Abstract
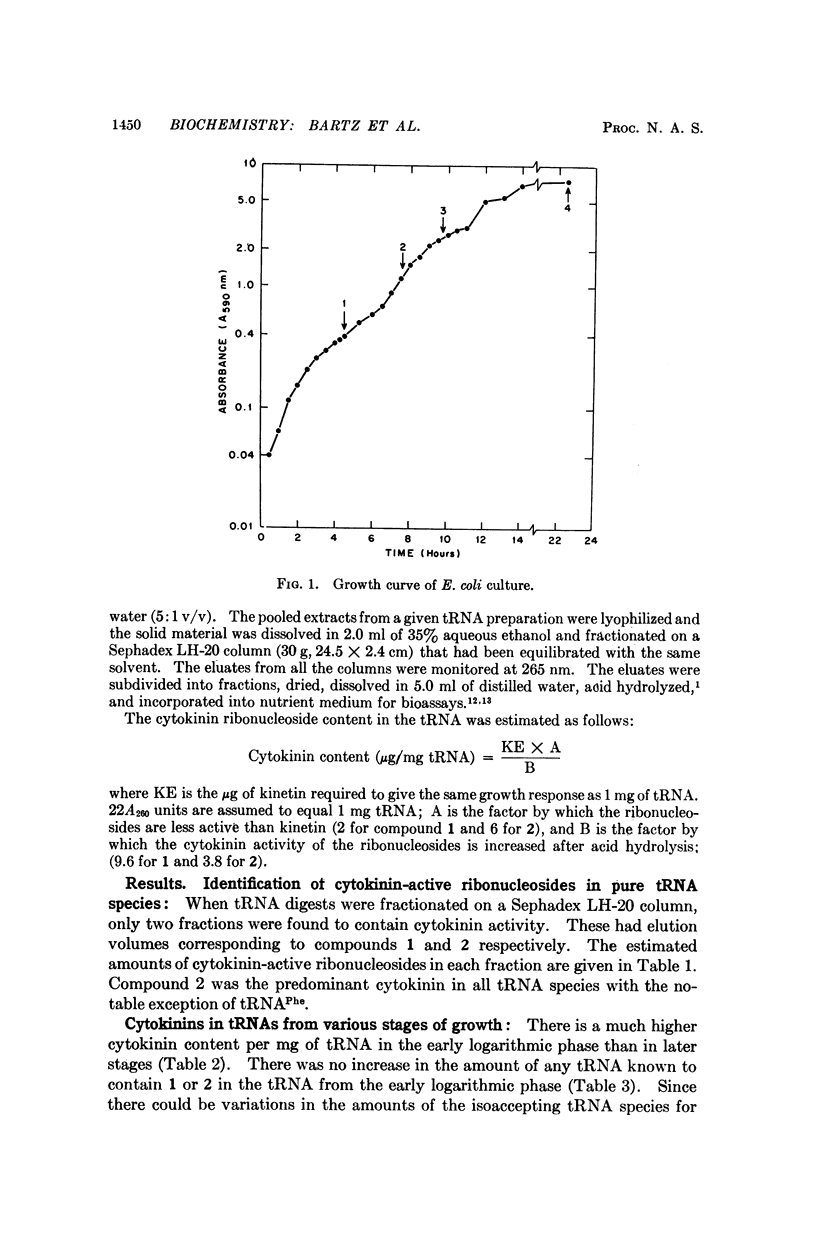
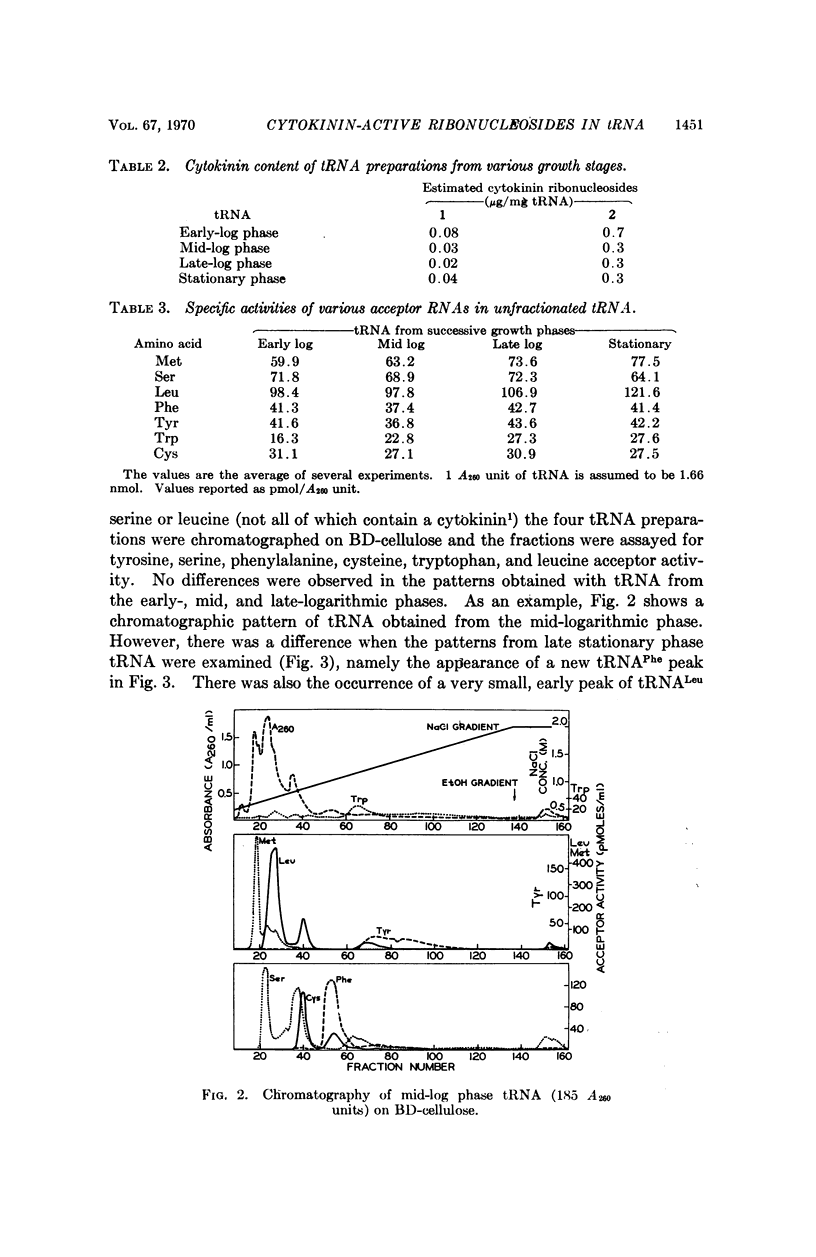
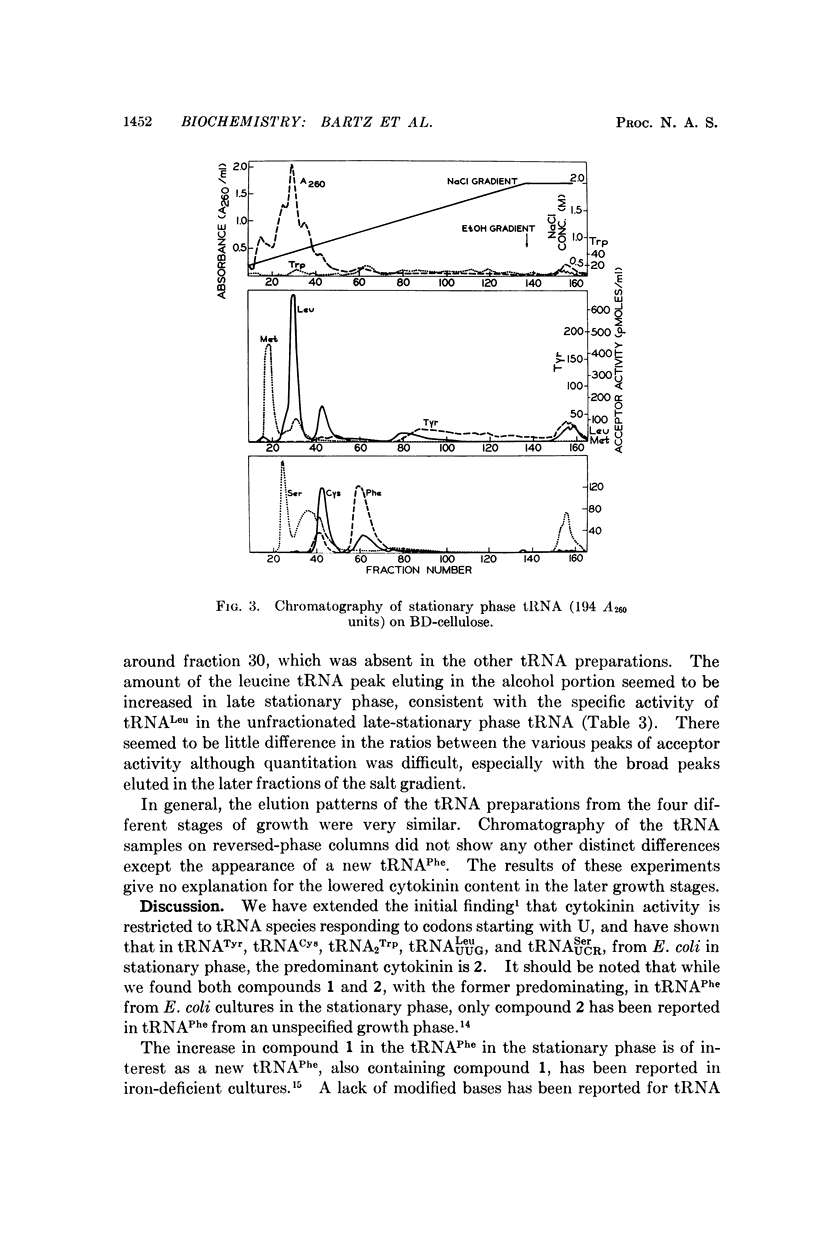
The distribution of the two cytokinin-active ribonucleosides 6-(3-methyl-2-butenylamino)-9-β-D-ribofuranosylpurine (1) and 6-(3-methyl-2-butenylamino)-2-methylthio-9-β-D-ribofuranosylpurine (2) in tRNAPhe, tRNAUUGLeu, tRNAUCRSer, tRNATyr, tRNACys, and tRNA2Trp from stationary phase Escherichia coli has been studied. Compound 2 predominated in all these species except tRNAPhe, in which compound 1 was greatly in excess. Compound 1 was present in relatively small amounts in tRNAUUGLeu, tRNACys, and tRNA2Trp, and was not detected in tRNAUCRSer and tRNATyr. Cells from the early logarithmic phase had the highest cytokinin content in their tRNA. Stationary phase tRNA had a slight increase in the amount of 1, which may be explained by the appearance of the new tRNAPhe. No differences were observed between the chromatographic patterns of tRNAs from early-, mid-, and latelogarithmic phases.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arceneaux J. L., Sueoka N. Two species of Bacillus subtilis tyrosine transfer ribonucleic acid. Biological properties and alteration in their relative amounts during growth. J Biol Chem. 1969 Nov 10;244(21):5959–5966. [PubMed] [Google Scholar]
