Abstract
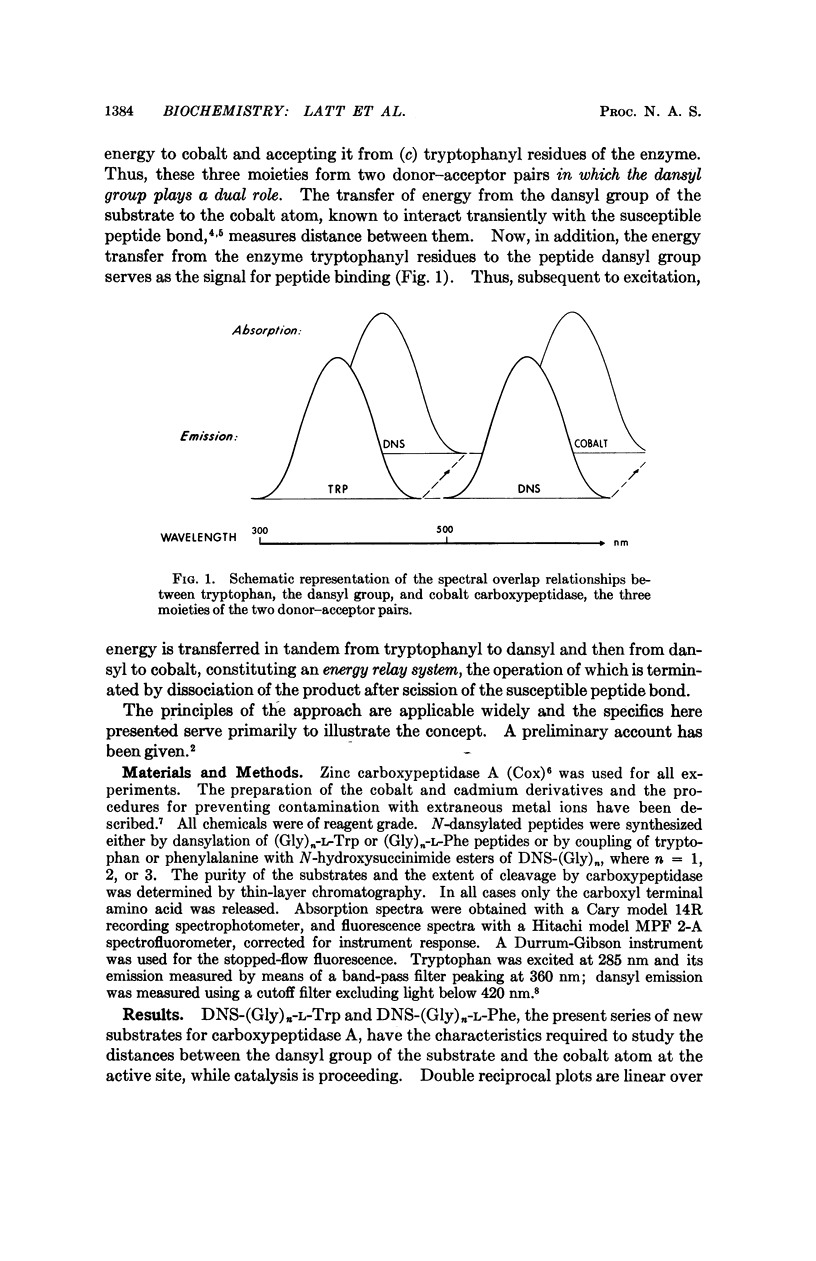
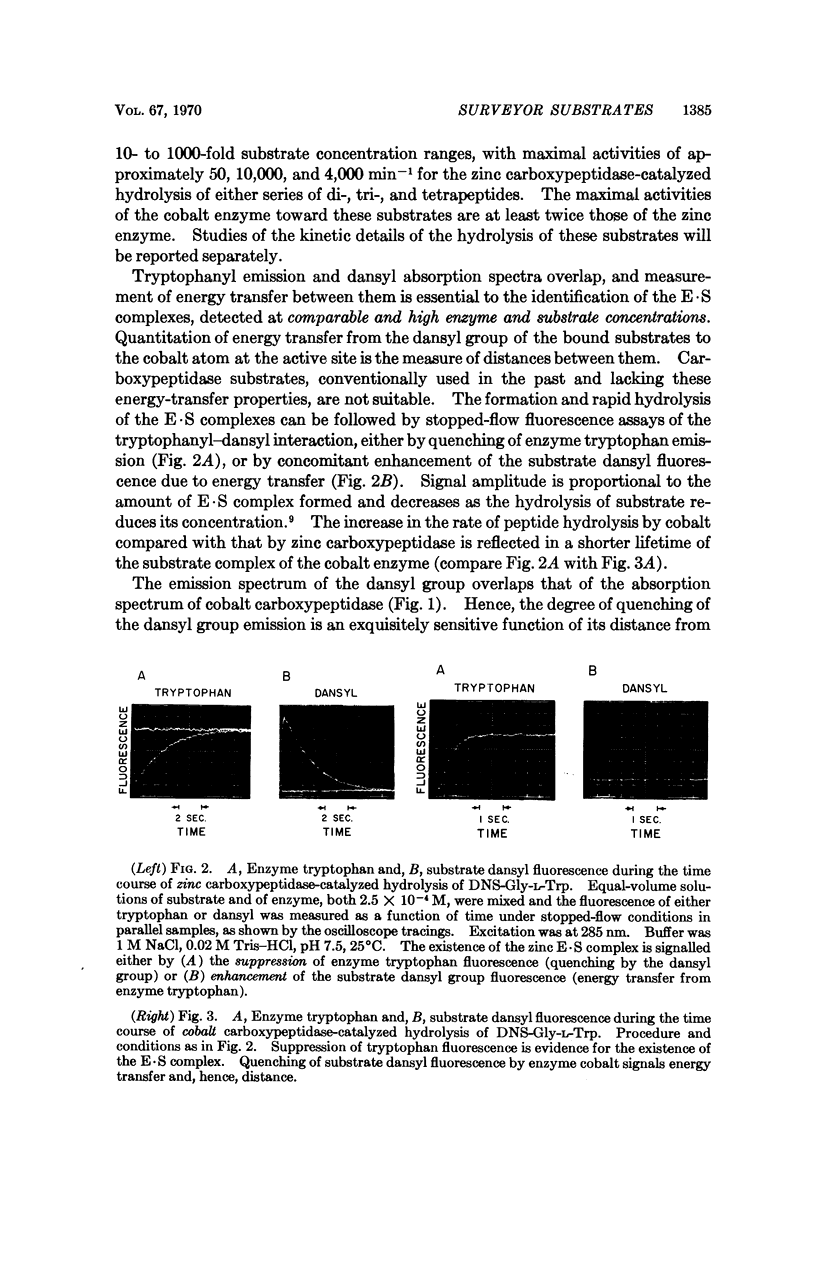
An approach is described for the simultaneous measurement of catalysis, the distance from an active-site moiety to fluorescent substrate moieties of an enzyme substrate complex, and the microenvironment of such substrate groups. It is illustrated by means of a three-component energy transfer relay system, consisting of cobalt carboxypeptidase, its fluorescent dansylated peptide substrates, and tryptophanyl residues of the enzyme. The mode of procedure can be applied generally to yield conjoint information on functional and structural aspects of active centers of enzymes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramowitz N., Schechter I., Berger A. On the size of the active site in proteases. II. Carboxypeptidase-A. Biochem Biophys Res Commun. 1967 Dec 29;29(6):862–867. doi: 10.1016/0006-291x(67)90299-9. [DOI] [PubMed] [Google Scholar]
- Blow D. M., Steitz T. A. X-ray diffraction studies of enzymes. Annu Rev Biochem. 1970;39:63–100. doi: 10.1146/annurev.bi.39.070170.000431. [DOI] [PubMed] [Google Scholar]
- COLEMAN J. E., VALLEE B. L. Metallocarboxypeptidases: stability constants and enzymatic characteristics. J Biol Chem. 1961 Aug;236:2244–2249. [PubMed] [Google Scholar]
- COX D. J., BOVARD F. C., BARGETZI J. P., WALSH K. A., NEURATH H. PROCEDURES FOR THE ISOLATION OF CRYSTALLINE BOVINE PANCREATIC CARBOXYPEPTIDASE A. II. ISOLATION OF CARBOXYPEPTIDASE A-ALPHA FROM PROCARBOXYPEPTIDASE A. Biochemistry. 1964 Jan;3:44–47. doi: 10.1021/bi00889a008. [DOI] [PubMed] [Google Scholar]
- Christen P., Riordan J. F. Syncatalytic modification of a functional tyrosyl residue in aspartate aminotransferase. Biochemistry. 1970 Jul 21;9(15):3025–3034. doi: 10.1021/bi00817a014. [DOI] [PubMed] [Google Scholar]
