66)90261-9. [DOI] [PubMed] [Google Scholar]
- Katz L., Penman S. The solvent denaturation of double-stranded RNA from poliovirus infected HeLa cells. Biochem Biophys Res Commun. 1966 May 25;23(4):557–560. doi: 10.1016/0006-291x(66)90765-0. [DOI] [PubMed] [Google Scholar]
- Pinck M., Yot P., Chapeville F., Duranton H. M. Enzymatic binding of valine to the 3' end of TYMV-RNA. Nature. 1970 Jun 6;226(5249):954–956. doi: 10.1038/226954a0. [DOI] [PubMed] [Google Scholar]
- Trávnícek M. Some properties of amino acid-acceptor RNA isolated from avian tumour virus BAI strain A (avian myeloblastosis). Biochim Biophys Acta. 1969 Jun 17;182(2):427–439. [PubMed] [Google Scholar]
- Yaniv M., Barrell B. G. Nucleotide sequence of E. coli B tRNA1-Val. Nature. 1969 Apr 19;222(5190):278–279. doi: 10.1038/222278a0. [DOI] [PubMed] [Google Scholar]
- Yaniv M., Gros F. Studies on valyl-tRNA synthetase and tRNA from Escherichia coli. I. Purification and properties of the enzyme from normal Escherichia coli strains. J Mol Biol. 1969 Aug 28;44(1):1–15. doi: 10.1016/0022-2836(69)90401-x. [DOI] [PubMed] [Google Scholar]