- Armstrong D. J., Burrows W. J., Evans P. K., Skoog F. Isolation of cytokinins from tRNA. Biochem Biophys Res Commun. 1969 Oct 22;37(3):451–456. doi: 10.1016/0006-291x(69)90936-x. [DOI] [PubMed] [Google Scholar]
- Armstrong D. J., Burrows W. J., Skoog F., Roy K. L., Söll D. Cytokinins: distribution in transfer RNA species of Escherichia coli. Proc Natl Acad Sci U S A. 1969 Jul;63(3):834–841. doi: 10.1073/pnas.63.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows W. J., Armstrong D. J., Skoog F., Hecht S. M., Boyle J. T., Leonard N. J., Occolowitz J. Cytokinin from soluble RNA of Escherichia coli: 6-(3-methyl-2-butenylamino)-2-methylthio-9-beta-D-ribofuranosylpurine. Science. 1968 Aug 16;161(3842):691–693. doi: 10.1126/science.161.3842.691. [DOI] [PubMed] [Google Scholar]
- Burrows W. J., Armstrong D. J., Skoog F., Hecht S. M., Boyle J. T., Leonard N. J., Occolowitz J. The isolation and identification of two cytokinins from Escherichia coli transfer ribonucleic acids. Biochemistry. 1969 Jul;8(7):3071–3076. doi: 10.1021/bi00835a057. [DOI] [PubMed] [Google Scholar]
- Gefter M. L., Russell R. L. Role modifications in tyrosine transfer RNA: a modified base affecting ribosome binding. J Mol Biol. 1969 Jan 14;39(1):145–157. doi: 10.1016/0022-2836(69)90339-8. [DOI] [PubMed] [Google Scholar]