- Conrad R. H., Brand L. Intramolecular transfer of excitation from tryptophan to 1-dimethylaminonaphthalene 5-sulfonamide in a series of model compounds. Biochemistry. 1968 Feb;7(2):777–787. doi: 10.1021/bi00842a036. [DOI] [PubMed] [Google Scholar]
- Davies R. C., Riordan J. F., Auld D. S., Vallee B. L. Kinetics of carboxypeptidase A. I. Hydrolysis of carbobenzoxyglycyl-l-phenylalanine, benzoylglycyl-l-phenylalanine, and hippuryl-dl-beta-phenyllactic acid by metal-substituted and acetylated carboxypeptidases. Biochemistry. 1968 Mar;7(3):1090–1099. doi: 10.1021/bi00843a029. [DOI] [PubMed] [Google Scholar]
- LATT S. A., CHEUNG H. T., BLOUT E. R. ENERGY TRANSFER. A SYSTEM WITH RELATIVELY FIXED DONOR-ACCEPTOR SEPARATION. J Am Chem Soc. 1965 Mar 5;87:995–1003. doi: 10.1021/ja01083a011. [DOI] [PubMed] [Google Scholar]
- Lipscomb W. N., Reeke G. N., Jr, Hartsuck J. A., Quiocho F. A., Bethge P. H. The structure of carboxypeptidase A. 8. Atomic interpretation at 0.2 nm resolution, a new study of the complex of glycyl-L-tyrosine with CPA, and mechanistic deductions. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):177–214. doi: 10.1098/rstb.1970.0020. [DOI] [PubMed] [Google Scholar]
- Stryer L. Fluorescence spectroscopy of proteins. Science. 1968 Nov 1;162(3853):526–533. doi: 10.1126/science.162.3853.526. [DOI] [PubMed] [Google Scholar]
- VALLEE B. L., RIORDAN J. F., COLEMAN J. E. Carboxypeptidase A: approaches to the chemical nature of the active center and the mechanisms of action. Proc Natl Acad Sci U S A. 1963 Jan 15;49:109–116. doi: 10.1073/pnas.49.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]