- Gillam I., Blew D., Warrington R. C., von Tigerstrom M., Tener G. M. A general procedure for the isolation of specific transfer ribonucleic acids. Biochemistry. 1968 Oct;7(10):3459–3468. doi: 10.1021/bi00850a022. [DOI] [PubMed] [Google Scholar]
- Goodman H. M., Abelson J. N., Landy A., Zadrazil S., Smith J. D. The nucleotide sequences of tyrosine transfer RNAs of Escherichia coli. Eur J Biochem. 1970 Apr;13(3):461–483. doi: 10.1111/j.1432-1033.1970.tb00950.x. [DOI] [PubMed] [Google Scholar]
- Jacobson M., Hedgcoth C. Levels of 5,6-dihydrouridine in relaxed and chloramphenicol transfer ribonucleic acid. Biochemistry. 1970 Jun 9;9(12):2513–2519. doi: 10.1021/bi00814a018. [DOI] [PubMed] [Google Scholar]
- Lengyel P., Söll D. Mechanism of protein biosynthesis. Bacteriol Rev. 1969 Jun;33(2):264–301. doi: 10.1128/br.33.2.264-301.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muench K. H., Safille P. A. Transfer ribonucleic acids in Escherichia coli. Multiplicity and variation. Biochemistry. 1968 Aug;7(8):2799–2808. doi: 10.1021/bi00848a015. [DOI] [PubMed] [Google Scholar]
- Nishimura S., Harada F., Narushima U., Seno T. Purification of methionine-, valine-, phenylalanine- and tyrosine-specific tRNA from Escherichia coli. Biochim Biophys Acta. 1967 Jun 20;142(1):133–148. doi: 10.1016/0005-2787(67)90522-9. [DOI] [PubMed] [Google Scholar]
- Nishimura S., Yamada Y., Ishikura H. The presence of 2-methylthio-N6-(delta-2-isopentenyl) adenosine in serine and phenylalanine transfer RNA's from Escherichia coli. Biochim Biophys Acta. 1969 Apr 22;179(2):517–520. doi: 10.1016/0005-2787(69)90065-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Gefter M. L. An iron-dependent modification of several transfer RNA species in Escherichia coli. J Mol Biol. 1969 Dec 28;46(3):581–584. doi: 10.1016/0022-2836(69)90197-1. [DOI] [PubMed] [Google Scholar]
- Roy K. L., Söll D. Purification of five serine transfer ribonucleic acid species from Escherichia coli and their acylation by homologous and heterologous seryl transfer ribonucleic acid synthetases. J Biol Chem. 1970 Mar 25;245(6):1394–1400. [PubMed] [Google Scholar]
- Skoog F., Armstrong D. J., Cherayil J. D., Hampel A. E., Bock R. M. Cytokinin activity: localization in transfer RNA preparations. Science. 1966 Dec 9;154(3754):1354–1356. doi: 10.1126/science.154.3754.1354. [DOI] [PubMed] [Google Scholar]
- Söll D., Cherayil J. D., Bock R. M. Studies on polynucleotides. LXXV. Specificity of tRNA for codon recognition as studied by the ribosomal binding technique. J Mol Biol. 1967 Oct 14;29(1):97–112. doi: 10.1016/0022-2836(67)90183-0. [DOI] [PubMed] [Google Scholar]
- Wettstein F. O., Stent G. S. Physiologically induced changes in the property of phenylalanine tRNA in Escherichia coli. J Mol Biol. 1968 Nov 28;38(1):25–40. doi: 10.1016/0022-2836(68)90126-5. [DOI] [PubMed] [Google Scholar]



